Abstract
Objectives
To examine barriers to cervical cancer screening among women who have experienced intimate partner violence (IPV) and accessed domestic violence shelters. To compare barriers among those up-to-date (UTD) and not UTD on screening, and evaluate acceptability of HPV self-sampling.
Methods
This is a cross-sectional survey in which domestic violence shelters in Ohio were identified and women completed an anonymous survey assessing UTD screening status, barriers related to screening, history of IPV, intention to follow-up on abnormal screening, and acceptability of self-sampling. Characteristics of UTD and not UTD women were compared using Mann Whitney U tests.
Results
A total of 142 women from 11 shelters completed the survey. Twenty three percent of women were not UTD. Women who were not UTD reported more access-related barriers (mean 2.2 vs. 1.8, 0.006). There was no difference in reported IPV-related barriers between women who were not UTD and those UTD (mean 2.51 in not UTD vs. 2.24 in UTD, p=0.13). Regarding future screening, of the women who expressed a preference, more women not UTD preferred self-sampling than UTD women (32% vs. 14%, p= 0.05).
Conclusions
In this study, access-related barriers were more commonly reported among women not UTD with screening. Addressing these barriers at domestic violence shelters may improve screening among not UTD women. Self-sampling may also be one feasible approach to support screening in this population.
Keywords: cervical cancer, screening, intimate partner violence, prevention, self-sampling, barriers to healthcare
INTRODUCTION
In the United States, there were approximately 12,360 new cases of cervical cancer in 2014, and approximately 4,020 women died of this disease [1]. Both the incidence and mortality of cervical cancer have decreased significantly over the past several decades, largely due to cervical cancer screening in the United States [2]. While this is a tremendous success, more than 60% of cervical cancer cases occur in underserved and under-screened women [3]. Vulnerable women with low socio-economic status as well as racial/ethnic minorities are most affected by this disease [2]; other high-risk groups of women continue to be identified. Another vulnerable population at higher risk of cervical cancer is women who have experienced intimate partner violence (IPV) [4]. In the United States, 10–33% of women experience some form of IPV in their lifetime [5–7]. One study of 6,285 women showed a 32% decreased likelihood of undergoing screening in women who experienced violence compared to those who had not [8]. Other studies have shown that women with a history of IPV have approximately 2.6 to 4.2 times the odds of developing this disease [4, 5].
While many factors contribute to the increased incidence of disease in this population, barriers to screening and delay in treatment for pre-invasive disease play an important role. Women’s experience with IPV adds a unique element to matters of sexual health, [9] and likely influences screening practices in a complex way. Qualitative data suggests that both emotional and psychological factors play an important role in limiting preventive behaviors [10]. These factors require further investigation in larger samples. Self-sampling is a secondary prevention method which has successfully increased screening among other at risk populations [11], and it is unknown whether this method would be acceptable and effectively decrease barriers in this population. In order to reduce the burden of disease in this population, it is critical to hear directly from these women themselves to help identify barriers that prevent appropriate screening and treatment of pre-invasive disease.
The objectives of this study were to better define the barriers to cervical cancer screening identified by women who have experienced IPV, and compare them among women who are and are not up-to-date (UTD) on Pap smear screening. We also examined the willingness to follow-up on abnormal screening results in the future and the acceptability of HPV self-sampling as a screening method in this population.
METHODS
Study Sample
The study was a cross sectional survey via convenience sample that included a group of women actively served by domestic violence shelters in Ohio. Domestic violence shelters in Ohio were identified via online resources and contacted via phone and/or email to determine if they would be willing to participate in offering this survey to the women utilizing their services. Once a partnership was established, a data collection day was selected, and a study team member visited the shelter and presented the opportunity to participate in the survey. Data from 7 out of 11 shelters were collected in this way. The remaining 4 shelters opted to have surveys available at the shelter for women to complete, with no study team member present. All women served by the shelter and available at the time of the survey were eligible to participate, and the surveys were self-administered by the participants. For this study, the denominator of women visiting a shelter during the study data collection period is not known. However, among the women offered the opportunity to complete the survey by the study team members, all agreed to complete the survey.
Study Survey
The survey for this study was designed to assess 1) Barriers to Pap smear screening including access barriers and barriers specific to IPV; 2) History of screening and UTD status; 3) Intention to follow-up and 4) Acceptability of a self-sampling screening strategy. Several items in the survey were adapted from the Demographic and Pap Smear Test Stage Questionnaire. This is a validated questionnaire based on the Champion’s Health Belief Model [12]. Additionally, questions related to barriers to cervical cancer screening specifically among women who have experienced IPV were based on the findings from qualitative studies by Ackerson et al. [13]. We defined UTD as having a smear within the past 3 years. Any woman who reported not having a Pap smear within the past 3 years was therefore classified as being not UTD. [10, 12]
Access-related barriers were defined as those affecting access to care, such as financial constraints, proximity to clinical resources, expectations of test efficacy, competing priorities, or limited time. Eight items were used to measure access-related barriers (Table 2). IPV related barriers were measured with eight items and included the following: anticipating the Pap test would trigger IPV memories, fear of pain, embarrassment, feelings of loss of control, and lack of trust in medical providers. Barrier items were scored using a 5-point Likert scale (1= Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree) (Table 2).
Table 2.
Comparison of Access- and IPV-related barriers for up-to-date and not up-to-date women
| Barrier items | Up-to-Date (n=107) |
Not Up-to- Date (n=35) |
Total (n=142) |
|
|---|---|---|---|---|
| Access-Related Barriers | Mean (STD) | Mean (STD) | Mean (STD) | |
| Access Barrier Summary Score ** | 1.82 (0.73) | 2.22 (0.72) | 1.92 (0.75) | |
| Don’t know where to go * | 1.66 (1.09) | 2.27 (1.35) | 1.81 (1.19) | |
| Too much time * | 1.56 (0.87) | 1.94 (0.91) | 1.65 (0.89) | |
| Can not remember | 2.10 (1.14) | 2.39 (1.12) | 2.17 (1.14) | |
| Not a priority | 1.95 (1.10) | 2.38 (1.21) | 2.05 (1.14) | |
| Too far away | 1.69 (0.88) | 2.15 (1.23) | 1.80 (1.00) | |
| Expensive * | 1.93 (1.16) | 2.56 (1.34) | 2.08 (1.23) | |
| Will not help | 1.55 (0.86) | 1.59 (0.80) | 1.56 (0.84) | |
| Unfamiliar with the exam ** | 1.86 (1.05) | 2.48 (1.25) | 2.01 (1.13) | |
| IPV-Related Barriers | Mean (STD) | Mean (STD) | Mean (STD) | |
| IPV Barrier Summary Score | 2.24 (0.90) | 2.51 (0.93) | 2.30 (0.92) | |
| Fear of flashbacks to IPV | 2.07 (1.20) | 2.36 (1.34) | 2.14 (1.24) | |
| Afraid of result | 2.56 (1.31) | 2.82 (1.24) | 2.63 (1.29) | |
| Will be painful | 2.32 (1.25) | 2.74 (1.37) | 2.42 (1.28) | |
| Evoke feelings from IPV | 2.17 (1.21) | 2.45 (1.33) | 2.24 (1.24) | |
| No control | 2.10 (1.10) | 2.41 (1.34) | 2.17 (1.17) | |
| Male Doctor | 2.42 (1.35) | 2.53 (1.24) | 2.44 (1.32) | |
| Embarrassment | 2.16 (1.13) | 2.42 (1.29) | 2.22 (1.17) | |
| Mistrust of Provider | 1.97 (1.17) | 2.22 (1.07) | 2.03 (1.15) | |
Barrier items were scored using a 5-point Likert scale
(1= Strongly Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly Agree)
Differences between groups were evaluated using Mann-Whitney U test.
p<0.05
p<0.01
Intention to follow-up on abnormal screening was assessed with one question that addressed different levels of follow-up needed, ranging from counseling to biopsy. In order to assess the acceptability of self-sampling, self-sampling definitions and techniques were described in the survey; participants preferences for Pap smear, self-sampling or either were then addressed; finally, the participants were asked to assess whether IPV-barriers would play a role in acceptability of self-sampling and whether they would be as likely to follow-up on abnormal findings from self-sampling. The survey instrument can be found online in additional materials at: supplemental digital content #1.
The survey required approximately 15 minutes to complete. The survey was written at an eighth grade reading level and key medical terms like Pap smear were defined in order to help participants better understand the survey questions.
Data Collection and Analysis
Data from the surveys were manually entered into a database and checked for accuracy. Descriptive statistics are used to report about the sample of women. Summary scores for the IPV-related and access-related barrier measures were computed by adding the items and dividing by the number of items in the scale so that the resulting score was on the same metric as the original items (i.e. 1–5). The main analyses compared differences in mean score among access and IPV barriers for UTD women vs. not UTD women. These were analyzed using Mann-Whitney U test and significance was evaluated at p<0.05. Analyses were conducted using SPSS version 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). [14]. All procedures in this study were reviewed and approved by the Institutional Review Board of the Cleveland Clinic.
Role of the Funding Source
The study sponsor had no role in the design of the study, collection, analysis, interpretation of the data, writing of the report, or decision to submit for publication. The corresponding author had final responsibility for the decision to submit this manuscript for publication
RESULTS
A total of 142 women from 11 centers in the state of Ohio completed the survey. Twenty-three percent of women reported that they had not received a Pap smear within the past three years and were therefore classified as being not UTD. The average age of women was 37 years old, 53% of women were white, 85% earned <$20,000 per year, and 54% had only a high school education or less. (Table 1). Demographic characteristics of women who were UTD and those who were not UTD were similar (p>0.05), except with regards to insurance status. A higher percentage of women who were not UTD reported having no insurance or “other” insurance, and a lower percentage reported having Medicare/Medicaid or HMO/Employer insurance.
Table 1.
Demographic Characteristics
| Sample Characteristics | Up-to-Date (n=107) |
Not Up-to- Date (n=35) |
All (n=142) |
|
|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
Mean (SD) |
||
| Age | 36 (11) | 38 (13) | 37 (11) | |
| n (%) | n (%) | n (%) | ||
| Ethnicity | Latina/Hispanic | 7 (7) | 2 (6) | 9 (6) |
| Race | White | 47 (46) | 19 (59) | 66 (49) |
| Black | 52 (51) | 13 (41) | 65 (49) | |
| Other | 3 (3) | 0 (0) | 3 (2) | |
| Income | <$20,000 | 92 (86) | 29 (91) | 121 (87) |
| ≥$20,000 | 15 (14) | 3 (9) | 18 (13) | |
| Education | Less than high school | 20 (19) | 4 (12) | 24 (18) |
| High school or GED | 40 (38) | 10 (30) | 50 (36) | |
| Some college (no degree) | 32 (31) | 10 (30) | 42 (30) | |
| College or more | 13 (12) | 9 (27) | 22 (16) | |
| Insurance * | None | 19 (18) | 11 (32) | 30 (21) |
| Medicare/Medicaid | 62 (59) | 12 (35) | 74 (53) | |
| HMO/Employer | 12 (11) | 2 (6) | 14 (10) | |
| Other | 13 (12) | 9 (27) | 22 (16) | |
p<0.05
Access- and IPV-related Barriers
Table 2 displays the ratings on each of the barrier items and the summary scores for UTD vs. not UTD women. Compared to the UTD women, those who were not UTD with screening reported higher mean scores in agreement with access-related barriers (p=0.006). Specifically, not UTD women reported higher mean agreement that they don’t know where to go, that a Pap smear takes too much time, that it is too expensive, and that they felt unfamiliar with the exam. No significant differences were observed between UTD and not UTD women for the summary score or any of the items measuring IPV related barriers.
Intention to Follow-up and Future Screening
Both women who were UTD and not UTD agreed that they would be willing to follow-up on an abnormal Pap smear result either for advice (mean score for both groups was 4.37 [UTD 4.37, not UTD 4.38], p=0.98), for a repeat Pap smear (mean score 4.36 [UTD4.38, not UTD 4.27], p=0.65), or for a pelvic exam with biopsies of the cervix (mean score 4.27 [UTD 4.27, not UTD 4.24], p=0.90).
When offered a self-sampling option, 42.3% of women reported that they would prefer regular Pap smear screening at a physician’s office, 9.9% of women reported that they would prefer the self-sampling option, and 39.4% of women reported that they would do either. When responses were compared, 43% of women who were UTD and 43% of women who were not UTD did not have a preference between self-sampling and Pap smear, p=NS. Of the 57% of women who had a preference for one type of screening or the other, significantly more women who were not UTD preferred self-sampling (32%) compared to women who were UTD (14%) (p= 0.05) (Figure 1).
Figure 1.
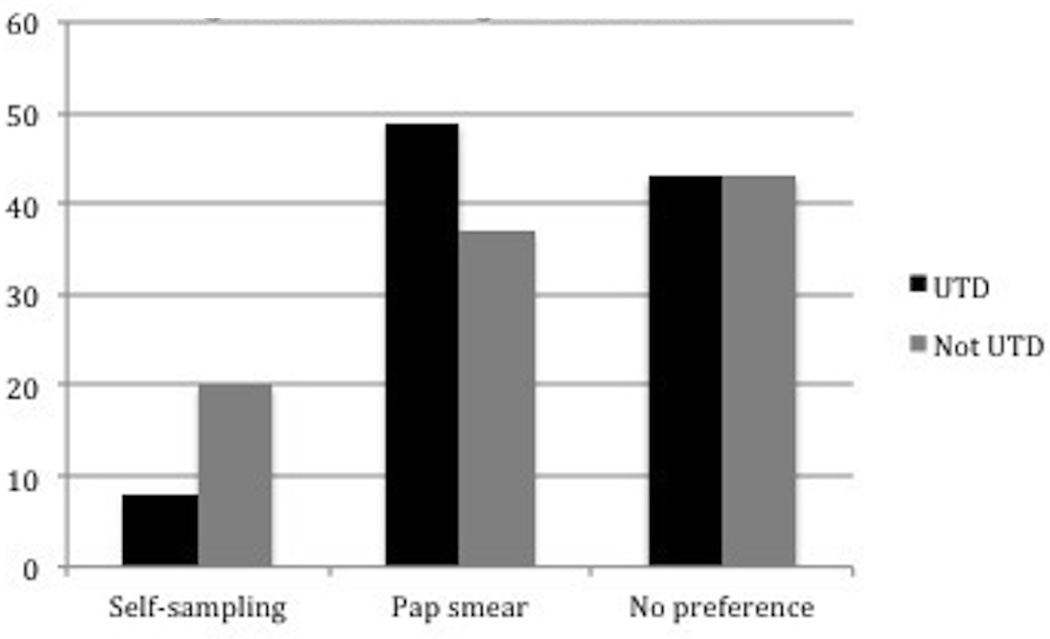
Screening Preferences
DISCUSSION
IPV is a significant problem in the United States, and studies suggest that this population of women is at increased risk for several adverse health outcomes including diagnosis and repercussions from cervical cancer [8]. Understanding the barriers to screening in at risk populations is a critical step towards preventing disease and improving outcomes. This study shows that access-related barriers were more commonly reported among women not UTD with screening. Furthermore, among women who preferred self-sampling, most were not UTD on their Pap smears. Thus, for women who are most at risk for being under-screened, self-sampling may be an option that helps overcome some barriers. Multiple social, demographic and contextual factors contribute to compliance with screening in this population, and self-sampling is likely one intervention which must occur within a context of additional interventions to address multiple barriers. This research offers a reflection of women’s voices that can help guide these future interventions.
From a public health perspective, the results of this study have important implications, as access-related barriers may be more easily addressed on a broad scale vs. barriers linked to IPV. Improving access to services for the IPV population could be implemented in various ways such as forming relationships between shelters and providers, providing financial vouchers, transportation, and even educational sessions about the importance of cervical cancer screening and the options for testing, tailored to women utilizing shelter services.
This study has several limitations that are important to recognize when interpreting the data. First, this study is limited to women who have accessed domestic violence shelters, and not all women from the shelters were included. This was a convenience sample and is therefore not reflective of the overall population of women who have experienced IPV. Furthermore, the interplay of various social and demographic factors, and the complexity of the broad social context that influence screening practices are not accounted for in this survey. Therefore, while there were no differences in IPV barriers between UTD and not UTD women, the broader interpretation of the role of IPV barriers cannot fully be defined in this context. Furthermore, while women expressed that they intended to be treated for abnormal findings, it is unclear whether this intention might translate into practice and how best to capitalize on this intention.
The data in this survey is based on self-report and therefore may be subject to recall bias, particularly with regard to being UTD on Pap smears. Previous studies have documented that recall bias in this context exists and likely underestimates the proportion of patients adequately screened as well as the number of patients who have abnormal results [15]. In this study, if women did in fact under report being UTD, our study would be biased towards finding no difference in barriers as the groups would be more similar. Finally, it is important to recognize that our definition of being UTD on Pap smear screening included women who have had a Pap smear within the past three years. While new guidelines suggest that women between the ages of 30–65 who have negative co-testing with cytology and HPV may extend screening to a 5-year interval, this guideline did not come out until 2012; therefore, this would not affect our assessment of UTD status of women in this sample [16–17].
The strength of this study is that it provides an opportunity for a previously understudied population of women to have a voice regarding the barriers that they face to care. While prior studies have examined both IPV-related and access-related barriers, to our knowledge, no prior study has evaluated the differences in barriers reported by UTD and non-UTD women. While it is important to recognize the possibility of reluctance to admit to IPV barriers, and the influences on IPV barriers cannot fully be reflected through this methodology, women’s responses in this context meaningfully contribute to the current knowledge regarding barriers in this specific population.
CONCLUSION
This study adds significant insight into the barriers to cervical cancer screening faced by women who have experienced IPV. While both IPV-related barriers and access-related barriers are critical to consider when providing sexual health and screening for this population, specific interventions to help address access-related barriers may help to improved screening among women who report not being UTD on screening. Furthermore, HPV self-sampling may be an important intervention, particularly for women who are not currently UTD. These results can help guide planning for future interventions for this population.
Supplementary Material
Acknowledgments
Funding: This research was supported by a grant through the Foundation for Gynecologic Oncology from the Gynecologic Oncology Group entitled Improving Outcomes in Gynecologic Oncology Patients Research and Education Award and the Behavioral Measurement Core Facility of the Case Comprehensive Cancer Center (P30 CA43703)
Study was approved by The Institutional Review Board of The Cleveland Clinic
Abbreviations/Acronyms
- IPV
Intimate Partner Violence
- UTD
Up-to-date
Footnotes
Conflict of Interest Statement: None of the authors has any competing financial interests.
Contributor Information
Kimberly L. Levinson, Email: klevins1@jhmi.edu.
Amelia M. Jernigan, Email: Amelia.jernigan@gmail.com.
Susan A. Flocke, Email: susan.flocke@case.edu.
Ana I. Tergas, Email: ait2111@cumc.columbia.edu.
Camille C. Gunderson, Email: ccgunder@gmail.com.
Warner K. Huh, Email: whuh@uabmc.edu.
Ivy Wilkinson-Ryan, Email: ivywilkry@gmail.com.
Peter J. Lawson, Email: peter.lawson@case.edu.
Amanda N. Fader, Email: afader1@jhmi.edu.
Jerome L. Belinson, Email: jlb@poiinc.org.
References
- 1. Cancer.org internet. Atlanta (GA): The American Cancer Society; [cited 2014 Dec 3]. c1913–2015 [updated 2014 Oct 13;]. Available from: http://www.cancer.org/cancer/cervicalcancer/detailguide/cervical-cancer-key-statistics. [Google Scholar]
- 2.Adegoke O, Kulasingam S, Birnig B. Cervical Cancer Trends in the United States: a 35-Year Population-Based Analysis. J Women’s Health. 2012;21:1–7. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarinci IC, Garcia FAR, Kobetz E, et al. Cervical Cancer Prevention. Cancer. 2010:2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coker AL, Hopenhayn C, DeSimone CP, et al. Violence against Women Raises Risk of Cervical Cancer. J Women’s Health. 2008;18:1179–1185. doi: 10.1089/jwh.2008.1048. [DOI] [PubMed] [Google Scholar]
- 5.Coker AL, Sanderson, Fadden MK, et al. Intimate Partner Violence and Cervical Neoplasia. J Women’s Health and Gender-Based Medicine. 2000;9:1015–1023. doi: 10.1089/15246090050200051. [DOI] [PubMed] [Google Scholar]
- 6.Brown MJ, Weitzen S, Lapane KL. Association between Intimate Parnter Violence and Preventive Screening Among Women. J Women’s Health. 2013;00:1–5. doi: 10.1089/jwh.2012.4222. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi S, Rovi S, Vega M, et al. Intimate partner violence and cancer screening among urban minority women. JABFP. 2010;23:343–353. doi: 10.3122/jabfm.2010.03.090124. [DOI] [PubMed] [Google Scholar]
- 8.Cronholm PF, Bowman MA. Women with Safety Concerns report Fewer Gender-Specific Preventive Healthcare Services. J Women’s Health. 2008;18:1011–1018. doi: 10.1089/jwh.2008.0968. [DOI] [PubMed] [Google Scholar]
- 9.Coker AL. Does Physical Intimate Partner Violence affect sexual health? A systematic Review. Trauma, Violence, & Abuse. 2007;8:149–177. doi: 10.1177/1524838007301162. [DOI] [PubMed] [Google Scholar]
- 10.Ackerson K. A History of Interpersonal Trauma and the Gynecological Exam. Qualitative Health Research. 2012;22:679–688. doi: 10.1177/1049732311424730. [DOI] [PubMed] [Google Scholar]
- 11.Sancho-Garnier H, Tamalet C, Halfon P, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening non-attenders from lower socioeconomic groups in France. Int J Cancer. 2013;1:133, 2681–2687. doi: 10.1002/ijc.28283. [DOI] [PubMed] [Google Scholar]
- 12.Guvenc G, Akyuz A, Acikel CH. Health Belief Model Scale for Cervical Cancer and Pap Smear Test: psychometric testing. J Adv Nurs. 2011;67:428–437. doi: 10.1111/j.1365-2648.2010.05450.x. [DOI] [PubMed] [Google Scholar]
- 13.Cox CL, Roghmann KJ. Empirical test of the interaction model of client health behavior. Res Nurs Health. 1984;7:275–285. doi: 10.1002/nur.4770070406. [DOI] [PubMed] [Google Scholar]
- 14.IBM Corp. Released 2012. IBM SPSS Statistics for Windows. Version 21.0. Armonk, NY: IBM Corp; [Google Scholar]
- 15.Newell S, Girgis A, Sanson-Fisher R, Ireland M. Accuracy of patients’ recall of Pap and cholesterol screening. Am J Public Health. 2000;90(9):1431–1435. doi: 10.2105/ajph.90.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, Amican Society for Colpoascopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. Ca Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC.gov internet. Atlanta (GA): The Centers for Disease Control and Prevention; [cited 2015 June 28]. c1946–2015 [updated 2015 May 20;]. Available from: www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


