Abstract
Chronic kidney disease is increasingly common in older adults. Estimating the glomerular filtration rate can be challenging in this population, with sarcopenia affecting the accuracy of various formulae. Competing risks of death influence the risk of progression to end stage kidney disease. In managing chronic kidney disease in this population, one must take into consideration other comorbidities including assessment of geriatric syndromes. More research is still needed to guide medical management in this heterogeneous population.
Introduction
Increasing longevity in most developing and developed countries is resulting in global aging. The United Nations projects that the global population age 65 and older will triple from 0.5 billion in 2010 to 1.5 billion by 2050.1 In the United States (US), the number of older individuals (age 65 and older) will double from 40 million to 80 million over the next 30 years. Global aging has far-reaching implications for health care systems which will need to grapple with rapidly increasing demands for health care services. The majority of older adults have two or more chronic health conditions. Syndromes such as frailty, cognitive impairment, and sensory impairment disproportionately affect older adults and result in reduced independence. Many of these issues are magnified in the chronic kidney disease (CKD) population. Herein, we discuss the epidemiology of CKD in older individuals and public health implications.
Estimation of Glomerular Filtration Rate in Older Adults
Chronic kidney disease is defined as a glomerular filtration rate (GFR) of <60 ml/min/1.73m2 or markers of kidney damage, such as albuminuria, for greater than 3 months.2 Various creatinine-based formulae are used to estimate GFR, including the Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. Few older adults were included in the development of these estimating equations, although validation of these equations using iohexol or measured creatinine clearance as the reference standard in older cohorts have generally shown that these equations are reasonably accurate.3 The MDRD equation is less accurate at higher levels of GFR, relative to the CKD-EPI equation, although the importance of these subtle differences in clinical practice may be subject to debate. More recently, the Berlin Initiative Study (BIS) developed an equation to estimate GFR using age, sex and serum creatinine in 600 participants aged 70 years or older.4,5 Compared to other creatinine-based equations, the BIS equation had comparable or better accuracy, reduced bias, and improved precision. In particular, the BIS equation tends to reclassify patients to a higher eGFR compared to other equations. External validation of the BIS equation has been performed in European and Brazilian cohorts with comparable performance characteristics.6
Cystatin C is an alternate biomarker of kidney function that is less affected by muscle mass than creatinine.7 For this reason, cystatin C has been suggested as a superior measure of kidney function in older individuals, since they are more likely to be affected by sarcopenia. Several GFR estimating equations, including the CKD-EPI8 and BIS equations,9 have versions which incorporate cystatin C measurements in addition to creatinine.
Epidemiology of Chronic Kidney Disease in Older Adults
Data from the National Health and Nutrition Examination Surveys 1999–2004 estimate that approximately 40% of adults above age 60 meet the current definition for CKD using the MDRD equation to estimate GFR. Among older adults, approximately 7% have CKD stage 1–2, 30% have CKD stage 3, and 5% have CKD stage 4–5.10 Using the CKD-EPI equation, the prevalence of CKD is lower among adults age 60–69 (due to a lower percentage of adults with stage 3 CKD), and similar among those age ≥70 years.11 Among adults age ≥80 years, the prevalence of CKD using the CKD-EPI equation was >50% between 2005–2010, representing an absolute increase of more than 11% compared to the prior decade.12
The adjusted incident rate of treated end stage renal disease (ESRD) in the US has grown 7.1% for patients age 75 and older, to 1,707 per million population in 2011. In contrast, incidence rates for patients age 45–64 and 65–74, are now 8.1–8.3 percent lower than in 2000, at 571 and 1,307 per million, respectively.2
Prognosis of CKD in Older Adults
In the absence of albuminuria or known cause of kidney disease, some question whether a low GFR in older persons is a true disease state. This is especially applicable to those with only a modest reduction in GFR (e.g. GFR 50–60 ml/min/1.73m2), a large proportion of the older adult population with CKD. Indeed, some argue that age-related structural changes in the kidney can be considered a normal part of aging, especially in the absence of other electrolyte or metabolic abnormalities.16
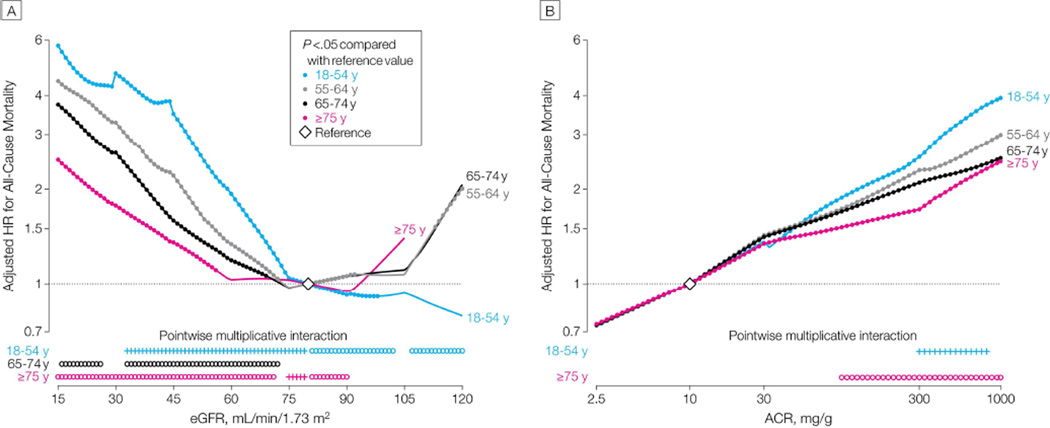
To help clarify the clinical relevance of CKD in older adults, several studies have examined the prognosis of older adults with varying stages of CKD. The largest of these analyses, from Chronic Kidney Disease Prognosis Consortium, included more than 2 million adults from 46 cohort studies from general, high-risk and CKD populations.17 In these analyses, mortality was higher with higher levels of albuminuria and lower levels of eGFR (using the creatinine CKD-EPI equation) in all age groups (FIGURE 1).18 The absolute risk for mortality was higher at older age groups at all levels of albuminuria and eGFR. For albuminuria, the relative risk for mortality was consistent by age, except at higher levels of albuminuria (e.g. >100 mg/g), where relative risk was slightly lower at older ages. For eGFR, interaction with age was evident across a broad range of eGFR. Among adults age 18–54, there was a linear relationship between lower eGFR and higher mortality risk. Among adults age ≥54, a J-shaped relation between eGFR and mortality was observed. There was an increased risk of mortality as eGFR dropped below 80 ml/min, but this association was less pronounced compared to younger adults. There was also a linear increase in mortality for eGFR levels >80 ml/min, which likely reflects sarcopenia rather than an elevated GFR. The absolute risk of ESRD was slightly lower at older vs. younger age groups for albuminuria levels >30 mg/g and eGFR levels <45 ml/min/1.73m2. The relative risk for ESRD was generally similar across age groups for eGFR <60 ml/min/1.73m2 or albuminuria >30 mg/g, and no significant age interaction was detected. The finding of lower absolute risk of ESRD among older adults should be interpreted with some caution, since this study considered only treated ESRD as an outcome. For example, in a Canadian population-based study, Hemmelgarn et al. identified patients with treated and untreated ESRD. In this study, the rate of untreated ESRD was five times higher in adults aged ≥ 85 compared to those aged 18–44.19
Figure 1.
A: Adjusted Hazard Ratio (HR) for All-Cause Mortality.
B: Adjusted HR for All-Cause Mortality by Albumin-Creatinine Ratio (ACR)
Reprinted with permission from: Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. Dec 12 2012;308(22):2349–2360
In older adults, the competing risk of death influences whether someone progresses to ESRD. Prediction of whether older patients will progress to ESRD will inform decisions as to whether and when preparation for renal replacement therapy should take place. In a large cohort of US Veterans with CKD stages 3–5, the absolute risk of death was much higher than risk of progression to ESRD in those >75 years of age, even in those with CKD stage 5. Conversely, in patients younger than age 45, the risk of progression to ESRD was greater than the risk of death, even for those with CKD stage 2–3.20 Similar findings have been observed in other populations. For example, in an Italian cohort of 1200 adults averaging 65 years of age, patients with less advanced CKD were more likely to die rather than progress to ESRD.21 In particular, ESRD occurred more frequently than death in stage 4 and 5 CKD, whereas the opposite was true in stage 3 CKD. Similarly, in a cohort of approximately 3000 older adults averaging 70 years of age, the risk of death and ESRD were similar for patients with CKD 4. In this study, factors such as older age, heart failure, lower hemoglobin level, lower phosphate level, malignancy, diabetes, and male sex were associated with higher risk of death prior to ESRD.22
In addition to death and ESRD, acute kidney injury (AKI) is also an important consequence of CKD in older adults. The incidence of AKI increases with age and caries a high morbidity and mortality.23,24 Older adults with CKD are more susceptible to AKI events, which in turn, may contribute to progression of CKD. Efforts to reduce of the number and severity of AKI episodes may be an important component of CKD management in older adults.
Clinical and public health implications of CKD in older adults
The high and increasing prevalence of CKD among older adults has several potential public health and clinical implications. One of the main issues to consider is whether all older adults with CKD benefit from nephrology care. It should be evident that there are not enough nephrologists to care for the large number of older adults with CKD. A variety of risk stratification approaches have been suggested in order to better target nephrology care to those at highest risk of adverse outcomes, including the incorporation of cystatin C for eGFR estimation, or the assessment of geriatric syndromes such as frailty. For example, KDIGO guidelines suggest the use of cystatin C as a confirmatory test for CKD.25 A related issue is whether a diagnosis of CKD meaningfully changes clinical management of older adults with eGFR levels between 45–59 ml/min/1.73m2. Agents such as angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) may slow progression of CKD especially when there is concomitant proteinuria, although few of these trials included patients older than 70 years.26 However, such agents may predispose older patients to AKI24 and older patients may not survive long enough to benefit from this therapy.27 Further, some argue that given slow average rate of progression of CKD in older patients with eGFR between 45–59 ml/min/1.73m2, managing these patients differently than their equal age counterparts with normal GFR may not change the outcome.28
Much heterogeneity exists among older adults of a similar age and eGFR. The presence of pre-existing comorbid conditions and geriatric syndromes limit the utility of a single therapeutic approach that may work reasonably well in younger populations. Frailty is a syndrome characterized by weight loss and loss of muscle strength and endurance, leading to increased vulnerability to stressors. Frailty is more prevalent in those with CKD,29,30 and is associated with an increased risk for death, ESRD, and earlier dialysis initiation.23,31 Thus, the identification of frailty may be useful for risk stratification of older adults with CKD. Drawz et al developed and validated a model to predict development of ESRD in Veterans age ≥65 with CKD and eGFR <30 ml/min.32 Younger age, congestive heart failure, higher systolic blood pressure, lower eGFR, lower potassium, and lower albumin were all associated with risk of progression to ESRD. Metabolic complications of CKD, such as a low serum bicarbonate concentration, may also predict progression of CKD among older adults.33
For older adults with established CKD, the high prevalence of co-existing conditions and geriatric syndromes may complicate CKD management and contribute to both under-treatment and over-treatment. For example, medications such as beta-blockers and ACE inhibitors appear to be under-prescribed post-myocardial infarction in older patients with CKD.34 Conversely, in other circumstances, CKD may increase the potential for medication side effects – for example from spironolactone35 or warfarin36. Similarly, guidelines are conflicting regarding the optimal blood pressure targets and choice of blood pressure-lowering agents for older patients with CKD.37 Given the heterogeneity in patient characteristics and preferences, it is important to consider which outcomes matter most for individual patients when making decisions about therapy. For example, some patients may care most about maximizing longevity, whereas others may consider quality of life and daily functioning equally important.38
Conclusions
CKD is increasingly common in older adults and is often, though not invariably, accompanied by other chronic conditions and geriatric syndromes. A major challenge of the next decade, therefore, will be in determining the optimal methods for risk stratification and management of this large, growing and heterogeneous group of patients. More clinical trials which include frail older adults and measure quality of life and other important geriatric outcomes, will help inform decisions about the management and intensity of care in older adults with CKD.
Acknowledgements
Dr. Fung is supported by T32 DK007357.
Dr. Kurella Tamura is supported by HX001262 from the Department of Veterans Affairs. Views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Hayutin A. Global Aging: The New New Thing: The Big Picture of Population Change. [Accessed August, 2014];Stanford Center on Longevity. 2007 http://longevity3.stanford.edu/publications/. [Google Scholar]
- 2.US Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 3.Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61(1):57–66. doi: 10.1053/j.ajkd.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Lopes MB, Araujo LQ, Passos MT, et al. Estimation of glomerular filtration rate from serum creatinine and cystatin C in octogenarians and nonagenarians. BMC Nephrol. 2013;14:265. doi: 10.1186/1471-2369-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peralta CA, Lee A, Odden MC, et al. Association between chronic kidney disease detected using creatinine and cystatin C and death and cardiovascular events in elderly Mexican Americans: the Sacramento Area Latino Study on Aging. J Am Geriatr Soc. 2013;61(1):90–95. doi: 10.1111/jgs.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alshaer IM, Kilbride HS, Stevens PE, et al. External validation of the Berlin equations for estimation of GFR in the elderly. Am J Kidney Dis. 2014;63(5):862–865. doi: 10.1053/j.ajkd.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowling CB, Sharma P, Fox CS, O'Hare AM, Muntner P. Prevalence of reduced estimated glomerular filtration rate among the oldest old from 1988–1994 through 2005–2010. JAMA. 2013;310(12):1284–1286. doi: 10.1001/jama.2013.252441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obi Y, Kimura T, Nagasawa Y, et al. Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin J Am Soc Nephrol. 2010;5(9):1558–1565. doi: 10.2215/CJN.08061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muntner P, Bowling CB, Gao L, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6(9):2200–2207. doi: 10.2215/CJN.02030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 16.Winearls CG, Glassock RJ. Classification of chronic kidney disease in the elderly: pitfalls and errors. Nephron Clin Pract. 2011;119(Suppl 1):c2–c4. doi: 10.1159/000328013. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita K, Ballew SH, Astor BC, et al. Cohort profile: the Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol. 2013;42(6):1660–1668. doi: 10.1093/ije/dys173. [DOI] [PubMed] [Google Scholar]
- 18.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stengel B, Metzger M, Froissart M, et al. Epidemiology and prognostic significance of chronic kidney disease in the elderly--the Three-City prospective cohort study. Nephrol Dial Transplant. 2011;26(10):3286–3295. doi: 10.1093/ndt/gfr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 21.De Nicola L, Minutolo R, Chiodini P, et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int. 2012;82(4):482–488. doi: 10.1038/ki.2012.174. [DOI] [PubMed] [Google Scholar]
- 22.Sud M, Tangri N, Pintilie M, Levey AS, Naimark D. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation. 2014;130(6):458–465. doi: 10.1161/CIRCULATIONAHA.113.007106. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22(1):28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 25.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 26.O'Hare AM, Kaufman JS, Covinsky KE, Landefeld CS, McFarland LV, Larson EB. Current guidelines for using angiotensin-converting enzyme inhibitors and angiotensin II-receptor antagonists in chronic kidney disease: is the evidence base relevant to older adults? Ann Intern Med. 2009;150(10):717–724. doi: 10.7326/0003-4819-150-10-200905190-00010. [DOI] [PubMed] [Google Scholar]
- 27.O'Hare AM, Hotchkiss JR, Kurella Tamura M, et al. Interpreting treatment effects from clinical trials in the context of real-world risk information: end-stage renal disease prevention in older adults. JAMA Intern Med. 2014;174(3):391–397. doi: 10.1001/jamainternmed.2013.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakash S, O'Hare AM. Interaction of aging and chronic kidney disease. Semin Nephrol. 2009;29(5):497–503. doi: 10.1016/j.semnephrol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm-Leen ER, Hall YN, M KT, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7):664–671. e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drawz PE, Goswami P, Azem R, Babineau DC, Rahman M. A simple tool to predict end-stage renal disease within 1 year in elderly adults with advanced chronic kidney disease. J Am Geriatr Soc. 2013;61(5):762–768. doi: 10.1111/jgs.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenstein L, Driver TH, L FF, et al. Serum Bicarbonate Concentrations and Kidney Disease Progression in Community-Living Elders: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmayer WC, Charytan DM, Brookhart MA, Levin R, Solomon DH, Avorn J. Kidney function and use of recommended medications after myocardial infarction in elderly patients. Clin J Am Soc Nephrol. 2006;1(4):796–801. doi: 10.2215/CJN.00150106. [DOI] [PubMed] [Google Scholar]
- 35.Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110(6):438–441. doi: 10.1016/s0002-9343(01)00642-8. [DOI] [PubMed] [Google Scholar]
- 36.Sakaan SA, Hudson JQ, Oliphant CS, et al. Evaluation of warfarin dose requirements in patients with chronic kidney disease and end-stage renal disease. Pharmacotherapy. 2014;34(7):695–702. doi: 10.1002/phar.1445. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser EA, Lotze U, Schafer HH. Increasing complexity: which drug class to choose for treatment of hypertension in the elderly? Clin Interv Aging. 2014;9:459–475. doi: 10.2147/CIA.S40154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried TR, Tinetti ME, Towle V, O'Leary JR, Iannone L. Effects of benefits and harms on older persons' willingness to take medication for primary cardiovascular prevention. Arch Intern Med. 2011;171(10):923–928. doi: 10.1001/archinternmed.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]