Abstract
The c-kit oncogene is an important target in the treatment of gastrointestinal tumors. A potential approach to inhibition of the expression of this gene involves selective stabilization of G-quadruplex structures that may be induced to form in the c-kit promoter region. Here we report on the structure of an unprecedented intramolecular G-quadruplex formed by a G-rich sequence in the c-kit promoter in K+ solution. The structure represents a new folding topology with several unique features. Most strikingly, an isolated guanine is involved in G-tetrad core formation, despite the presence of four three-guanine tracts. There are four loops: two single-residue double-chain-reversal loops, a two-residue loop, and a five-residue stem-loop, which contain base-pairing alignments. This unique structural scaffold provides a highly specific platform for the future design of ligands specifically targeted to the promoter DNA of c-kit.
Introduction
The proto-oncogene c-kit encodes for a 145-160 kDa tyrosine kinase receptor,1 which regulates key signal transduction cascades to control cell growth and proliferation. As the c-kit protein plays such a critical role in establishing normal cell growth, mutations in structurally important regions of the protein, or its overexpression, result in impaired function, which leads to oncogenic cellular transformations.2 Gain-of-function mutations are found in several highly malignant human cancers.3-5 Human gastrointestinal stromal tumors (GIST) have been associated with mutations around the two main autophosphorylation sites in the juxtamembrane region,3 whereas kinase domain mutations are found in myeloid leukemias and human germ cell tumors.4,5 These oncogenic mutations result in increased dimer formation by bringing together two c-kit kinases into close proximity and facilitating autophosphorylation.
The c-kit protein is currently the principal therapeutically important target in the treatment of GIST.6 The small molecule Gleevec (imatinib), originally developed as an inhibitor of the c-abl kinase in chronic myelogenous leukaemia (CML), was subsequently found to be an effective inhibitor in Vitro and in ViVo of c-kit kinase activity.7 It is now widely used as a therapy for GIST, where it has made a major difference to survival rates.6,8 However, as with CML, resistance to Gleevec occurs as a result of deactivating mutations in the active site, which diminish binding and clinical effectiveness of the drug.9 A number of new c-kit kinase inhibitors are currently being developed and evaluated with the aim of circumventing this resistance,10 although they themselves may well produce new patterns of resistance mutations.
An alternative approach to c-kit inhibition would involve selective gene regulation at the transcriptional level. Such an approach is currently being explored in the case of the c-myc oncogene,11,12 which involves the induction of G-quadruplex DNA structures in a G-rich region of the c-myc promoter nuclease hypersensitivity element (NHE) III1 that is responsible for up to 90% of c-myc transcription.13 G-quadruplexes, which may have transient stability by themselves when embedded within the double-stranded DNA of a eukaryotic gene, may be stabilized further by a small-molecule ligand such as the porphyrin molecule TMPyP4.12 The topology and structure of the c-myc DNA quadruplexes containing four14 and five15 G-tracts have been determined by nuclear magnetic resonance (NMR) spectroscopy, together with that of a TMPyP4 complex for the five-guanine-tract quadruplex.15 The latter are parallel-stranded quadruplexes, with several strand-reversal loops and base-pair platforms.15 They have structural complexity beyond that of quadruplexes formed from telomeric DNA repeats,16-21 suggesting the possibility of identifying ligands that are selective for the c-myc quadruplexes.
Recently, two G-rich sequences have been identified in the promoter region of the human c-kit gene, and biophysical data, including NMR, have established that these sequences can form G-quadruplex structures.22,23 In particular, the c-kit87up d(AGGGAGGGCGCTGGGAGGAGGG) sequence, 87 nucleotides upstream of the transcription start site of the human c-kit gene, forms a single G-quadruplex species.22 Because this sequence contains four tracts of three consecutive guanines separated by linkers of one or four residues, it was initially assumed to form a conventional G-quadruplex structure involving these G-tracts in the G-tetrad core and the linkers in the loops. Surprisingly, modifications in the linker sequences were found to be detrimental to formation of a folded quadruplex structure,22 suggesting that they were more intimately involved in the quadruplex fold than would be expected for a conventional parallel or antiparallel arrangement.
Here we report on the NMR-based solution structure of the G-quadruplex formed by this sequence in K+ solution. The structure reveals a highly unusual and unprecedented G-quadruplex folding topology with several unique elements. This structure rationalizes the data on previous sequence modifications,22 as well as that on many systematic sequence modifications performed in the present work. The unique scaffold of the c-kit87up quadruplex may provide a platform for designing ligands that specifically target the G-quadruplex topology in the c-kit promoter.
Results
NMR Spectra
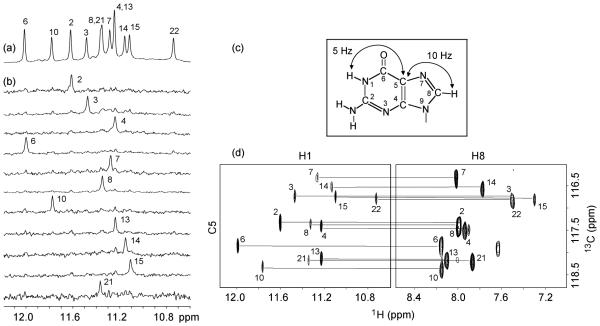
NMR spectra of c-kit87up in K+ solution are of excellent quality with well-resolved resonances (Figure 1a). The number of peaks is consistent with formation of a single conformation. Spectral line-widths (2-4 Hz for sharpest peaks at 25 °C) suggest formation of a monomeric structure, in agreement with previous data,22 which showed that the melting temperature of c-kit87up was independent of the oligonucleotide concentration.
Figure 1.
Spectra and assignments of c-kit87up. (a) The 600 MHz imino proton spectrum of the c-kit87up sequence in K+ solution at 25 °C. Unambiguous assignments are indicated. (b) Imino protons were assigned in 15N-filtered spectra of samples, 2% 15N-labeled at the indicated positions. (c) Long-range J couplings in a guanine used for imino-H8 correlation. (d) H8 proton assignments of c-kit87up by through-bond correlations between imino and H8 protons via 13C5 at natural abundance, using long-range J couplings shown in (c).
We observe 12 sharp peaks of guanine imino protons at 25 °C, which likely belong to the G-tetrad core. This suggests formation of a G-quadruplex structure containing three G-tetrad layers. Three other imino protons (two for guanines and one for a thymine) are visible at lower temperatures (Figure S1, Supporting Information).
In contrast to the narrow and well-resolved NMR spectrum of c-kit87up in K+ solution (Figure S2b, Supporting Information), its counterpart in Na+ solution exhibits a broad and unresolved envelope probably associated with an aggregated species (Figure S2a, Supporting Information).
Spectral Assignments
We prepared samples (Table S1, Supporting Information) in which only one guanine was 2% 15N-labeled at a time, using deliberately diluted 15N-labeled phosphoramidite.24 Guanine imino protons were unambiguously assigned by the site-specific low-enrichment approach24 (Figure 1b). Unexpectedly, these unambiguous assignments revealed that G10, an isolated guanine within the C9-G10-C11-T12 “linker” sequence, was among 12 sharp imino protons, but not that of G20 from the last G-tract.
Most guanine H8 protons were assigned by natural-abundance through-bond correlations25 to the already assigned imino protons via 13C5 (Figure 1c, d). Guanine H8 proton assignments were completed (or confirmed) by multi-bond correlations to 15N nuclei in site-specific 2% 15N-labeled samples.24 The spectral assignments for other resonances were completed by through-bond (COSY and TOCSY) and through-space (NOE-SY) correlations between protons.26
G-Quadruplex Folding Topology
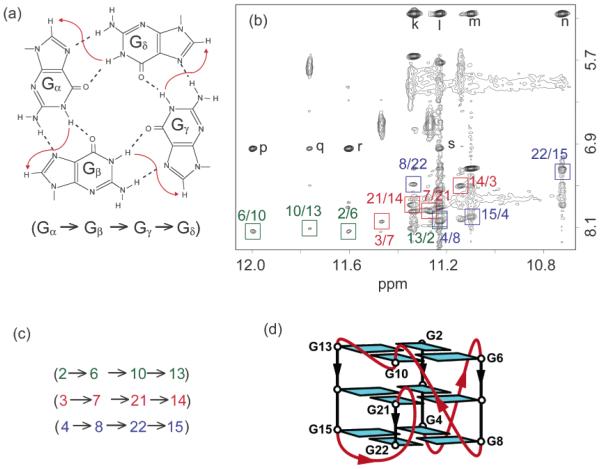
On the basis of the characteristic NOEs between imino and H8 protons (Figure 2), we established an unprecedented G-quadruplex fold for c-kit87up, involving three G-tetrads: G2•G6•G10•G13, G3•G7•G21•G14, and G4•G8•G22•G15 (Figure 2d). The glycosidic conformations of all guanine are anti, as reflected by the observed H1′-H8 NOE intensities (not shown). These glycosidic conformations are consistent with the G-tetrad core containing all parallel G-tracts (Figure 2d). There are four loops in the structure. Two single-residue linkers (A5 and C9) form two double-chain-reversal loops that bridge three G-tetrad layers. The third loop, C11-T12, connects two adjacent corners (G10 and G13) of the G-tetrad core (Figure 2d). The five-residue segment A16-G17-G18-A19-G20 forms the fourth loop that allows the terminal G21-G22 stretch to be inserted back to the G-tetrad core. This folding topology is also supported by proton exchange data, which showed that imino protons of the central G-tetrad (G3, G7, G21, and G14) are the most protected from exchange with water (Figure S3, Supporting Information).
Figure 2.
G-tetrad alignments and folding topology of c-kit87up. (a) Schematic of a G-tetrad with characteristic guanine imino-H8 connectivity patterns indicated by arrows. (b) NOESY spectrum (mixing time, 200 ms). The characteristic guanine imino-H8 cross-peaks for G-tetrads are framed and labeled with the imino proton assignment in the first position and that of the H8 proton in the second position. Peaks k, l, m, and n show close distances between G20(NH2) and the imino protons of guanines from the bottom tetrad; peaks p, q, r, and s show close distances between A1(H2) and the imino protons of guanines from the top tetrad. (c) Summary of characteristic guanine imino-H8 patterns observed for c-kit87up. (d) Topology of c-kit87up based on the G-tetrad alignments in (c).
Solution Structure of c-kit87up G-Quadruplex
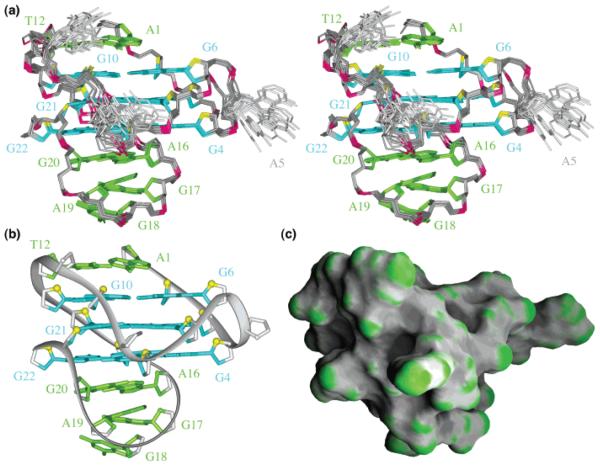
The structure of the c-kit87up quadruplex (stereo pair, Figure 3a; representative structure, Figure 3b, c) was calculated on the basis of NMR restraints (Table 1). The G-tetrad core is well defined. We observe three base pairs formed within the top and bottom loops (Figure 3): A1•T12 (Figure 4a), A16•G20 (Figure 4b, c), and G17•A19 (Figure 4c, d). The stacking of the A1•T12 (Figure 4a) and A16•G20 (Figure 4b) on the top and the bottom of the G-tetrad core, respectively, is reflected by the observation of NOE cross-peaks between A1(H2) to the imino protons of guanines from the top tetrad (G2, G6, G10, G13) and those between G20(NH2) to the imino protons of guanines from the bottom tetrad (G4, G8, G22, G15) (Figure 2b). Formation of the Watson-Crick A1•T12 base pair is supported by the observation of the imino proton of T12 at 5 °C (Figure S1, Supporting Information) and the NOE between this proton and the A1(H2) proton (not shown). A1 adopts a syn conformation, consistent with the observation of a strong intraresidue H8-H1′NOE cross-peak (not shown).
Figure 3.
Solution structure of the c-kit87up quadruplex in K+ solution. (a) Stereoview of 11 superpositioned refined structures. Guanine bases in the G-tetrad core are colored cyan; other bases, green; the backbone, gray; phosphorus atoms, red; O4′atoms, yellow. (b) Ribbon and (c) surface views of a representative refined structure.
Table 1.
NMR Restraints and Structure Statistics
| A. NMR Restraints | ||
|
| ||
| distance restraints | nonexchangeable | exchangeable |
| intra-residue distance restraints | 200 | 0 |
| sequential (i, i + 1) distance restraints | 72 | 6 |
| long-range (i, ≥ i + 2) distance restraints | 13 | 38 |
| other restraints | ||
| hydrogen bond restraints | ||
| (H–N, H–O, and heavy atoms) | 60 | |
| torsion angle restraints | 54 | |
| intensity restraints | ||
| nonexchangeable protons | 240 | |
| B. Statistics for 11 Structures following Intensity Refinement | ||
|
| ||
| NOE violations | ||
| number (>0.2 Å) | 0.182 ± 0.404 | |
| maximum violation (Å) | 0.284 ± 0.024 | |
| rmsd of violations | 0.018 ± 0.004 | |
| deviations from the ideal covalent geometry | ||
| bond lengths (Å) | 0.004 ± 0.000 | |
| bond angles (deg) | 0.974 ± 0.018 | |
| impropers (deg) | 0.349 ± 0.008 | |
| NMR R-factor (R1/6) | 0.017 ± 0.005 | |
| pairwise all heavy atom rmsd values | ||
| all heavy atoms except A5, C9, C11 | 0.50 ± 0.09 | |
| all heavy atoms | 0.83 ± 0.12 | |
|
| ||
Figure 4.
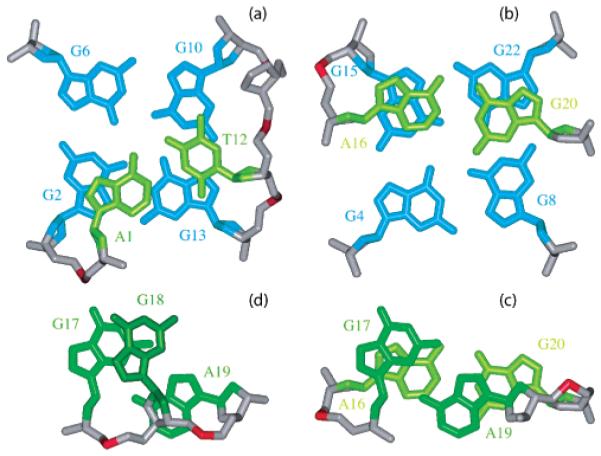
Base pairs in loops. Stacking (a) between the Watson-Crick A1•T12 pair and the G2•G6•G10•G13 tetrad, (b) between the Watson-Crick A16•G20 pair and the G4•G8•G22•G15 tetrad, (c) between the Watson-Crick A16•G20 pair and the sheared G17•A19 pair, and (d) between the sheared G17•A19 pair and the single-base G18.
The C9-G10-C11-T12 fragment forms an interesting configuration in the structure (Figure 5), where the middle G10 participates in the G-tetrad core.
Figure 5.
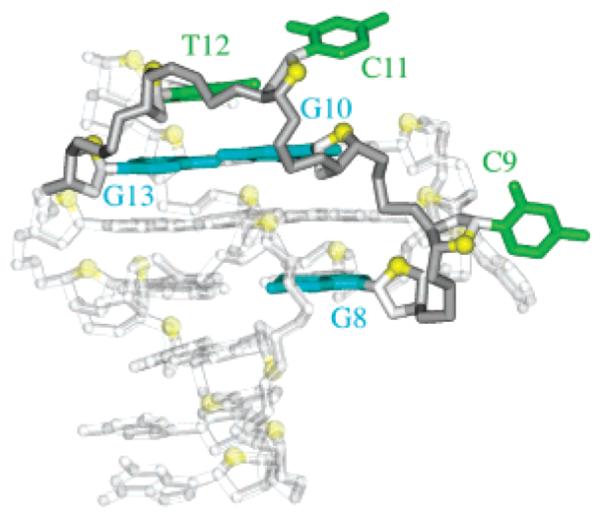
Highlight of the C9-G10-C11-T12 fragment within the c-kit87up quaduplex.
Formation of the Watson-Crick-type A16•G20 base pair was supported by the observation of G20 imino proton at 12.8 ppm (Figure S1, Supporting Information) and the strong NOE between A16(H2) and G20(NH2). This was independently confirmed in the sample ck26 (Table 2), in which G20 was substituted by an inosine. NMR-restrained structure calculation revealed formation of the sheared G17•A19 pair (Figure 4c, d) and the single-residue turn G18 (Figure 4d). The positions of bases in the five-residue A16-G17-G18-A19-G20 loop were supported by several unusual chemical shifts: position of G18 over the G17•A19 base pair would explain the upfield shifts of its H1′and H5′/H5′p′rotons; upfield shifts of H5′a′nd H2′′ (but not H5′and H2′) protons of A19 are consistent with the positions of these protons over the aromatic rings of both G18 and G20. The configuration of this unusual stem-loop resembles a hairpin loop observed previously,27 which consists of a single residue turn closed by a sheared G•A pair, flanked by a Watson-Crick pair. Imino proton of the sheared G•A pair was also observed at 10.7 ppm,27 as G17 in c-kit87up.
Table 2.
Investigated G-Rich Sequences in the c-kit Promotera
| sequence |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| name | 1 | 2 | 3 | 4 | ||||||
| c-kit87up | A | GGG | A | GGG | C | G | CT | GGG | AGGAG | GG |
| ck16 | T | GGG | A | GGG | C | G | CT | GGG | AGGAG | GG |
| ck17 | A | GGG | T | GGG | C | G | CT | GGG | AGGAG | GG |
| ck18 | A | GGG | A | GGG | T | G | CT | GGG | AGGAG | GG |
| ck19 | A | GGG | A | GGG | C | G | TT | GGG | AGGAG | GG |
| ck20 | A | GGG | A | GGG | C | G | CT | GGG | TGGAG | GG |
| ck21 | A | GGG | A | GGG | C | G | CT | GGG | AGGTG | GG |
| ck22 | A | GGG | A | GGG | C | G | CT | GGG | ATGAG | GG |
| ck23 | A | GGG | A | GGG | C | G | CT | GGG | AGTAG | GG |
| ck24 | A | GGG | A | GGG | C | G | CT | GGG | AGGAT | GG |
| ck25 | A | GGG | A | GGG | C | T | CT | GGG | AGGAG | GG |
| ck26 | A | GGG | A | GGG | C | G | CT | GGG | AGGAI | GG |
| ck27 | A | GGG | A | GGG | C | G | CT | GGG | AIGAG | GG |
Modifications are in boldface. Loop numbers are listed in the column head.
Analysis of Modified c-kit87up Sequences
We systematically probed the importance of different structural elements in the c-kit87up quadruplex fold by individual modifications of several residues (Table 2).
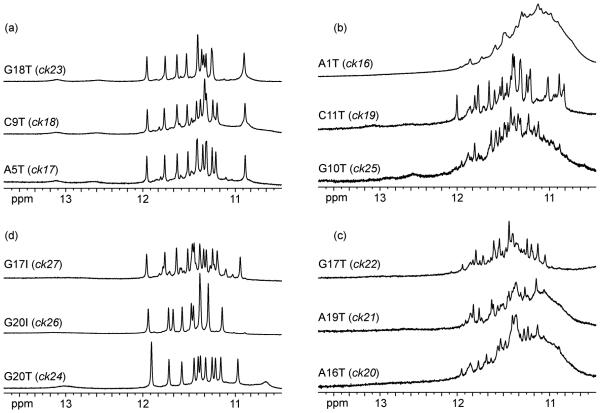
The single-residue loops A5 and C9 were substituted by T in the sequences ck17 and ck18 (Table 2), respectively, without altering the general fold, as suggested by their NMR spectra (Figure 6a). This was anticipated, as the structure of a single-residue double-chain-reversal loop bridging three G-tetrad layers has been shown to be independent of the nature of the base in it.28
Figure 6.
The 600 MHz imino proton spectra of the modified c-kit87up sequences in K+ solution at 25 °C.
Formation of the Watson-Crick A1•T12 base pair appears to be important for the structure, as the A1T mutation in the ck16 sequence altered the general fold (Figure 6b). The ck19 sequence containing the C11T mutation showed a doublings of imino proton peaks (Figure 6b). It is possible that for this sequence there are two conformations in which A1 can be paired with either T11 or T12.
Base pair alignments in the five-residue loop are also important for the fold since mutations that alter them (A16T, G17T, and A19T) would affect the general fold (Figure 6c). In this loop, however, the single-residue turn G18 could be substituted by a T in the ck23 sequence, without altering the general fold (Figure 6a). This is not unexpected since this is the sole nucleotide in the loop not to be involved in tertiary interactions other than stacking onto the preceding guanine base. Substitution G20T (ck24) resulted in a similar general fold, except that the Watson-Crick A16•T20 may be formed instead of A16•G20. This possibility is structurally feasible. The G20I substitution (ck26) also resulted in a single conformation of the same general fold (Figure 6d), whereas the G17I substitution (ck27) maintained the general fold for the major conformation of only about 80%, indicating the significant role of the amino group of G17 in the G17•A19 pair (Figure 6d).
Finally, G10 should be important for the unique folding topology of c-kit87up, as NMR spectra (Figure 6b) suggest that the G10T mutation (ck25) favored an alternative G-quadruplex fold.
Discussion
We have solved the structure of an unprecedented intramolecular G-quadruplex formed by a promoter sequence from the c-kit oncogene in K+ solution. Initial inspection of the sequence, with four equally sized G-tracts, suggested that the fold would be a straightforward parallel-type one, in accord with the biophysical data.22 The present study has shown that this is not the case. The structure contains two single-residue double-chain-reversal loops spanning three G-tetrad layers, a robust type of loop reported previously15,28 that could play an important role in the folds of many G-quadruplexes29 in K+ solution. This quadruplex is remarkable in requiring the active participation of 18 out of the 22 nucleotides in the structural organization. The sole exceptions are the single-nucleotide loops (A5 and C9) and turns (C11 and G18).
The structure also revealed several new topological elements. The most striking feature of the structure is the participation of an isolated non-G-tract guanine (G10) in formation of the G-tetrad core, despite the presence of the four G-tracts, each containing three consecutive guanines. This is counter-intuitive: generally, it was thought that G-tracts are most favorable for forming columns that support the G-tetrad core. This new folding principle, that G residues in non-G-tract regions can participate in forming the structural core, should be kept in mind in future studies that attempt to predict G-quadruplex topologies from sequence data alone.
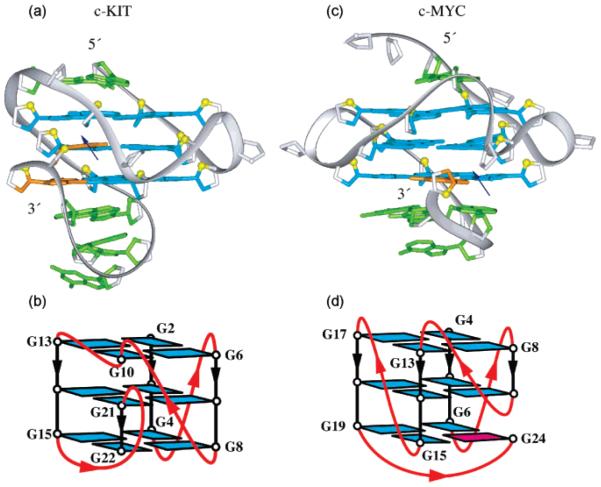
Another notable feature of the structure is the snapback parallel-stranded G-tetrad core. The snapback arrangement was first observed in the G-quadruplex formed by a five-G-tract sequence from the c-myc promoter.15 The comparison between the snapback G-quadruplexes from c-kit and c-myc promoters is shown in Figure 7. In both cases, the G-tetrad core is interrupted30 and base pairings in the loops are important in stabilizing the snapback scaffold. The difference between c-kit and c-myc quadruplexes is that the latter involves insertion of a single syn guanine (Figure 7c, d), the former involves insertion of two anti guanines (Figure 7a, b). The snapback feature of this structure would also allow for continuation of the DNA sequence in both directions without significant steric hindrance.
Figure 7.
Comparison between G-quadruplex structures observed in (a, b) c-kit (this work) and (c, d) c-myc15 promoters. In (a) and (c): color coded as in Figure 3, except that snapback residues are colored orange; the blue arrows indicate break points within interrupted G-tetrad cores. In (b) and (d): loops are colored red; anti and syn guanines are colored cyan and magenta, respectively.
Formation of G-quadruplex structures in the c-myc promoter has been shown to inhibit the transcription of the c-myc oncogene.12 The same approach may also apply to the c-kit oncogene. Because the G-quadruplex structure formed by c-kit87up is quite unique, it appears to be a specific target for drug design. In particular, there is a pronounced cleft in the structure between the bottom G-tetrad and the stem-loop that has a complex topology, suggesting that it could be a recognition site for ligands.
Methods
Sample Preparation
The unlabeled and the site-specific low-enrichment (2% 15N-labeled) oligonucleotides were synthesized and purified as described previously.24 Some site-specific 15N-labeled samples were independently resynthesized to definitively verify assignments. Unless otherwise stated, the strand concentration of the NMR samples was typically 0.5-5 mM; the solutions contained 70 mM of KCl and 20 mM of potassium phosphate (pH 7).
NMR Spectroscopy
Experiments were performed on 600 MHz Varian and 800 MHz Bruker NMR spectrometers at 25 °C, unless otherwise specified. Resonances were assigned unambiguously by using site-specific low-enrichment labeling24 and through-bond correlations at natural abundance.25,26 Assignments for some residues were verified and confirmed in different independently synthesized labeled samples. Spectral assignments were also assisted and supported by COSY, TOCSY, and NOESY spectra.26 Interproton distances were measured by using NOESY experiments at different mixing times.
Structure Calculation
The structures of the c-kit87up quadruplex were calculated using the X-PLOR program.31 NMR-restrained molecular dynamics computations were performed essentially as described previously.20a Folding of the five-residue A16-G17-G18-A19-G20 loop was facilitated by hydrogen bond restraints on the Watson-Crick A16•G20 base pair. On the basis of the observation of a cross-peak between I20(H2) and A19(H2) in the G20I modified sample (ck26), the position of A19(H2) was additionally restrained with respect to G20(N2). The loop configurations from independent folding computations were inspected visually. Two groups of folds emerged, differing by the position of G18. Selection for the G18 configuration that satisfies the observed upfield chemical shifts of H1′and H5′/H5′’ resulted in the well-converged loop structure (Figure 3).
Data Deposition
The coordinates for the c-kit87up quadruplex have been deposited in the Protein Data Bank (accession code 2O3M).
Supplementary Material
Acknowledgments
This research was supported by NIH Grant GM34504 to D.J.P. and CRUK Programme Grant C129/A4489 to S.N. D.J.P. is a member of the New York Structural Biology Center supported by NIH Grant GM66354.
Footnotes
Supporting Information Available: Table S1, a list of sitespecific low-enrichment 15N-labeled sequences used for resonance assignments; Figures S1-S3, imino proton spectra of c-kit87up at 5 °C (Figure S1), in Na+ and K+ solution (Figure S2), and in real-time hydrogen exchange experiments (Figure S3). This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Anh Tuân Phan, Email: phantuan@ntu.edu.sg.
Stephen Neidle, Email: stephen.neidle@pharmacy.ac.uk.
Dinshaw J. Patel, Email: pateld@mskcc.org.
References
- (1).Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Roskoski R., Jr. Biochem. Biophys. Res. Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- (3).Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Cancer Res. 1999;59:4297–4300. [PubMed] [Google Scholar]
- (4).Kitamura Y, Hirota S, Nishida T. Mutation Res. 2001;477:165–171. doi: 10.1016/s0027-5107(01)00117-8. [DOI] [PubMed] [Google Scholar]
- (5).Tian Q, Frierson HF, Jr., Krystal GW, Moskaluk CA. Amer. J. Pathol. 1999;154:1643–1647. doi: 10.1016/S0002-9440(10)65419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tarn C, Godwin AK. Curr. Treat. Options Oncol. 2005;6:473–486. doi: 10.1007/s11864-005-0026-x. [DOI] [PubMed] [Google Scholar]
- (7).Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CDM, Fletcher JA, Demetri GD. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- (8).von Mehren M, Watson JC. Hematol. Oncol. Clin. North Am. 2005;19:547–564. doi: 10.1016/j.hoc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- (9).Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- (10).Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schöffski P, Van Oosterom A, Marynen P, Debiec-Rychter M. Clin. Cancer Res. 2006;12:2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- (11).Simonsson T, Pecinka P, Kubista M. Nucleic Acids Res. 1998;26:1167–1172. doi: 10.1093/nar/26.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Cooney M, Czernuszewicz G, Postel EH, Flint SJ, Hogan ME. Science. 1988;241:456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- (14).Phan AT, Modi YS, Patel DJ. J. Am. Chem. Soc. 2004;126:8710–8716. doi: 10.1021/ja048805k. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ambrus A, Chen D, Dai J, Jones RA, Yang D. Biochemistry. 2005;44:2048–2058. doi: 10.1021/bi048242p. [DOI] [PubMed] [Google Scholar]
- (15).Phan AT, Kuryavyi V, Gaw HY, Patel DJ. Nat. Chem. Biol. 2005;1:167–173. doi: 10.1038/nchembio723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Smith FW, Feigon J. Nature. 1992;356:164–168. doi: 10.1038/356164a0. (a) [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Patel DJ. J. Mol. Biol. 1995;251:76–94. doi: 10.1006/jmbi.1995.0417. [DOI] [PubMed] [Google Scholar]; (c) Smith FW, Schultze P, Feigon J. Structure. 1995;3:997–1008. doi: 10.1016/s0969-2126(01)00236-2. [DOI] [PubMed] [Google Scholar]
- (17).Wang Y, Patel DJ. Structure. 1993;1:263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
- (18).Parkinson GN, Lee MPH, Neidle S. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- (19).Wang Y, Patel DJ. Structure. 1994;2:1141–1155. doi: 10.1016/s0969-2126(94)00117-0. [DOI] [PubMed] [Google Scholar]
- (20).Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. J. Am. Chem. Soc. 2006;128:9963–9970. doi: 10.1021/ja062791w. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phan AT, Luu KN, Patel DJ. Nucleic Acids Res. 2006;34:5715–5719. doi: 10.1093/nar/gkl726. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang D. Nucleic Acids Res. 2006;34:2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu Y, Noguchi Y, Sugiyama H. Bioorg. Med. Chem. 2006;14:5584–5591. doi: 10.1016/j.bmc.2006.04.033. [DOI] [PubMed] [Google Scholar]; (e) Zhang N, Phan AT, Patel DJ. J. Am. Chem. Soc. 2005;127:17277–17285. doi: 10.1021/ja0543090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Phan AT, Patel DJ. J. Am. Chem. Soc. 2003;125:15021–15027. doi: 10.1021/ja037616j. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phan AT, Modi YS, Patel DJ. J. Mol. Biol. 2004;338:93–102. doi: 10.1016/j.jmb.2004.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Haider S, Parkinson GN, Neidle S. J. Mol. Biol. 2002;320:189–200. doi: 10.1016/S0022-2836(02)00428-X. [DOI] [PubMed] [Google Scholar]; (d) Parkinson GN, Ghosh R, Neidle S. Biochemistry. 2007;46:2390–2397. doi: 10.1021/bi062244n. [DOI] [PubMed] [Google Scholar]
- (22).Rankin S, Reszka AP, Huppert J, Zloh M, Parkinson GN, Todd AK, Ladame S, Balasubramanian S, Neidle S. J. Am. Chem. Soc. 2005;127:10584–10589. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Fernando H, Reszka AP, Huppert J, Ladame S, Rankin S, Venkitaraman AR, Neidle S, Balasubramanian S. Biochemistry. 2006;45:7854–7860. doi: 10.1021/bi0601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Phan AT, Patel DJ. J. Am. Chem. Soc. 2002;124:1160–1161. doi: 10.1021/ja011977m. [DOI] [PubMed] [Google Scholar]
- (25).Phan AT. J. Biomol. NMR. 2000;16:175–178. doi: 10.1023/a:1008355231085. [DOI] [PubMed] [Google Scholar]
- (26).Phan AT, Guéron M, Leroy JL. Methods Enzymol. 2001;338:341–371. doi: 10.1016/s0076-6879(02)38228-4. [DOI] [PubMed] [Google Scholar]
- (27).Zhu L, Chou SH, Xu J, Reid BR. Nat. Struct. Biol. 1995;2:1012–1017. doi: 10.1038/nsb1195-1012. [DOI] [PubMed] [Google Scholar]
- (28).Phan AT, Kuryavyi V, Ma JB, Faure A, Andréola ML, Patel DJ. Proc. Natl. Acad. Sci. U.S.A. 2005;102:634–639. doi: 10.1073/pnas.0406278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Todd AK, Johnston M, Neidle S. Nucleic Acids Res. 2005;33:2901–2907. doi: 10.1093/nar/gki553. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huppert JL, Balasubramanian S. Nucleic Acids Res. 2005;33:2908–2916. doi: 10.1093/nar/gki609. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Phan AT, Kuryavyi V, Luu KN, Patel DJ. In: Quadruplex Nucleic Acids. Neidle S, Balasubramanian S, editors. Royal Society of Chemistry; U.K.: 2006. pp. 81–99. [Google Scholar]
- (31).Brünger AT. X-PLOR: A system for X-ray crystallography and NMR. Yale University Press; New Haven, CT: 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.