Abstract
Objective
Everyday physical activity (EPA) is an important modifiable contributor to age-related variability in executive functioning (EF). However its role may be moderated by non-modifiable genetic factors. We tested independent and interactive effects of Brain-derived neurotrophic factor (BDNF rs6265) and Insulin degrading enzyme (IDE rs6583817) on EF and EPA-EF relationships.
Method
The sample consisted of genotyped older adults (N=577, M age=70.47 years) over three waves (~9 years) of the Victoria Longitudinal Study. Analyses included (a) confirmatory factor analysis establishing a single latent EF factor from four standard EF tasks, (b) latent growth modeling over a 40-year band of aging (ages 53-95), and (c) structural regression to investigate the independent and interactive effects of BDNF, IDE and EPA.
Results
First, higher levels of EPA were associated with better EF performance at the centering age (75 years) and less EF decline. Second, IDE G+ (protective) carriers exhibited better EF performance at age 75 than their G− (non-protective) peers. Third, within the IDE G+ carrier group, those with higher EPA exhibited better EF performance and slower decline over time than those with lower EPA. Fourth, for the BDNF homozygote Val group higher EPA was associated with better EF performance and more gradual EF change; however, this beneficial effect was not seen for Met carriers.
Conclusion
The effect of modifiable physical health factors on EF is moderated by biological mechanisms associated with risk-protection genetic polymorphisms.
Keywords: everyday physical activity, executive function, BDNF Val66Met, IDE rs6583817, Victoria Longitudinal Study
Variability in trajectories of age-related cognitive decline can be attributed to multiple modifiable and non-modifiable factors, including those from biological, health, genetic, and lifestyle domains (Anstey, 2014; Dixon, Small, MacDonald & McArdle, 2012; Fotuhi, Hachinski & Whitehouse, 2009). Such factors can be examined independently or in interactive combinations that may reflect magnified risk-elevating or even counter-acting influences (Ferencz et al., 2014; McFall et al., 2014; Sapkota, Vergote, Westaway, Jhamandas & Dixon, 2015). We examine the independent and interactive associations between everyday physical activity (EPA), a modifiable influence, and two non-modifiable genetic polymorphisms (Insulin degrading enzyme (IDE) rs6563817; Brain derived neurotrophic factor (BDNF) rs6265) on concurrent and longitudinal change for a latent executive function (EF) variable in older adults from the Victoria Longitudinal Study (VLS).
EF encompasses higher-level cognitive processes required to make and execute plans, solve problems, set goals, shift between stimulus and response, and inhibit responses (e.g., Luszcz, 2012; West, 1996). These complex processes, mediated by the prefrontal cortex, are often categorized into three dimensions, namely, updating, shifting, and inhibition (Miyake et al., 2000). EFs are thought to be among the most age-sensitive cognitive functions (de Frias, Dixon & Strauss, 2006; Glisky, 2007; McFall et al., 2013, Raz, Dahle, Rodrigue, Kennedy & Land, 2011) due to significant age-related neurodegeneration occurring in the prefrontal cortices (Raz & Rodrigue, 2006). However, not all individuals show the same decline in EF performance as they age. Substantial individual differences suggest other factors, such as genetics or lifestyle, may influence age-related EF decline. Therefore, age-related prefrontal volume loss and subsequent decline in cognitive performance may be mitigated by cognitive reserve and regular participation in leisure pursuits such as physical activity (Ferencz et al., 2014; Hultsch, Hertzog, Small & Dixon, 1999; Small, Dixon, McArdle & Grimm, 2011; Solé-Padullés et al., 2009; Whalley, Deary, Appleton & Starr, 2004).
The benefits of controlled exercise interventions and fitness training to brain and general health are well known (Erickson et al., 2010, 2011; Kelly et al., 2014; Voss et al., 2013). However, there has been growing interest in EPA, a modifiable lifestyle factor which encompasses everyday leisure participation in a wide variety of activities available to older adults in voluntary moderate doses. Examples include walking, tennis, jogging, exercise, and gardening. Some longitudinal research has found higher baseline EPA is associated with better scores on reasoning and memory (Lindwall et al., 2012), and less decline in episodic memory, executive function, and verbal fluency (Blasko et al., 2014; Wang et al., 2013). In addition, reductions in EPA over time have been associated with declines in episodic memory (Small et al., 2011), reasoning, fluency, memory, and semantic knowledge (Lindwall et al., 2012). Taken together, these studies add to the mounting evidence demonstrating that the effect of EPA on cognition may be broad, diverse, and relevant to non-demented aging.
It is widely accepted that genetic variation is also a major contributor to heterogeneity in cognitive performance (Harris & Deary, 2011; Laukka et al., 2012) and these effects may be magnified in aging when additional risk factors are considered (Lindenberger et al., 2008; Nagel et al., 2008). Genetic influences also exert domain-specific effects on cognition (Ferencz et al., 2014), accounting for up to 79 percent of the variance in individual differences in EF (Swan & Carmelli, 2002). A number of candidate genes have been associated with cognitive aging (Harris & Deary, 2011; Laukka et al., 2013; Mengel-From, Christensen, McGue & Christiansen, 2011). Due to recent evidence of associations with EF or physical exercise IDE and BDNF are the candidate genes of interest to this study (Alfimova, Korovaiteseva, Lezheiko & Golimbet, 2012; Erickson et al., 2008; McFall et al., 2013, 2014; Nagel et al., 2008).
IDE. IDE is highly expressed in the brain and is recognized primarily for its role in degradation of insulin and human amyloid precursor protein (Authier, Posner & Bergeron, 1996), thus preventing the accumulation of intracellular insulin levels (Schuh, Reider, Rizzi, Chaves & Roriz-Cruz, 2011). IDE rs6383817 has an A and G allele and is associated with IDE expression; the A allele is correlated with higher levels of IDE (Carrasquillo et al., 2010). We have recently examined the association between EF performance and this polymorphism in relation to two health factors, type 2 diabetes (T2D) and pulse pressure (McFall et al., 2013, 2014). In both studies, the IDE G+ polymorphism was associated with better EF outcomes in older adults. The hypothesized mechanism for these results is associated with insulin levels in the prefrontal cortex (McFall et al., 2013, 2014), thus providing a reasonable bridge and underpinning to observed benefits for EF performance among adults (Awad, Gagnon & Messier, 2004).
Of note, however, is another function. IDE binds to and degrades insulin-like growth factor 1 (IGF-I; Schuh et al., 2011), a substrate of IDE which mediates the effect of exercise on cognition in animal models by increasing BDNF signaling in response to exercise (Cotman & Berchtold 2007). Specifically, exercise increases the level and uptake of circulating IGF-1 thus increasing BDNF expression and signaling (Carro et al., 2000; Cotman, Berchtold & Christie, 2007; Ding, Vaynman, Akhavan, Ying & Gomez-Pinnilla, 2006) until IGF-1 circulation is limited by IDE binding and degradation (Schuh et al., 2011). The resulting improved trophic factor signaling has been implicated in the neuroprotective effects of exercise on brain function (Phillips, Baktir, Srivatsan & Salehi, 2014; Voss, Nagamatsu, Liu-Ambrose & Kramer, 2011). Whether similar effects occur as a result of EPA is not yet known. However, it is conceivable that EPA could also increase the level and uptake of IGF-1. Thus, due to decreased expression of IDE, degradation of IGF-1 could occur more slowly in G+ allele carriers, resulting in increased trophic factor signalling and increased cognitive benefits, perhaps especially for EF.
BDNF. BDNF is a protein highly expressed in the central nervous system. It is involved in several important brain functions such as neuronal growth, differentiation, repair, plasticity, and survival (Liu et al., 2014; Poo, 2001). A single nucleotide polymorphism in the BDNF gene (rs6265) leads to a substitution of valine by methionine at codon 66 of the BDNF precursor protein. The Met allele has been implicated in disruption of neuronal processing, activity-dependent secretion, and intracellular trafficking of BDNF in animal models (Chen, Bath, McEwen, Hempstead & Lee, 2008; Egan et al., 2003), resulting in a significant decrease in available BDNF which is required for long-term potentiation and depression (Poo, 2001). Given the age-related decrease in BDNF expression (Hattiangady, Rao, Shetty & Shetty, 2005), BDNF rs6265 has become a prime candidate for association studies in cognitive aging.
Inconsistent results among genetic association studies examining the relationship between BDNF and cognitive function in non-demented older adults may be partly attributed to an age-specific effect of the BDNF gene or other moderating factors (Erickson et al., 2008; Mandelman & Grigorenko, 2012; Payton, 2009). In addition, other genetic or physiological factors may influence BDNF production, and thus the effect of BDNF may operate through moderating factors (Erickson, Miller & Roeckin, 2012; Sapkota et al., 2015). Specifically, BDNF expression is regulated by the BDNF polymorphism and influenced by physical exercise (Coelho et al., 2014; Erickson et al., 2012; Ferris, Williams & Shen, 2007; Neeper, Gόmez-Pinilla, Choi & Cotman, 1996; Rasmussen et al., 2009). As the amount of available BDNF affects cognitive function (Dincheva, Glatt & Lee, 2012), it follows that the BDNF gene may moderate the effect of exercise and possibly EPA on cognitive performance in aging. In fact, Erickson and colleagues (2013) examined the interactive association between BDNF and EPA on working memory in adults ranging in age from 30-54 years. Results indicated greater amounts of EPA were associated with better working memory performance, but selectively for the Met+ allele carriers, attenuating the genetic risk typically associated with this genotype.
Research Goals
The overall aim of this study was to examine concurrent and longitudinal relationships between EPA and EF as potentially moderated by two genetic polymorphisms associated with EF performance and change in older adults. We assembled a 3-wave (up to 9 years) VLS data set, covering a 40-year age span (55 – 95 years), that included manifest measures of the key constructs, EF, EPA, and two theoretically selected genetic polymorphisms (i.e., IDE rs6583817 and BDNF rs6265). We used structural equation modeling to investigate four research goals. Research goal 1 was to (a) confirm that a single-factor EF latent variable model used in previous VLS research (McFall et al., 2013, 2014) applied to this slightly different sub-sample of participants and (b) test this particular latent variable model for measurement invariance across three waves. Research goal 2 was to determine the best fitting latent growth models for EF and EPA. Research goal 3 was to use conditional growth models to explore how EPA, IDE, and BDNF independently affect level and change in EF. Research goal 4 was to determine whether the EPA-EF relationship was modified by IDE or BDNF.
Method
Participants
Participants were community dwelling older adults drawn from the Victoria Longitudinal Study (VLS). The VLS is a Canadian large-scale, long-term investigation of neurocognitive aging as influenced by biological, medical, health, lifestyle, environmental and other factors (Dixon & de Frias, 2004). Three main sequential samples (initially aged 55-95 years) are followed at about 4-year intervals (M =4.4). As the focus of this study is to examine change in EF relative to everyday physical activity as moderated by genetic variants, participants were limited to a source subsample of approximately 683 (bridging all three main VLS core samples). This source sample provided biofluid for genotyping between 2009 and 2011. Following previous protocols within the VLS (Dixon et al., 2012; McFall et al., 2014), a longitudinal data set from the same time frame was assembled, with a total individualized duration of up to 9 years. Specifically, the present study consisted of Sample 1 (S1) waves 6 and 7, Sample 2 (S2) waves 4 and 5, and Sample 3 (S3) waves 1, 2, and 3. For efficiency, the first wave in each sample will be henceforth termed as W1, the second W2, and the third W3.
Exclusionary criteria were applied, including: (a) a diagnosis of Alzheimer's Disease or other forms of impairment and dementia, (b) Mini Mental State Exam score of < 24 (MMSE; Folstein, Folstein & McHugh, 1975), (c) a self-report of “severe” for conditions such as epilepsy, spinal or thyroid conditions, depression, head injury, (d) reported alcohol or drug dependence, (e) reported use of anti-psychotic medications, (f) self-reported “moderate” cases of neurological conditions (Parkinson's or stroke), (g) reported or identified cases of diabetes (e.g., with the VLS multilevel diagnostic criteria), and (h) participants with no EF (cf. McFall et al., 2013) or EPA data. A total of 106 participants were excluded.
The final sample contained N = 577 individuals (n = 380 females) all of whom contributed data to W1 (M age = 70.47, SD = 8.59, range 53.24 – 95.25). W2 consisted of n = 483 adults (M age = 74.63, SD = 8.46, range 57.27 – 94.53, n = 315 females). W3 consisted of n = 276 adults (M age = 74.91, SD = 7.20, range 62.44 – 94.90, n = 187 females). The design stipulated that S3 participants could contribute data to all three waves, but S1 and S2 participants contributed data to W1 and W2 (the third wave not available). This consideration is balanced by the advantage of testing genetic-activity influences on EF across a full accelerated longitudinal period of about 40 years, with individual 9-year trajectory contributions. Retention rates in this design for each available and defined interval are as follows (a) S1 W1-W2 = 84%; (b) S2 W1-W2 = 83%; (c) S3 W1-W2 = 84%; (d) S3 W2-W3 = 86%; (e) S3 W1-W3 = 76%. Table 1 presents basic demographic information. All missing data were estimated by multiple imputations using Mplus 7 (Enders, 2011; Little, 2013; Muthén & Muthén, 2010). By prevailing convention 20 or more imputations are recommended (e.g., Enders, 2011; Graham, Olchowski, & Gilreath, 2007; Rubin, 1987). We included 50 imputations, as we have done in previous studies (see McFall et al., 2014).
Table 1.
Descriptive Statistics for Sample by Genotype and Longitudinal Wave
| IDE | G+ (G/G & G/A) | G− (A/A) | ||||
|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W1 | W2 | W3 | |
| n (%) | 497 (22 & 64) | 416 (22 & 64) | 251(21 & 69) | 80 (14) | 67 (14) | 25 (9) |
| Age | 70.0 (8.48) | 73.1 (7.93) | 74.7 (6.97) | 73.3 (8.76) | 76.9 (8.49) | 76.8 (9.18) |
| Range | 53.2 - 95.2 | 57.3 - 91.9 | 62.4 - 92.9 | 54.6 - 90.7 | 58.9 - 94.5 | 63.2 - 94.9 |
| Gender (% female) | 66.6 | 66.0 | 67.6 | 60.0 | 62.5 | 67.6 |
| EPA | 16.01 (5.06) | 15.16 (4.97) | 14.85 (4.99) | 15.29 (5.38) | 14.35 (6.11) | 13.83 (5.86) |
| EF Factor Score | .03 (.83) | .25 (1.05) | .51 (.98) | −.27 (.93) | −.12 (1.22) | .26 (1.02) |
| BDNF | Met+ (Met/Met & Met/Val) | Met− (Val/Val) | ||||
|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W1 | W2 | W3 | |
| n (%) | 197 (4 & 30) | 173 (5 & 31) | 99 (4 & 32) | 380 (66) | 310 (64) | 177 (64) |
| Age | 70.8 (8.33) | 74.2 (7.93) | 75.6 (7.16) | 70.3 (8.73) | 73.2 (8.18) | 74.5 (7.21) |
| Range | 54.1 - 95.2 | 58.1 - 94.5 | 62.4 - 94.9 | 53.2 - 90.8 | 57.3 - 91.1 | 62.8 - 92.9 |
| Gender (% female) | 68.5 | 67.4 | 70.6 | 64.2 | 64.6 | 66 |
| EPA | 15.98 (5.05) | 14.78 (5.14) | 14.01 (4.98) | 15.87 (5.15) | 15.19 (5.13) | 15.18 (5.10) |
| EF Factor Score | −.03 (.88) | .08 (1.17) | .31 (1.14) | .02 (.84) | .27 (1.03) | .59 (.87) |
Note. Results presented as Mean (Standard Deviation); EPA = Everyday Physical Activity. The genotypic distribution for IDE is not in Hardy-Weinberg equilibrium, χ2 = 49.93. The genotypic distribution for BDNF is in Hardy-Weinberg equilibrium, χ2 = .95. W1 = Wave 1; W2 = Wave 2; W3 = Wave 3.
Measures
Executive Function (EF)
The four EF measures used were Hayling sentence completion test (Burgess & Shallice, 1997), Stroop test (Taylor, Kornblum, Lauber, Minoshima, & Koeppe, 1997), Brixton spatial anticipation test (Burgess & Shallice, 1997), and Color trails test part two (CTT; D'Elia, Satz, Uchiyama, & White, 1996). All four measures have all been frequently used in standard form with older adults in VLS studies reporting psychometric (Bielak, Mansueti, Strauss & Dixon, 2006), structural and neuropsychological (de Frias, Dixon & Strauss, 2006, 2009), genetic (Sapkota et al., 2015), health (McFall et al., 2013, 2014), and lifestyle (de Frias & Dixon, 2014) factors.
Everyday Physical Activity (EPA)
The measure was the four-item physical activity subscale from the VLS-Activity Lifestyle Questionnaire (VLS-ALQ; Hultsch et al., 1999; Small et al., 2011). Each item indexed frequency of participation in a variety of everyday physical activities (e.g., jogging, gardening) over a period of two years on a scale of 0 (never) to 8 (daily). Responses were totalled, producing a continuous measure with scores ranging from 0 – 32. Higher scores indicate more participation in everyday leisure physical activities. Notably, the range (0 - 31) within this sample suitably encompassed the scope of possible EPA engagement. Psychometric and other details are available (Hultsch et al., 1999; Lindwall et al., 2012). To confirm inter-item reliability (consistency) for the present sample, we tested a single factor latent variable model. All four EPA items loaded on the EPA latent variable and fit the data well (χ2 = 6.296, df = 3, p = .098, RMSEA = .035, CFI = .954, SRMR = .021).
DNA Extraction and Genotyping
Saliva was collected according to DNA Genotek technology, including all recommended practices for biofluid collection, stabilization, and preparation (for details see McFall et al., 2013). Genotyping was carried out by using a PCR-RFLP strategy to analyze the allele status for IDE (rs6583817) and BDNF (rs6265).
For the genetic analyses we grouped the allelic combinations into dichotomous variables representing the relative risk versus neutral or protective categories. Accordingly, the IDE genotype was categorized by the presence of a G allele (G+ = G/G, homozygous major allele, and G/A, heterozygous allele) or the absence of a G allele (G− =A/A, homozygous minor allele) in accordance with other VLS research indicating an effect on EF performance for this allelic combination (McFall et al., 2013, 2014). In previous VLS research, we reported that the presence of the G allele was associated with protection from age related decrements in EF performance but that this G allele was the most susceptible to the influence of a modifiable health factor (i.e., pulse pressure). The BDNF genotype was categorized by the presence or absence of a Met (risk) allele (Met+ = Met homozygotes or Met/Val vs. Met− = Val homozygotes). See Table 1 for allele distribution percentages across waves.
Statistical Analyses
Structural equation modeling was conducted using Mplus 7 (Muthén & Muthén, 2010). Confirmatory factor analysis and latent growth modeling were used to test the four research goals. Model fit for all analyses was determined using standard indices: (a) χ2 for which a good fit would produce a non-significant test (p > .05), indicating the data are not significantly different than the model estimates, (b) comparative fit index (CFI) for which ≥ .95 was judged a good fit and between .90 and .94 was judged an adequate fit, (c) Tucker-Lewis index (TLI) for which ≥.95 was judged a good fit and between .90 and .94 was judged an adequate fit, (d) root mean square error of approximation (RMSEA), for which ≤ .05 would be judged good and between .06 and .08 would be judged adequate, and (e) standardized root-mean-square residual (SRMR) for which good fit is judged by a value of ≤ .08 (Kline, 2011; Little, 2013).
Analyses for RG 1: EF latent model and invariance verification
First, confirmatory factor analysis was performed to verify that a single latent EF factor (previously observed: McFall et al., 2013, 2014) fit this particular sub-sample of participants. Second, we verified this model had 3-wave measurement invariance including (a) configural invariance, for which the same indicator variables load onto the latent variable to determine if the same EF measures represent the latent variable at each wave of data collection, (b) metric invariance, for which factor loadings are constrained to be equal for each latent variable indicating that each latent variable was measuring the same construct, and (c) scalar invariance, for which indicator intercepts are constrained to be equal allowing mean differences to be evident at the latent mean level. Third, EF factor scores were estimated in Mplus and used in all subsequent growth models. Multiple imputations for EPA, age, and EF factor scores were used.
Analyses for RG 2: Latent growth modeling for EF and EPA
Consistent with other VLS research, EPA and age were coded as continuous factors. Age was centered at age 75, the approximate mean of the 40-year span of data. This is a commonly observed inflection period in cognitive aging (e.g., Dixon et al., 2012; Schaie, 2013; Small et al., 2011) and has been used in previous related research (McFall et al., 2014). Latent growth modeling was performed to establish the functional form of change for EF and EPA. We tested (a) a fixed intercept model, which assumes no inter- or intraindividual variation (b) a random intercept model, which models interindividual variability in overall level but no intraindividual change (c) a random intercept fixed slope model, which allows interindividual variability in level but assumes all individuals exhibit the same rate of change and (d) a random intercept, random slope model which allows interindividual variability in level and change (Singer & Willett, 2003).
Analyses for RG 3 and RG4: Independent effects of EPA, IDE, and BDNF on the EF growth model (RG 3); Interactive effects of IDE x EPA and BDNF x EPA on the EF growth model (RG4)
The best unconditional EF growth model was used as the baseline model against which conditional growth models with predictors of change (EPA, BDNF and IDE genotype) were tested (Little, 2013). First, three independent models were tested, one each for baseline level of EPA, IDE, and BDNF genotype. We used path analyses to determine the effect of each predictor on level of EF performance at age 75 and 9-year EF change. Second to examine gene x EPA interactions, we tested a conditional growth model for EF with EPA measured at W1 as a predictor, on level of EF performance at age 75 and 9-year EF change using the four genotype groups. Two models were tested: (a) one for IDE G+ (G/G, G/A) and IDE G− (A/A) groups and (b) one for BDNF Met+ (Met/Met, Met/Val) and BDNF Met− (Val/Val) groups. Education and gender were separately and simultaneously included as covariates; however, none of these analyses changed the pattern of results. Pulse pressure (PP) was considered and included as a potential covariate; however, results did not indicate a relationship between EPA and PP. Therefore, PP, education, and gender were not included in the final model.
Results
RG 1: EF Latent Model and Invariance Verification
We first confirmed that a single-factor model consisting of the four EF indicators fit this sub-sample of participants (see Table 2 for all goodness of fit indices). Second, we verified by testing longitudinal measurement invariance that the EF model measured the same construct over time and that the same indicator variables marked EF at each wave. The partial scalar invariance model was retained and this allowed us to compare latent variable means (see Table 2 for all goodness of fit indices; Kline, 2011).
Table 2.
Goodness of Fit Indices for Executive Function Confirmatory Analysis Models and Measurement Invariance Testing
| Model | AIC | BIC | χ2 | df | p | RMSEA | CFI | TLI | SRMR | Δχ2 | Δdf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| One Factor EF (W1) | 9138.884 | 9191.18 | 2.46 | 2 | 0.29 | .02 (.00-.09) | 0.99 | 0.99 | 0.01 | ||
| One Factor EF (W2) | 7842.417 | 7892.40 | 0.01 | 2 | 0.99 | .00 (.00-.00) | 1 | 1.03 | 0.01 | ||
| One Factor EF (W3) | 5308.477 | 5351.92 | 3.53 | 2 | 0.17 | .03 (.00-.14) | 0.98 | 0.95 | 0.05 | ||
| Configural Invariance | 20277.10 | 20516.78 | 32.45 | 35 | 0.52 | .00 (.00 -.03) | 1 | 1.00 | 0.03 | ||
| Metric Invariance | 20274.00 | 20487.54 | 41.36 | 41 | 0.46 | .00 (.00-.03) | 1 | 0.89 | 0.05 | 8.91 | 6 |
| Scalar Invariance | 20386.38 | 20565.05 | 169.72 | 49 | <.001 | .07 (.06-.08) | 0.92 | 0.89 | 0.10 | 128.36* | 8 |
| Partial Scalar Invariance* | 20292.95 | 20489.05 | 68.30 | 45 | 0.014 | .03 (.01-.04) | 0.98 | 0.98 | 0.06 | 26.94* | 4 |
Note. EF = Executive Function; W1 = Wave 2; W2 = Wave 2; W3 = Wave 3; CTT = Color Trails; HAY = Hayling; AIC = Akaike information criterion; BIC = Bayesian information criterion; RMSEA = Root Mean Square Error of Approximation; CFI = Comparative Fit Index; TLI = Tucker-Lewis Index; SRMR = Standardized Root Mean Square Residual
p < .001
a Best fitting model used for Factor Score Analysis.
RG 2: Latent Growth Models for EF and EPA
The best fitting unconditional growth model for EF was established as a random intercept, random slope model (see Table 3). First, at age 75, older adults varied significantly in level of EF performance (b = 1.14, p < .001). Second, there was significant decline in EF performance (M = −.013, p = .022). Third, there was significant individual variability in the rate of decline (b = .003, p < .001).
Table 3.
Goodness of Fit Indices for EF and EPA Latent Growth Models
| Model | −2LL | Parameters Free | AIC | BIC | D | Δdf |
|---|---|---|---|---|---|---|
|
Executive Function (EF)
| ||||||
| Fixed intercept | 5612.52 | 2 | 5616.52 | 5625.23 | ||
| Random intercept | 3406.82 | 3 | 3412.82 | 3425.89 | 2205.70 | 1 |
| Random intercept, fixed slope | 3162.74 | 4 | 3170.74 | 3188.17 | 244.08 | 1 |
| Random intercept, random slope* | 1927.28 | 6 | 1939.28 | 1965.42 | 1235.46 | 2 |
|
Everyday Physical Activity (EPA) | ||||||
| Fixed intercept | 2723.37 | 4 | 2731.37 | 2748.80 | ||
| Random intercept | 2074.14 | 5 | 2084.14 | 2105.93 | 649.23 | 1 |
| Random intercept, fixed slope* | 1874.28 | 6 | 1886.28 | 1912.43 | 199.86 | 1 |
| Random intercept, random slope** | 1872.72 | 8 | 1888.72 | 1923.58 | 1.56 | 2 |
Note. H0 = log likelihood value; −2LL = −2 log likelihood; AIC = Akaike information criterion; BIC = Bayesian information criterion; S = sample variance
p < .001
a Preferred model.
b This model was not retained as the variance of the slope was not significant
Next for EPA, the preferred model was a random intercept, fixed slope model (see Table 3). First, at age 75, there was significant variability in level of EPA (b = .161, p < .001). Second, older adults exhibited significant decline in EPA level (M = −.021, p < .001), but without individual differences in rate as evidenced by the non-significant random slope (p > .05) in the random intercept, random slope model.
RG 3: Independent Effects of EPA, IDE and BDNF on EF Growth
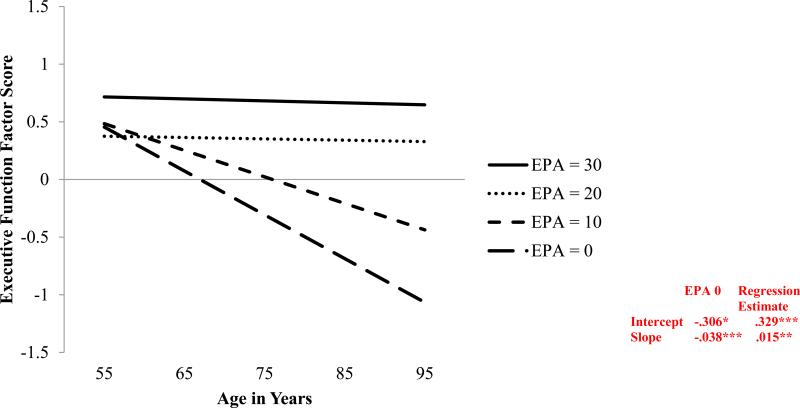
We tested two growth models with EPA as a predictor of EF level and change. The first model used the EPA growth model in parallel process with the EF growth model. Time-varying EPA did not predict EF performance at age 75 (b = −.005, p >.05) nor 9-year EF change (b = .452, p >.05). The second time-invariant model with initial (W1) level of EPA revealed significant predictions for both EF performance at age 75 (b= .329, p <.001) and 9-year change (b = .015, p = .001. Specifically, at W1 lower levels of EPA were associated with significantly worse EF performance (M = -.306) than were higher levels of EPA (M = .023,). Moreover, lower initial levels of EPA were associated with greater 9-year EF decline (M = −.038) than were higher levels (M = −.023). Thus, the time-invariant EPA (W1) model was used in subsequent analyses.
We tested two models with either IDE or BDNF as a predictor of EF level and change. First, IDE significantly predicted level of EF performance at age 75 (b = −.337, p = .013). Specifically, the IDE G+ group had better EF performance at the centering age of 75 (M = .262) than did the IDE G− group (M = −.069). IDE genotype did not predict 9-year EF change (b = −.013, p =.135). Second, BDNF did not predict level of EF performance at age 75 (b = .062, p = .528) nor 9-year EF change (b = .005, p = .358).
In an exploratory follow-up analysis, we tested the IDE x BDNF interaction on EF performance and 9-year change. This model did not predict level of EF performance at age 75 (b = −.094, p = .068) nor 3-wave change (b = −.003, p = .381).
RG 4: Interactive Effects of IDE x EPA and BDNF x EPA on EF Growth
We tested two models to examine whether IDE or BDNF moderated the effect of EPA on EF. The moderation hypotheses were supported for both genes as evidenced by the differing effects of EPA on EF performance and change at the two levels (each) of IDE and BDNF.
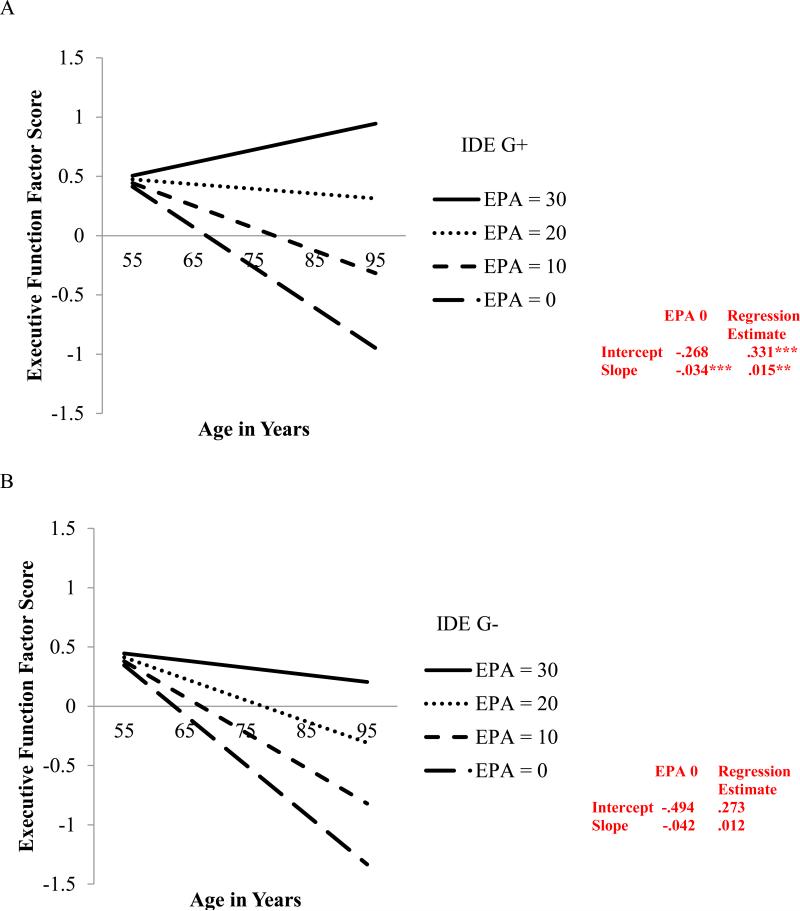
A significant interaction was indicated for IDE. This was produced by a significant effect of EPA on EF level (intercept) and change (9-year) for one genotype group (IDE G+) but not the other (IDE G−; see Figure 2 for model fit indices). Specifically, for the IDE G+ group, level of EPA at W1 predicted both level of EF performance at age 75 (b = .326, p < .001) and 9-year EF change (b = .015, p = .006). Within the protective IDE G+ group, older adults with low levels of EPA at W1 exhibited poorer EF performance (M = −.268) and steeper 9-year decline (M = −.034) than did their peers with high levels of EPA (M = .063 and M = −.019, respectively; see Figure 2a). This pattern was not present for the G− group, as level of EPA did not alter level of EF at age 75 (b = .276, p = .305) nor the 9-year EF change (b = .012, p = .425; see Figure 2b).
Figure 2.
Predicted growth curves for executive function factor scores by IDE using everyday physical activity (EPA) level (0 = low, 30 = high) as a predictor where age is a continuous variable centered at age 75. −2 log likelihood = 1899.76; Akaike information criterion = 1929.76; Bayesian information criterion = 1995.12. Figure 2(A) is IDE G+ (i.e., A/G, G/G). Figure 2(B) is IDE G− (i.e., A/A). * p < .05; ** p < .01; ***p < .001
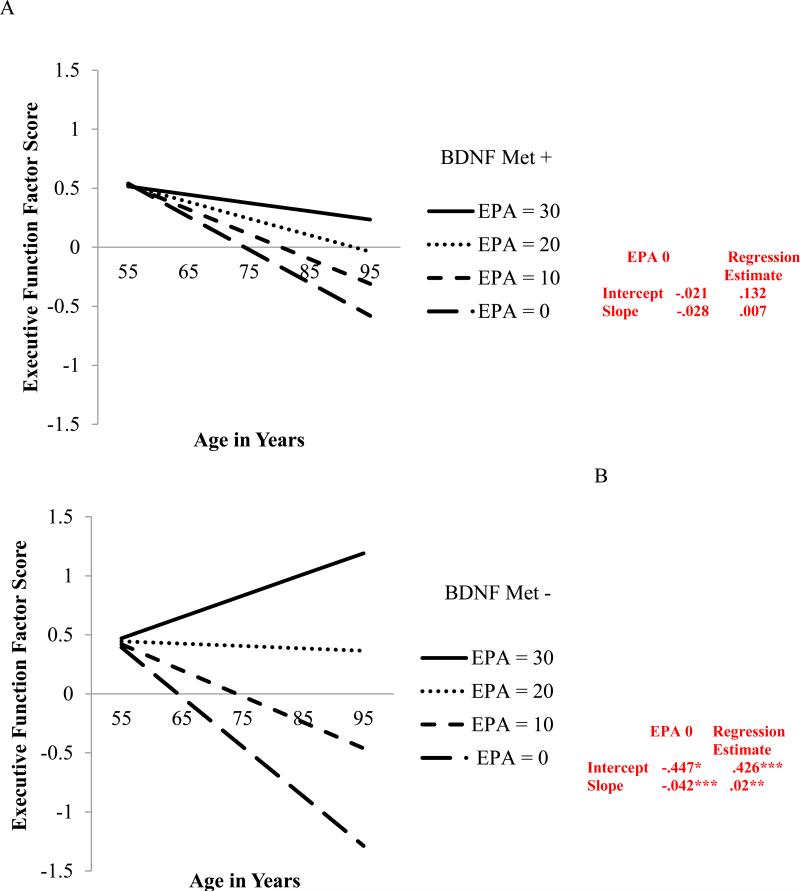
Similarly, a significant interaction was indicated for BDNF (see Figure 3 for model fit indices). Specifically, for the Met− group, level of EPA at W1 predicted both the level of EF performance (b = .426, p < .001) and 9-year EF change (b = .020, p = .003). Within the Met – group, older adults with lower levels of EPA at W1 exhibited poorer EF performance at age 75 (M = −.447) and steeper 9-year EF decline (M = −.042) than their peers with higher EPA levels at W1 (M = −.021 and M = −.022, respectively; see Figure 3b). In contrast, for the BDNF Met+ (risk) group, level of EPA at W1 did not affect level of EF performance (b = .132, p = .385) nor 9-year EF change (b = .007, p = .432; see Figure 3a).
Figure 3.
Predicted growth curves for executive function factor scores by BDNF using everyday physical activity (EPA) level (0 = low, 30 = high) as a predictor where age is a continuous variable centered at 75 years. −2 log likelihood = 1897.80; Akaike information criterion = 1927.79; Bayesian information criterion = 1993.16. Figure 3(A) is BDNF Met+ (i.e., Met/Met, Met/Val). Figure 3(B) is BDNF Met – (i.e., Val/ Val). * p < .05; ** p < .01; ***p < .001
As an exploratory follow-up analysis, we examined whether there was a significant IDE x BDNF x EPA interaction. Specifically, we tested the effect of EPA, measured at W1, on level of EF performance at age 75 and 9-year change as a function of four genetic combinations of IDE and BDNF (G+/Met+, G+/Met−, G−/Met+, G−/Met−). Notably, the analyses showed a significant effect exclusively for the G+/Met− group (i.e., the most protective/non-risk group). Specifically, level of EPA at W1 significantly predicted both the level of performance (b =.040, p = .001) and 9-year change (b = .002, p = .006) in EF performance. Within the G+/Met− group, older adults with higher levels of EPA at W1 exhibited better EF performance at the age of 75 (M = −.316 ) and less 9-year decline (M = −.037) than did their genetically corresponding peers with lower levels of EPA at W1 (M = −.356 and M = −.039, respectively). EPA at W1 did not predict performance or 9-year change in any group characterized by the presence of one or both genetic risk alleles. These exploratory results are consistent with the pattern observed for each of the two-way gene x EPA analyses.
Discussion
The overall aim of this research was to examine concurrent and longitudinal associations among a modifiable lifestyle factor (EPA), two genetic polymorphisms (IDE and BDNF) related to neurocognitive aging, and performance and change in EF. We distributed this aim into four goals. Research goals 1 and 2 confirmed (a) a single-factor EF model fit our data well and (b) previously observed variability in EF performance and decline (McFall et al., 2013, 2014). These results provided the groundwork for testing cross-domain moderating effects that could produce evidence supportive of potential synergistic risk-reduction for a non-demented aging group. We now highlight the key findings for Research goals 3 and 4.
Research Goal 3 (independent effects of EPA, IDE, and BDNF on the unconditional EF growth model) revealed several notable results. The results for the EPA time-invariant model indicated that older adults with higher initial levels of EPA had better initial EF performance and more gradual decline over the three waves. This is consistent with emerging research which indicates that moderate and everyday physical activity engagement may buffer against some cognitive decline in aging (e.g., Bielak, Cherbuin, Bunce & Anstey, 2014; Blasko et al., 2014; Ferencz et al., 2014; Hamer, Lavoie & Bacon, 2013; Lindwall et al., 2012; Rovio et al., 2005; Wang et al., 2013; Woodard et al., 2012).
A possible mechanism for this emerging pattern is that EPA, like moderate aerobic exercise, may favorably affect gray matter volume in older adults, either through increases or slower degradation. In fact, for older adults moderate exercise has been found to increase gray matter volume in the prefrontal cortex (Colcombe et al., 2006), an area which mediates the association between aerobic exercise and EF (Weinstein et al., 2012). Erickson and colleagues (2010) found that greater amounts of walking predicted greater gray matter volume over a period of 9 years. Walking is an everyday moderate-intensity activity included in the range of activities represented in the construct of EPA. Although it awaits targeted intervention-based research, it is conceivable that other modalities of moderate or everyday physical activities could also benefit prefrontal gray matter volume, thereby favorably influencing EF in non-demented older adults.
The present results serve to broaden the scope of physical activity that can be beneficial to cognitive functioning in non-demented older adults. As demonstrated in clinical trials, the organized exercise subcategory (i.e., aerobic, resistance) has well-known positive effects on cognitive performance. Some recent observational research implicates moderate everyday physical activity as also providing some cognitive benefits, at least under some conditions, the details of which are yet to be determined (e.g., dosage, duration). As a modifiable influence, the fact of effective doses at moderate levels implies that EPA may be an accessible and durable dementia risk reducing lifestyle factor for many older adults.
The corresponding analyses of the independent effects of IDE or BDNF as predictors of EF level and change revealed two main results. First, IDE genotype predicted level of EF performance (See McFall et al., 2014) but not rate of change in normal aging older adults. Little research on IDE-neuropsychological associations has been conducted; however, whether IDE significantly predicts EF rate of change appears to depend in part on methodological (e.g., length of longitudinal period), demographic (e.g., age of sample), or moderation characteristics (e.g., moderators included and analyzed). Second, BDNF genotype did not independently predict either initial level or 9-year change in EF. Previous BDNF research reveals inconsistent relationships with cognitive performance and decline over time in a variety of human groups (Das et al., 2014, Egan et al., 2003; Erickson et al., 2008; Gajewski et al., 2011, 2012; Harris et al., 2006; Miyajima et al., 2007). Reviews suggest that differences across studies may be due to issues of domain specificity, low predictive independent associations, or non-evaluated but relevant moderation effects (Mandelman & Grigorenko, 2012; Sapkota et al., 2015). The latter are the ultimate target of the present study. Therefore, the main result of RG3 was to establish the EPA-EF association. We now turn to the pivotal results of RG4, which was designed to test linkages between our modifiable risk factor (EPA) and potential genetic moderators (IDE and BDNF).
For Research Goal 4, we tested whether BDNF or IDE moderated the EPA-EF relationship. Several key findings supported our expected genetic moderation effects. First, results from the IDE conditional growth model indicated that IDE indeed moderated the EPA-EF relationship, as indicated by the observation that the EPA-EF patterns were different for the G+ as compared with the G− groups (see Figure 2). Specifically, older adults with two risk-reducing factors (i.e., carriers of the G allele and with high EPA) had better EF performance at age 75 and had more gradual 9-year EF decline than did genetically corresponding (G+) adults with low EPA. In contrast, for the non-protective IDE G− group, level of EPA did not significantly affect either level of EF performance or 9-year change. This pattern is consistent with a “two-boost” (or synergistic) effect, in that risk reduction is enhanced even when the sources of protection are from different domains (activity, genetic). Overall these results suggest that individuals with the protective IDE G+ allele are more likely to experience beneficial effects from EPA.
Mechanisms promoting these effects are still to be determined. However, one possible mechanism which could account for these results is an EPA induced increase in neurotrophic signalling for G+ carriers. The IDE G allele is related to a decrease in IDE expression (McFall et al., 2013). IDE binds to and degrades IGF-1 (Schuh et al., 2011), which influences the levels of circulating IGF-1. IGF-1 mediates BDNF exercise-induced changes in the brain (Voss, Vivar, Kramer & van Praag, 2014). As the EPA measure reflects participation in different types of everyday physical activities, it is conceivable that EPA could initiate a neurotrophic response similar to (but of less intensity than) aerobic exercise. Therefore, the effect of EPA for the IDE G+ carriers could be attributable to the decrease in IDE expression, which increases the amount of circulating IGF-1, thus positively affecting a neurotrophic cascade. As this was the first known study examining the interactive effects of EPA and IDE on EF, future research could investigate whether neurotrophic factor signaling is increased by EPA, as well as the interaction between EPA and IDE within other cognitive domains. Such studies would further elucidate the IDE- EPA relationship within non-demented and relatively healthy older adults.
As noted above, BDNF, when tested as a single candidate gene, produces inconsistent associations with cognition in a variety of groups (Mandelman & Grigorenko, 2012). However, in this study we observed that BDNF actually moderated the EPA-EF relationship (see Figure 3). For Val homozygotes (Met−; non-risk), EPA was significantly and positively related to EF performance (at age 75) and 9-year change. These results show that older adults in the genetic risk-reduced group (without the BDNF Met allele) had better EF outcomes both concurrently and longitudinally, but only if they also had high initial levels of EPA. Even low-risk (Met−) adults who had low initial EPA levels produced lower levels of EF performance and steeper longitudinal decline in EF. As can be seen in the figure, the results were different for carriers of the Met allele (i.e., genetic risk); EPA level was not associated with EF performance (at age 75) or 9-year change. This pattern is also consistent with an interactive association between genetic risk (low) and activity engagement (high)—a combination that produces better and more stable EF performance in non-demented older adults.
Regarding potential mechanisms and qualifications, it is interesting to consider the present results in the context of a recent report by Erickson and colleagues (2013). As previously described, their results (with midlife participants) indicated that greater levels of EPA-like measures offset genetic risk associated with the Met allele for working memory performance. In contrast, the current results with older adults indicated that the BDNF x EPA interaction was observed only for those without the risk allele. The apparent differences in results between these two studies may point to a complementary pattern. The general BDNF-EPA interaction effect could come from a combination of factors; for example, the Met allele is associated with lower availability of BDNF in the brain and lower brain volume (Egan et al., 2003; Miyajima et al., 2007) and there is an age-related decrease in BDNF levels (Lommatzsch et al., 2005; Zeigenhorn et al., 2007). Taken together, it is possible that these results reflect an aging-selective limit to the potential effect that environmental or lifestyle factors can have on older adult Met allele carriers. Accordingly, older BDNF Val homozygotes (Met−) appear to be more influenced by the beneficial effects of lifestyle factors.
As brain and general health can be modified by several lifestyle factors, it is possible a similar effect could be seen in future studies examining the relation between BDNF, cognitive function and other influential and interacting health factors. Although different in several respects, a recent study reported differential effects for older adult Met carriers with cardiovascular disease, as compared with older Val homozygotes of the same health condition (Szabo et al., 2013). This susceptibility to health and lifestyle factors for older Val homozygotes might explain some of the complexity and conflicting results found in research examining BDNF-cognition relationships in adults. Future research on BDNF-cognition associations in aging could include approaches that reflect moderation or interaction with other genetic, health, or lifestyle factors (Sapkota et al., 2015).
We note two points of interest regarding IDE and BDNF. First, in an exploratory analysis, we observed a significant three-way interaction which indicated older adults with three risk-reducing factors (i.e., IDE G+, BDNF Met− and higher levels of EPA) had better EF performance at the centering age and less 9-year decline than genetically corresponding peers with lower levels of EPA. As this effect was not observed for any other of the risk combinations, the results can be interpreted provisionally as supporting and extending the two-way interactions reported earlier. However, future research could examine these promising higher-order risk-related associations in larger samples. Second, IDE and BDNF are prominent but are not the only genes that could moderate the effect of EPA on cognition. For example, future research might examine the cognitive risks for older adult carriers of the APOE ε4 allele which have recently been associated with EPA (Smith, Nielson, Woodard, Seidenberg & Rao, 2013). Similarly, Ferencz et al., (2014) found that EPA attenuated the effect of genetic risk (consisting of PICALM, CLU, BIN1 polymorphisms) on episodic memory functioning for older adults. Conceivably, the effect of EPA on cognitive functioning could also be differentially modified by combinations within genetic (e.g., Sapkota et al., 2015) and other risk factors (e.g., Anstey, 2014).
There are several limitations associated with this study. First, we use a self-report measure of EPA and thus not all aspects of the construct domain are represented or observed. Although more direct measures of everyday activities could be valuable (e.g., Erickson et al., 2011), self-report inventories have been used successfully in converging behavioral studies (e.g., Hultsch et al., 1999; see also Lindwall et al., 2012, who compared four EPA indicators) Future research may consider including both observational and self-report indicators in order to establish validity and create composite indicators. Second, due to the design of the VLS, W3 data had not been collected for the first two samples contributing to this study. Therefore, only participants from the third VLS sample contributed to W3. A more complete design would have included participants from all three samples in W3; however, the results of the current 3-wave data were quite informative. Third, the present participants are positively selected and may possess several risk-reducing factors, such as access to national health care, above-average in years of education, community dwelling, and relatively healthy (e.g., free of known neurodegenerative disease). As a group, they may not be representative of the broadest population of older adults; however, they could reflect a growing proportion of older adults in western countries.
There are also several strengths associated with this study. First, we had a relatively large and well-characterized sample (W1 n = 577) which comprised a span of 40 years of aging. Second, we measured age as a continuous variable through an accelerated longitudinal design which allowed us to examine a span of 9 years. Third, we included four standard, reliable neuropsychological manifest variables in our EF latent variable. In addition, the research goals were analyzed within a modern statistical approach.
We conclude that EPA and genetic factors contribute synergistically to the observed variability in EF performance in non-demented aging. IDE G+ carriers and BDNF Met− homozygotes were more likely to benefit from the effect of higher levels of EPA on the aging of executive functions—a “two-boost” effect in non-demented aging. Our results complement recent literature (e.g., Erickson et al., 2013; Ferencz et al., 2014); specifically, genetically advantaged individuals are also sensitive to modifiable lifestyle factors, indicating a potential for expanded application of EPA interventions. Notably, by its very nature, EPA may be easily translated and converted to intervention programs that would likely be maintained over long periods. Such characteristics allow for a broad application of EPA interventions for non-demented older adults in a wide variety of settings – from community to long-term care facilities.
Figure 1.
Predicted growth curve for executive function factor scores using everyday physical activity (EPA) at W1 as a predictor with age as a continuous variable centered at 75 years. −2 log likelihood = 1912.68; Akaike information criterion = 1928.65; Bayesian information criterion = 1963.54.
Acknowledgments
This research was supported by grants from the (a) National Institutes of Health/National Institute on Aging (R01 AG008235) to RAD, (b) Alberta Health Services (University Hospital Foundation) to RAD, Jack Jhamandas, and David Westaway, and (c) the Canada Research Chairs program (to RAD). KJA is supported by NHMRC Research Fellowship #1002560.
Contributor Information
Sherilyn Thibeau, Department of Psychology, University of Alberta.
G. Peggy McFall, Department of Psychology, University of Alberta.
Sandra A. Wiebe, Department of Psychology, University of Alberta
Kaarin J. Anstey, Centre for Research on Ageing, Health and Wellbeing, Australian National University
Roger A. Dixon, Department of Psychology, University of Alberta.
References
- Alfimova MV, Korovaitseva GI, Lezheiko TV, Golimbet VE. Effect of BDNF Val66Met polymorphism on normal variability of executive functions. Bulletin of Experimental Biology and Medicine. 2012;152(5):606–609. doi: 10.1007/s10517-012-1587-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22803145. [DOI] [PubMed] [Google Scholar]
- Anstey KJ. Optimizing cognitive development over the life course and preventing cognitive decline: Introducing the cognitive health environment life course model (CHELM). International Journal of Behavioral Development. 2014;38(1):1–10. doi:10.1177/0165025413512255. [Google Scholar]
- Authier F, Posner BI, Bergeron JJM. Insulin-degrading enzyme. Clinical and Investigative Medicine. 1996;19(3):149. Retrieved from http://europepmc.org/abstract/MED/8724818. [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes and cognitive function. Journal of Clinical and Experimental Neuropsychology. 2004;26:1044–1088. doi: 10.1080/13803390490514875. doi:10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Mansueti L, Strauss E, Dixon RA. Performance on the Hayling and Brixton tests in older adults: Norms and correlates. Archives of Clinical Neuropsychology. 2006;21(2):141–149. doi: 10.1016/j.acn.2005.08.006. doi:10.1016/j.acn.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Cherbuin N, Bunce D, Anstey KJ. Preserved differentiation between physical activity and cognitive performance across young, middle and older adulthood over 8 years. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2014;69(4):523–532. doi: 10.1093/geronb/gbu016. doi:10.1093/geronb/gbu016. [DOI] [PubMed] [Google Scholar]
- Blasko I, Jungwirth S, Kemmler G, Weissgram S, Tragl KH, Fischer P. Leisure time activities and cognitive functioning in middle European population-based study. European Geriatric Medicine. 2014;5(3):200–207. doi:10.1016/j.eurger.2013.09.003. [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton tests. Thames Valley Test Company; Thurston, Suffolk, England: 1997. [Google Scholar]
- Carrasquillo MM, Belbin O, Zou F, Allen M, Ertekin-Taner N, Ansari M, Morgan K. Concordant association of insulin degrading enzyme gene (IDE) variants with IDE mRNA, Aß, and Alzheimer's disease. Plos One. 2010;5(1) doi: 10.1371/journal.pone.0008764. doi:10.1371/journal.pone.0008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. Journal of Neuroscience. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. Retrieved from http://www.jneurosci.org/content/20/8/2926.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports. 1985;100(2):126–131. Retrieved from http://www.jstor.org/stable/20056429. [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Foundation Symposia. 2008;289:180–195. doi: 10.1002/9780470751251.ch14. doi:10.1002/9780470751251.ch14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FGDM, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Santos-Galduróz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. Journal of Alzheimer's Disease. 2014;39(2):401–408. doi: 10.3233/JAD-131073. doi:10.32233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neuroscience. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. doi:10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: Learning from animal models. Alzheimer's and Dementia. 2007;3(2):30–37. doi: 10.1016/j.jalz.2007.01.013. doi:10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- D'Elia LA, Satz P, Uchiyama CL, White T. Psychological Assessment Resources. Odessa, FL.: 1996. Color Trails Test: Professional Manual. [Google Scholar]
- Das D, Tan X, Bielak AAM, Cherbuin N, Easteal S, Anstey KJ. Cognitive ability, intraindividual variability and common genetic variants of catechol-O-methyltransferase and brain-derived neurotrophic factor: A longitudinal study in a population-based sample of older adults. Psychology and Aging. 2014;29(2):393–403. doi: 10.1037/a0035702. doi:10.1037/a0035702. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA. Lifestyle engagement affects cognitive status differences and trajectories on executive functions in older adults. Archives of Clinical Neuropsychology. 2014;29(1):16–25. doi: 10.1093/arclin/act089. doi:10.1093/arclin/act089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20(2):206–214. doi: 10.1037/0894-4105.20.2.206. doi:10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23(6):778–791. doi: 10.1037/a0016743. doi:10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. doi:10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Glatt CE, Lee FS. Impact of the BDNF Val66Met polymorphism on cognition: Implications for behavioral genetics. Neuroscientist. 2012;18(5):439–451. doi: 10.1177/1073858411431646. doi:10.1177/1073858411431646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. doi:10.1080/13825580490511161. [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, McArdle JJ. Yes, memory declines with aging-but when, how, and why? In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. Psychology Press; New York: 2012. pp. 325–347. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. doi:10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Enders CK. Analyzing longitudinal data with missing values. Rehabilitation Psychology. 2011;56:267–288. doi: 10.1037/a0025579. doi:10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Banducci SE, Weinstein AM, MacDonald AW, Ferrell RE, Halder I, Manuck SB. The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychological Science. 2013;24(9):1770–1779. doi: 10.1177/0956797613480367. doi:10.1177/0956797613480367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: A 10-year longitudinal study of COMT and BDNF polymorphisms. Frontiers in Human Neuroscience. 2008;2(11):1–9. doi: 10.3389/neuro.09.011.2008. doi:10.3389/neuro.09.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and BDNF. The Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. doi:10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The cardiovascular health study. Neurology. 2010;75(16):1415–1422. doi: 10.1212/WNL.0b013e3181f88359. doi:10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. doi:10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz B, Laukka EJ, Welmer AK, Kalpouzos G, Angleman S, Keller L, Bäckman L. The benefits of staying active in old age: Physical activity counteracts the negative influence of PICALM, BIN1, and CLU risk alleles on episodic memory functioning. Psychology and Aging. 2014;29(2):440–449. doi: 10.1037/a0035465. doi:10.1037/a0035465. [DOI] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen C. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and Science in Sports and Exercise. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. doi:10.1249/mss.0b13e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nature Reviews Neurology. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. doi:10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiology of Aging. 2011;32(12):2327.e7–2327.e19. doi: 10.1016/j.neurobiolaging.2011.06.010. doi:10.1016/j.neurobiolaging.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The metgenotype of the BDNF Val66Met polymorphism is associated with reduced stroop interference in elderly. Neuropsychologia. 2012;50(14):3554–3563. doi: 10.1016/j.neuropsychologia.2012.09.042. doi:10.1016/j.neuropsychologia.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Riddle DR, editor. Changes in cognitive function in human aging. Brain aging: Models, methods, and mechanisms (Chapter 1) 2007 Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK3885/ [PubMed]
- Graham JW, Olchowski AE, Gilreath TD. How many multiple imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9. doi:10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Hamer M, Lavoie KL, Bacon SL. Taking up physical activity in later life and healthy ageing: The English longitudinal study of ageing. British Journal of Sports Medicine. 2013;48:239–243. doi: 10.1136/bjsports-2013-092993. doi:10.1136/bjsports-2013-092993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive aging in healthy older people. Trends in Cognitive Sciences. 2011;15(9):388–394. doi: 10.1016/j.tics.2011.07.004. doi:10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The brain-derived neurotrophic factor val66met polymorphism is associated with age-related change in reasoning skills. Molecular Psychiatry. 2006;11(5):505–513. doi: 10.1038/sj.mp.4001799. doi:10.1038/sj.mp.4001799. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Experimental Neurology. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. doi:10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Small BJ, Hertzog C, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. doi:10.1037/0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews. 2014;16(0):12–31. doi: 10.1016/j.arr.2014.05.002. doi:10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd Edition Guilford Press; New York, NY: 2011. [Google Scholar]
- Laukka EJ, Lövdén M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, Lövdén M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, Bäckman L. Genetic effects on old-age cognitive functioning: A population based study. Psychology and Aging. 2013;28(1):262–274. doi: 10.1037/a0030829. doi:10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heerkeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2(2):234–244. doi: 10.3389/neuro.01.039.2008. doi:10.3389/neuri.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall M, Cimino CR, Gibbons LE, Mitchell MB, Benitez A, Brown CL, Piccinin A. Dynamic associations of change in physical activity and change in cognitive function: Coordinated analyses of four longitudinal studies. Journal of Aging Research. 2012:1–12. doi: 10.1155/2012/493598. doi:10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD. Longitudinal structural equation modeling. Guilford Press; New York, NY: 2013. [Google Scholar]
- Liu M, Huang C, Chen M, Yang AC, Tu P, Yeh H, Tsai S. Effect of the BDNF Val66Met polymorphism on regional gray matter volumes and cognitive function in the Chinese population. Neuromolecular Medicine. 2014;16(1):127–136. doi: 10.1007/s12017-013-8265-7. doi:10.1007/s12017-013-8265-7. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiology of Aging. 2005;26(1):115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7th ed. Academic Press; San Diego, CA: 2011. pp. 59–72. doi:10.1016/B978-0-12-380882-0.00004-8. [Google Scholar]
- Mandelman SD, Grigorenko EL. BDNF val66met and cognition: all, none, or some? A meta-analysis of the genetic association. Genes, Brain and Behavior. 2012;11:127–136. doi: 10.1111/j.1601-183X.2011.00738.x. doi:10.1111/j.1601.183.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Dixon RA. IDE (rs6583817) polymorphism and type 2 diabetes differentially modify executive function in older adults. Neurobiology of Aging. 2013;34(9):2208–2216. doi: 10.1016/j.neurobiolaging.2013.03.010. doi:10.1016/j.neurobiolaging.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GM, Wiebe SA, Vergote D, Jhamandas J, Westaway D, Dixon RA. IDE (rs6583817) and pulse pressure are independently and interactively associated with level and change in executive function in older adults. Psychology and Aging. 2014;29(2):418–430. doi: 10.1037/a0034656. doi:10.1037/a0034656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel-From J, Christensen K, McGue M, Christiansen L. Genetic variations in the CLU and PICALM genes are associated with cognitive function in the oldest old. Neurobiology of Aging. 2011;32(3):554 e7–11. doi: 10.1016/j.neurobiolaging.2010.07.016. doi:10.1016/j.neurobiolaging.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Payton A. Brain-derived neurotrophic factor polymorphism val66met influences cognitive abilities in the elderly. Genes, Brain and Behaviour. 2007;7:411–417. doi: 10.1111/j.1601-183X.2007.00363.x. doi:10.1111/j.1601-183.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6th ed Muthén and Muthén; Los Angeles, CA: 2010. [Google Scholar]
- Nagel IE, Chicherio C, Li S, von Oertzen T, Sander T, Villringer A, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience. 2008;2:1–8. doi: 10.3389/neuro.09.001.2008. doi:10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726(1–2):49–56. doi:10.1016/0006-8993(96)00273-9. [PubMed] [Google Scholar]
- Payton A. The impact of genetic research on our understanding of normal cognitive ageing: 1995 to 2009. Neuropsychology Review. 2009;19(4):451–477. doi: 10.1007/s11065-009-9116-z. doi:10.1007/s11065-009-9116-z. [DOI] [PubMed] [Google Scholar]
- Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Frontiers in Cellular Neuroscience. 2014;8(170):1–16. doi: 10.3389/fncel.2014.00170. doi:10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2(1):24–32. doi: 10.1038/35049004. doi:10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. doi:10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. doi:10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiology of Aging. 2011;32(6):1124–1137. doi: 10.1016/j.neurobiolaging.2009.05.015. doi:10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kåreholt I, Helkala E, Viitanen M, Winblad B, Tuomilehto J, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. The Lancet Neurology. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. doi:10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputations for nonresponse in surveys. Wiley; Hoboken, NJ: 1987. doi:10.1002/9780470316696. [Google Scholar]
- Sapkota S, Vergote D, Westaway D, Jhamandas J, Dixon RA. Synergistic associations of catechol-O-methyltransferase and brain-derived neurotrophic factor with executive function in aging are selective and modified by apolipoprotein E. Neurobiology of Aging. 2015;36(1):249–256. doi: 10.1016/j.neurobiolaging.2014.06.020. doi:10.1016/j.neurobiolaging.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence. 2nd ed. Oxford University Press; New York, NY: 2013. [Google Scholar]
- Schuh AF, Rieder CM, Rizzi L, Chaves M, Roriz-Cruz M. Mechanisms of brain aging regulation by insulin: Implications for neurodegeneration in late-onset Alzheimer's disease. ISRN Neurology. 2011:1–9. doi: 10.5402/2011/306905. doi:10.5402/2011/306905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. doi:10.1093/acprof:oso/9780195152968.001.0001. [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2011;26:144–155. doi: 10.1037/a0026579. doi:10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Rao SM. Physical activity and brain function in older adults at increased risk for Alzheimer's disease. Brain Sciences. 2013;3:54–83. doi: 10.3390/brainsci3010054. doi:10.3390/brainsci3010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C, Bartrés-Faz D, Junqué C, Vendrell P, Rami L, Clemente IC, Molinuevo JL. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiology of Aging. 2009;30(7):1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. doi:10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Evidence for genetic mediation of executive control: A study of aging male twins. Journals of Gerontology - Series B Psychological Sciences and Social Sciences. 2002;57(2):133–143. doi: 10.1093/geronb/57.2.p133. Retrieved from http://psychsocgerontology.oxfordjournals.org/content/57/2/P133.short. [DOI] [PubMed] [Google Scholar]
- Szabo AJ, Alosco ML, Miller LA, McGeary JE, Poppas A, Cohen RA, Gunstad J. Brain-derived neurotrophic factor Val66Met polymorphism and cognitive function in persons with cardiovascular disease. Psychogeriatrics. 2013;13(4):206–212. doi: 10.1111/psyg.12013. doi:10.1111/psyg.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. doi:10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Voss. MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, Behaviour, and Immunology. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. doi:10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. Journal of Applied Physiology. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. doi:10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 2014;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. doi:10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jin Y, Hendrie HC, Liang C, Yang L, Cheng Y, Gao S. Late life leisure activities and risk of cognitive decline. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(2):205–213. doi: 10.1093/gerona/gls153. doi:10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Erickson KI. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain, Behavior, and Immunity. 2012;26(5):811–819. doi: 10.1016/j.bbi.2011.11.008. doi:10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):256–271. doi: 10.1037/0033-2909.120.2.272. doi:10.1037-0033-2909.120.2.256. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3:369–392. doi: 10.1016/j.arr.2004.05.001. doi:10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Sugarman MA, Nielson KA, Smith JC, Seidenberg M, Durgerian S, Rao SM. Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Current Alzheimer Research. 2012;9:436–446. doi: 10.2174/156720512800492477. doi:10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Hellweg R. Serum neurotrophins—A study on the time course and influencing factors in a large old age sample. Neurobiology of Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. doi:10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]