Abstract
Background
Neoplasm-related precocious puberty (PP) is a rare presenting feature of childhood cancer. Moreover, evaluation of suspected PP in a child is complex, and cancer is often not considered. We characterized the clinicopathologic features of patients presenting with PP at a large pediatric cancer center, reviewed the relevant literature, and developed an algorithm for the diagnostic work-up of these patients.
Methods
We examined the records of all patients with a neoplasm and concomitant PP treated at St. Jude Children’s Research Hospital from January 1975 through October 2011, reviewed the available literature, and analyzed the demographic, clinical, endocrine, and neoplasm-related features.
Results
Twenty-four of 13,615 children and adolescents (0.18%) were diagnosed with PP within 60 days of presentation. Primary diagnoses included brain tumor (12), adrenocortical carcinoma (5), hepatoblastoma (4), and others (3). PP was observed 0–48 months before diagnosis of neoplasm; 17 patients had peripheral PP and 7 had central PP.
Conclusions
Neoplasm-related PP is rare and takes the form of a paraneoplastic syndrome caused by tumor production of hormones or by alteration of physiologic gonadotropin production. PP can precede diagnosis of malignancy by months or years, and neoplastic causes should be considered early to avoid delayed cancer diagnosis. Treatment of the primary malignancy resolved or diminished PP in surviving patients with an intact hypothalamic–pituitary–gonadal axis.
Keywords: adrenocortical carcinoma, brain tumor, gonadal tumor, hepatoblastoma, paraneoplastic endocrinopathy, paraneoplastic precocious puberty
INTRODUCTION
Puberty begins with activation of the hypothalamic–pituitary–gonadal system, and its normal parameters are well defined. Precocious puberty (PP) is a more common pathological variant than absence or delay of puberty [1,2]. PP is conventionally defined as the onset of puberty before age 8 years in females and 9 years in males [2]. It is categorized as either “true” or central PP (gonadotropin-dependent, with premature activation of the hypothalamic–pituitary–gonadal axis) or precocious pseudopuberty, also called peripheral PP (independent of gonadotropin and axis maturation) [1,3]. While the gonadotropin pattern is normal in central PP, peripheral PP induces secondary sexual characteristics by androgen and estrogen secretion not physiologically mediated by gonadotropins [4]. Central PP occurs in one in 5,000–10,000 children and is 5–10 times as common in females as in males [5,6]. While normal Tanner-stage progression is approximately 12 months per stage, exceptionally rapid puberty may approach or exceed twice that rate.
In large studies of PP, the underlying cause is usually benign or unclear, with no evidence of organ lesions [7,8]. PP is caused by malignant neoplasms and is described as a presenting sign of childhood cancer in only a small number of case reports [8–12]. Therefore, awareness of the possibility of malignancy is necessary to avoid delayed cancer diagnosis. Here we review our experience of PP caused by neoplasm, estimate its incidence at the time of tumor diagnosis, and describe the demographic, clinical, endocrine, and neoplasm-related features of 24 cases treated at our pediatric cancer center. We also searched available reports for models of the underlying pathophysiology of neoplastic PP and for diagnostic clues to neoplasm in children who present with central or peripheral PP.
METHODS
We reviewed the records of all patients at St. Jude Children’s Research Hospital who had a new diagnosis of neoplasm between January 1, 1975 and October 30, 2011 to identify those who had a secondary diagnosis of PP or age-inappropriate pubertal development and velocity within 2 months of presentation. The 2-month period ensured inclusion of PP not initially appreciated or that emerged during early antineoplastic treatment. Abnormal pubertal velocity was identified by comparing pubertal development signs to skeletal maturation and/or the presence of elevated serum human chorionic gonadotropin (hCG) or hypothalamic–pituitary–gonadal axis hormones in males older than 8 years and females older than 9 years.
The time when PP signs were first observed by the caregiver or primary care physician was obtained from patient records. Physical, laboratory, and radiological findings consistent with PP or with pathologic pubertal course and velocity were reviewed to determine the relation of endocrine changes to underlying tumor type and treatment, including low-grade tumors or tumors typically considered benign in children. For brevity, all tumors may be referred to as cancers or neoplasms. This study was approved by the St. Jude Institutional Review Board.
RESULTS
Patients
Of 13,615 patients with neoplasms diagnosed during the study period, 24 (0.18%, 16 male, 8 female) had PP with onset before or within 2 months after presentation at St. Jude (Table I). PP was diagnosed or confirmed by clinical, laboratory, or radiological findings. Age at onset or at detection of pathologic pubertal velocity was 11 months to 11 years (median, 6.63 years). Twenty-one patients had signs of PP for more than 1-month (and 16 of them had signs for 3 months or more) before tumor diagnosis. In three patients, PP was appreciated within 60 days after presentation (patients 11, 12, 24, Table I).
TABLE I.
Patient Characteristics
| No. | Sex | Age at cancer diagnosis (y) | Age at diagnosis of PP (y) | Cancer diagnosis | Time from onset of PP to cancer diagnosis (mo) | Endocrine testing before cancer diagnosis (Y/N) | Main endocrine finding | Cancer outcome |
|---|---|---|---|---|---|---|---|---|
| CNS tumors | ||||||||
| 1 | Male | 9.2 | 5.5 | Hypothalamic astrocytoma | 44 | Yes | CPP, no tumor seen in initial work-up | CR |
| 2 | Male | 10.9 | 9.4 | Suprasellar JPA | 18 | Yes | CPP per hormone panel, mass on MRI | Stable disease |
| 3 | Male | 7.3 | 6.8 | Hypothalamic ganglion cell tumor | 6 | Yes | Pubarche, diagnosis of panhypopituitarism and cerebral mass | Relapse 3 |
| 4 | Female | 8.7 | 7.7 | Cerebellar JPA | 12 | Yes | Bone age >4 years advanced, menarche at 8 y, mass on MRI | CR |
| 5 | Male | 3.4 | 1.4 | Optic pathway glioma | 24 | No | Tanner G Ph II–III; MRI for hyperactivity, finding of mass | Unknown |
| 6 | Male | 15.1 | 11.1 | Pinealis germinoma | 48 | No | PP by history, age-appropriate at 1st visit | CR |
| 7 | Male | 11.1 | 10.8 | NGGCT | 3 | Yes | Diagnosis of GH deficiency 2 years prior, re-eval for PP showed elevated HCG/testosterone | CR |
| 8 | Male | 12.4 | 11.75 | NGGCT | 8 | Yes | GH deficiency noted age 4, DI age 7, panhypopituitarism age 11; re-imaged for PP | Unknown |
| 9 | Female | 7.25 | 6.8 | Craniopharyngioma | 6 | Yes | CPP and mass on MRI | Stable disease |
| 10 | Male | 9.8 | 9.3 | Pineal mixed germ cell tumor | 6 | No | Imaging for headaches and PP by PCP | CR |
| 11 | Female | 3.8 | 3.8 | Pineal PNET | 0 | No | Tanner B Ph II at cancer diagnosis | Death |
| 12 | Female | 11 | 11 | Pineal NGGCT | 0 | No | HCG and testosterone elevated at cancer diagnosis | CR |
| Extracranial tumors | ||||||||
| 13 | Male | 3.9 | 3 | Metastatic shepatoblastoma | 11 | Yes | Tanner G III–IV, abdominal mass | Death |
| 14 | Male | 10.5 | 6.5 | Metastatic ACC | 48 | Yes | Advanced bone age and elevated testosterone, no malignancy found on scans before age 10 | Death |
| 15 | Female | 1.2 | 0.9 | Metastatic ACC | 3 | Yes | Elevated DHEA +androgens, mass on US | CR, |
| 16 | Male | 2.1 | 2 | Hepatoblastoma | 1 | Yes | Tanner G II, mass detected by endocrinologist | CR |
| 17 | Female | 6.8 | 5.8 | Ovarian JGCT | 12 | Yes | CPP, “benign” enlarged ovarian follicles, no response to GnRH analog | CR |
| 18 | Male | 10.5 | 9.8 | Metastatic ACC | 8 | No | Rapid puberty with secondary sexual characteristics only | Death |
| 19 | Female | 3.5 | 3 | Ovarian JGCT | 6 | Yes | Pubarche, ovarian mass | CR |
| 20 | Female | 1.3 | 1.2 | ACC | 2 | Yes | Virilization, adrenal mass on US | Unknown |
| 21 | Male | 8.6 | 8.25 | ACC | 4 | No | Pubarche, penile enlargement, incidental finding of adrenal mass on trauma work-up CT | CR |
| 22 | Male | 9.9 | 7 | Leydig cell tumor | 35 | Yes | Tanner G IV, CPP per hormone panel, refractory to suppression × 12 months, imaging identified gonadal mass | CR |
| 23 | Male | 1.8 | 1.6 | Metastatic hepatoblastoma | 3 | No | Tanner G III–IV, hCG elevation | CR |
| 24 | Male | 3.1 | 3.2 | Metastatic hepatoblastoma | Within 1 month of begin of therapy | No | Tanner G III and hCG elevation after start of chemotherapy | On therapy |
ACC, adrenocortical carcinoma; CPP, central precocious puberty; CR, complete remission; DHEA, dehydroepiandrosterone; DI, Diabetes insipidus; G, grade; GH, growth hormone; HCG, human chorionic gonadotropin; JGCT, juvenile granulosa cell tumor; JPA, juvenile pilocytic astrocytoma; mo, months; MRI, magnetic resonance imaging; NGGCT, non-germinomatous germ cell tumor; PNET, primitive neuroectodermal tumor; PCP, primary care physician; PP, precocious puberty; Tanner G, Tanner stage for male genitals; Tanner Ph, Tanner stage for pubic hair; US, ultrasonography; y, years.
Tumors
Brain tumors (n = 12/24, six low-grade) were most common and resided in the pineal (n = 6) or optic pathway/hypothalamic (n = 4) regions. Of the 12 extracranial tumors, adrenocortical carcinoma (ACC, n = 5) and hepatoblastoma (n = 4) were most common (Table I). Most extracranial neoplasms were high-grade (9 of 12) and metastatic (6 of these 9) at diagnosis. Two of the three patients with PP discovered within 60 days after tumor diagnosis had brain tumors and presented with headache. The third had hepatoblastoma and, interestingly, developed PP after initiation of chemotherapy. This patient (patient 24) had normal hCG and testosterone levels at tumor diagnosis. After one course of chemotherapy, he showed signs of PP and significantly increased hCG and testosterone but a decrease in the primary tumor marker α-fetoprotein (AFP). A patient with metastatic hepatoblastoma (patient 23, Table I) had elevated AFP and hCG at cancer diagnosis; after one course of chemotherapy he had reduced AFP but markedly increased hCG and testosterone. He also experienced a greater frequency and duration of erections and mild acne. Both patients had reduced hormone levels and signs of PP after course two.
Endocrine Features, Treatment, and Outcome
Median age at onset of PP was 8.5 years in patients with brain tumors and 3.1 years in those with extracranial tumors. Seventeen patients (10 male, 7 female) met the age-based definition of PP; seven (6 brain tumors, 1 ACC) were older (6 males aged 9.3–11.75 years, 1 female aged 11.0 years) but demonstrated age-inappropriate pubertal velocity. In six of these seven patients, bone age was 6–50 months (median, 27 months) greater than chronological age at the time of tumor diagnosis. The remaining patient had prominent secondary sexual characteristics and elevated hCG and testosterone.
Fifteen patients did undergo evaluation by an endocrinologist which led to tumor diagnosis in 11 patients. Six of the seven females <8 years old at onset of PP were evaluated by a pediatric endocrinologist before tumor diagnosis. Only 6 of the 10 males <9 years old at onset of PP had undergone endocrine evaluation, despite onset of PP at a median of 4.3 years. In 3 of the 6 males age ≥9 years, malignancy was identified during endocrine evaluation. The one female with PP onset after age 8 years (patient 11) had no endocrine evaluation; her neurological symptoms (concurrent with abnormal pubertal signs) led to diagnosis of brain tumor.
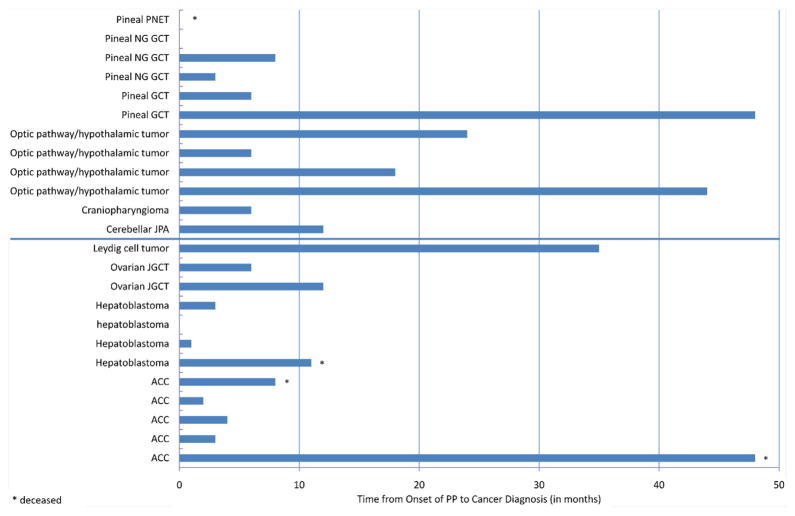
Median time from onset of PP to tumor diagnosis was 6 months (range, 0–48 months) in both sexes. Median time was 6 months in all patients with brain tumors and 5 months in those with extracranial tumors (Fig. 1). The six patients with low-grade brain tumors had a prolonged median period between PP onset and diagnosis (low grade, 18 months; high grade, 4.5 months). Remarkably, median time to diagnosis was greater among patients who had undergone endocrine evaluation (8 months vs. 4 months).
Fig. 1.
Months between onset of precocious puberty and cancer diagnosis in the 24 study patients. ACC, adrenocortical carcinoma; GCT, germ cell tumor; JGCT, juvenile granulosa cell tumor; JPA, juvenile pilocytic astrocytoma; NG GCT, non-germinomatous germ cell tumor; PNET, primitive neuroectodermal tumor.
Three of the 4 patients who died had extracranial tumors and a markedly longer time to diagnosis (8, 11, and 48 months) than the median of 5 months in other patients with extracranial tumors. All three patients had advanced solid tumors at diagnosis. One patient died of a malignant brain tumor (Table I).
Four patients were treated with a gonadotropin-releasing hormone (GnRH) agonist for presumed idiopathic central PP before the underlying neoplasms (2 brain tumors, 2 gonadal tumors; patients 1, 2, 17, 22) were discovered. All four had CNS imaging during their endocrine evaluations (3 had brain MRI after the year 2000, 1 had cranial CT before 1990), but no lesions were initially appreciated in the brain tumor patients. In one patient with a gonadal tumor (patient 17), the lesion was interpreted as benign. No records were found about review of images by radiologists at the cancer center. Patient 22 underwent endocrine evaluation and brain MRI 18 months after slowly progressive PP. Testosterone was elevated and basal LH and FSH were prepubertal, but the LH stimulation test indicated central PP, prompting treatment with a GnRH agonist. After 12 more months of progressive puberty and rising testosterone, ultrasonography revealed a testicular mass. After this Leydig cell tumor was resected, testosterone rapidly became undetectable while GnRH agonist treatment continued. With suspension of hormone suppression therapy, pubertal basal LH and FSH levels finally indicated central PP. Subsequent resumption of pubertal suppression was effective.
All four patients continued PP treatment concurrently with tumor therapy; two additional patients (with brain tumors) received GnRH agonist therapy after endocrine evaluation and during tumor treatment. Of the 20 surviving patients, 2 were lost to follow-up; the remaining 18 have been followed for a median of 7.6 years (range, 6 mo–31.9 y). Four developed panhypopituitarism after brain tumor treatment, and one had secondary central PP (patient 22). The remaining 13 patients needed no further endocrine therapy while in cancer remission.
DISCUSSION
Although rare, malignancy can cause central or peripheral PP and should be considered early in evaluation (Table II) [4,9,13]. Signs of pubertal abnormality were misinterpreted or underappreciated in half of our cohort reflected by a marked delay in evaluation of PP or falsely diagnosed idiopathic central PP. In 16 of 24 patients, signs of PP had been observed for more than 3 months before tumor diagnosis, often emerging before tumor-related signs and symptoms. Seven children had normal pubertal timing but accelerated pubertal velocity, as reflected by rapid progression through the Tanner stages and advanced bone age, that is, accelerated more than two standard deviations above the chronologic age [14]. This finding demonstrates that recognition of pathologic variants of puberty requires not only familiarity with the age definition of PP, but a thorough knowledge of the normal spectrum of pubertal development.
TABLE II.
Differential Diagnosis of Precocious Puberty
| Central precocious puberty (gonadotropin-dependent) | Peripheral precocious puberty (gonadotropin-independent) |
|---|---|
| Idiopathic | Exogenous steroids |
| CNS tumors | Hormone-secreting tumor (hCG, adrenal androgens/cortisol, gonadal testosterone/estradiol) |
| Anatomic or developmental abnormality (congenital or acquired) | Genetic abnormalities |
| Arachnoid cyst | Mutation of LH receptor |
| Hydrocephalus | McCune Albright syndrome |
| Hamartoma | Defects in adrenal steroid biosynthesis |
| CNS injuries | |
| Posttraumatic | Leydig-cell hyperplasia |
| Postinfectious | |
| Posthemorrhagic | Ovarian follicle cyst |
| Postradiation |
CNS, central nervous system; hCG, human chorionic gonadotropin; LH, luteinizing hormone.
Time from PP onset to tumor diagnosis did not differ notably between brain tumors and other diagnoses. However, most extracranial malignancies were high-grade and metastatic at diagnosis, and three of the four patients who died had metastatic extracranial malignancies with above-median intervals from PP onset to cancer diagnosis. The prolonged median interval between PP onset and diagnosis followed by favorable outcome in patients with low grade brain tumors can likely be explained by the less aggressive nature of the tumor, and the adolescent age of most of the patients which may have masked pathologic puberty. This study did not include neurological, neurocognitive, or comprehensive neuroendocrine outcomes, which may have been adversely affected by delayed diagnosis of malignancy despite good cancer outcome measures. Timely recognition of paraneoplastic PP would expedite cancer diagnosis, and thus potential resolution of PP, and might prevent metastatic disease.
A narrow majority of patients underwent endocrine evaluation which did not appear to expedite but rather delay cancer diagnosis. Misinterpretation as idiopathic CPP and failed hormone suppression therapy were remarkably frequent. It is very likely that tumors were present at the time of evaluation, as in patients 17 and 22. Although patients who did not undergo endocrine evaluation had a shorter median time from PP onset to tumor diagnosis, their concurrent symptoms (e.g., headache, abdominal distension) likely facilitated sooner cancer diagnosis. However, continued education should be provided to endocrinologists to raise awareness early for malignancy as a rare cause for PP.
Pathophysiology
A review of the literature reveals that brain tumors, gonadal and adrenal tumors, and hepatoblastoma may be associated with PP [15]; all of these tumors were represented in our series. An understanding of the pathophysiology of PP in relation to these tumors is essential to their detection. Our patients fell into the three pathophysiological groups shown in Table III [15].
TABLE III.
Neoplasms Associated with Precocious Puberty
| Tumor | Hormone/tumor marker | Tumor localization | Age/sex distribution | Specific characteristic |
|---|---|---|---|---|
|
Paraneoplastic endocrinopathy
| ||||
| Germ cell tumor (GCT) [17] | hCG (AFP) | Intragonadal | Older adolescents | hCG production depends on histological composition (yolk sac tumors never produce hCG) |
| Embryonal carcinoma | Extragonadal
|
|||
| Choriocarcinoma | Teratoma: infants/toddlers | |||
| Yolk sac tumor | Young adolescents | |||
| Teratoma | M/F 3:1 | |||
| Mixed cellular GCT | ||||
| Hepatoblastoma [12] | hCG (AFP) | Liver | <3 years of age M/F 1:1 |
AFP is main tumor marker |
|
| ||||
|
Excessive production of sex hormones
| ||||
| Juvenile granulosa cell tumor [22], Granulosa theca cell tumor | Estradiol | Ovaries | First decade of life | Endocrine abnormalities more frequent in younger patients |
| Leydig cell tumor [23] | Testosterone | Testes | Age 5–10 years | Only 0.8–3% of pediatric testicular tumors |
| Sertoli cell tumor | Testosterone or estrogen | Testes and ovaries | Bimodal (young children and adolescents) | Isosexual precocity; possible virilization in females and gynecomastia in males |
| Sertoli-Leydig cell tumor | Testosterone | Testes and ovaries | Less often hormonally active in females | |
| Adrenocortical carcinoma [26] | Androstenedione; dehydroepiandrosterone ≫estrogen | Adrenal gland | Age <6 years and adults M/F 1:1.6 |
Feminization in <10% of cases via aromatization of androgens or estrogen secretion |
|
| ||||
|
Alteration of physiologic gonadotropin production
| ||||
| Brain tumors [27] | None | Hypothalaic-pituitary region ≫other intracerebral localization | Young adolescents | Premature physiologic activation of hypothalamic–pituitary–gonadal axis |
| Hypothalamo-chiasmatic glioma and pilocytic astrocytoma of other location | Age 5–10 years, M = F | |||
| Parenchymal tumors of pineal gland Craniopharyngioma Ependymoma | Peak at age 5 years | |||
Paraneoplastic endocrinopathy
Nine of our 24 patients had true paraneoplastic PP. Five were diagnosed with germ cell tumors (GCTs), four had hepatoblastoma. Their symptoms likely reflected tumor ectopic production of hormone, most commonly hCG, a glycoprotein whose α-subunit is identical to that of luteinizing hormone (LH). HCG may be expressed by GCTs and hepatoblastomas, the latter classically produce AFP [16–18]. Because of its similarity to LH, hCG can stimulate testosterone production by Leydig cells, causing penile enlargement, pubic and axillary hair growth, and voice change in males [19]. Tumor lysis on initiation of chemotherapy can increase hCG release, potentiating testosterone production, and symptoms of PP. Testicular size often does not increase in the absence of FSH, which is necessary for spermatogenesis.
Basal and stimulated LH production is initially suppressed in hCG-induced PP. In females, ovarian follicles are not stimulated in the absence of FSH, and hCG-induced PP is less common, as are some hCG-secreting tumors [20]. Some case reports, however, describe females with isosexual PP, defined as secondary sexual characteristics matching the genetic sex, associated with hCG-secreting tumors, usually brain tumors. Disinhibition of GnRH, coincidental expression of estradiol or aromatase, or hCG’s weak intrinsic FSH-like activity are speculated to induce female hCG-mediated PP [21,22]. As previously reported [12,18], true paraneoplastic endocrinopathy was more frequent in males in our study (eight males and one female with a pineal non-germinomatous GCT). This female had age-inappropriate puberty and elevated hCG, LH, estradiol, and testosterone but undetectable FSH, supporting a hypothesis of FSH-like activity or co-expression of estradiol and/or aromatase. All hormone levels normalized during treatment.
Proliferative process with excessive sex hormone production
Eight of our patients had neoplasms that cause PP by inducing excessive sex hormone secretion by the tumor’s organ or tissue of origin. Three had a gonadal neoplasm and five had ACC. Key diagnostic findings include high levels of testosterone, estrogen or its precursors, and prepubertal basal or stimulated FSH and LH levels [23–25]. However, secondary central PP with pubescent LH and FSH levels induced by persistently high sex hormones may mask the underlying pathologic mechanism [26], as in patients 17 and 22 (Table I), who had gonadal neoplasms.
Childhood ACC, another entity in this group, usually secretes excessive steroids. The most common presenting sign is virilization with elevated androstenedione and dehydroepiandrosterone (DHEA). Estrogen secretion or aromatization of androgens to estrogen causes feminization in less than 10% of cases [27]. In our group, females with ACC were younger than males (12 and 16 months vs. 8.6–10.5 years) and had a shorter history of PP before cancer diagnosis (2 and 3 months vs. 8 months–4 years). Two of the males with prolonged unattended PP died of metastatic disease, while both females remain in remission. Although the number of patients does not allow definitive conclusions, younger age, and conspicuous virilization in females may prompt earlier medical evaluation.
Tumor alteration of gonadotropin production
In the remaining 7 of our 24 patients, tumor tissue was not hormonally active but likely disrupted hypothalamic and pituitary function. Such tumors are typically hypothalamic–chiasmatic gliomas, parenchymal pineal tumors, and craniopharyngiomas (rarely, ependymoma or other brain tumors) [28]. Their location may prematurely activate the hypothalamic–pituitary–gonadal axis, causing central PP. The mechanisms of activation are complex and not entirely clear. The neoplasm can cause hydrocephalus or exert a mass effect on the hypothalamus, which is thought to contribute to pubertal GnRH release and subsequent secretion of LH and FSH [28]. In such cases, PP is rarely the only presenting symptom. Headache, emesis, altered vision, cerebellar dysfunction, and seizures are common, depending on tumor location [29]. However, as observed in our study, patients with low-grade brain tumors may have a long history of neuroendocrine dysregulation before other signs and symptoms prompt additional evaluation.
Diagnostic Recommendations
It is essential to recognize pathologic puberty in a child, both premature onset and increased velocity (progression from Tanner II to IV in <12 months). For example, 7 of our 24 patients had abnormal pubertal velocity but were older than the recognized gender-specific threshold of PP. Discordant signs and symptoms should raise suspicion of an underlying problem. It should also be kept in mind that PP is more common in females but is more often pathological in males, partly explained by a higher incidence of benign central PP in females due to environmental factors and childhood obesity [3,5] but higher incidence of some hCG-secreting tumors and hCG-induced PP in males [12,18,20,30].
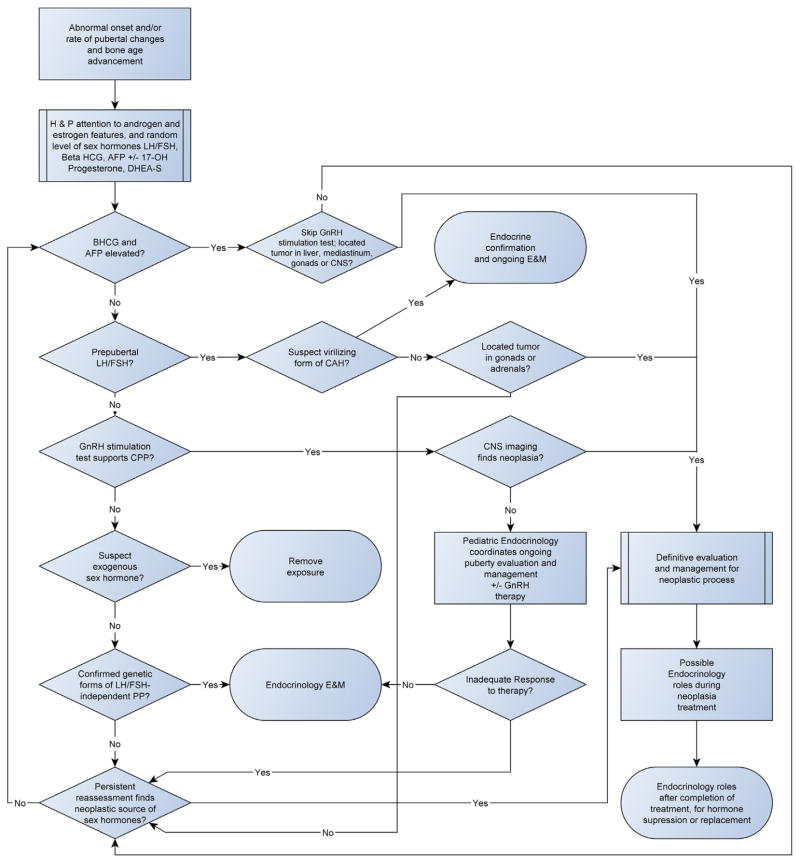
Basic evaluation of a child with PP should include a comprehensive medical history, including parental pubertal history and longitudinal body height history, and a thorough physical examination focused on growth, Tanner staging, asymmetry of genitals and gonads, other signs of puberty (e.g., changes in skin and voice), abdominal mass, and neurological abnormalities. Assessment of bone age completes a basic evaluation, although radiological bone maturation may lag behind physical findings or height velocity. Secondary sexual characteristics in prepubertal patients should prompt an evaluation for peripheral PP including tumor markers, sex hormones, and imaging of gonads and adrenal gland and, if appropriate, possible sites of hCG-producing tumors. Attention should also be given to females with signs of progressive virilization without thelarche and menarche. Central PP is more likely when initial changes include testicular enlargement in males and breast development in females, and MRI of the brain is necessary to detect small lesions in the pineal or hypothalamic-optic region. Figure 2 provides a diagnostic algorithm for central and peripheral PP, including neoplastic causes.
Fig. 2.
Diagnostic algorithm for precocious puberty, including malignancy as an underlying cause. AFP, alpha-fetoprotein; BHCG, beta-human chorionic gonadotropin; CAH, congenital adrenal hyperplasia; CNS, central nervous system; CPP, central precocious puberty; DHEA-S, dehydroepiandrosterone; E&M, evaluation and management; GnRH, gonadotropin–releasing hormone; H&P, history and physical examination; LH/FSH, luteinizing hormone and follicle stimulating hormone; MAS, McCune Albright syndrome; PP, precocious puberty.
CONCLUSION
Most children referred for a PP evaluation have benign normal variants [8]. Malignancy is a rare but important cause of PP, and early recognition and appropriate diagnosis are crucial. In our study, failure of caretakers and/or medical providers to recognize PP was the most common cause of delayed cancer diagnosis. PP may be misinterpreted as idiopathic central PP, and in rare cases central PP may be induced by peripheral PP. However, even when LH and FSH are elevated, inappropriately high testosterone or estradiol levels or inadequate response to a suppression regimen should raise early suspicion of an autonomous pathologic process. In such cases, close monitoring and repeated imaging of potential hormone-producing sites may be necessary to identify a tumor.
In our series, extracranial tumors, especially hepatoblastoma and ACC, were the most frequent cause of PP in young children. Patients with brain tumors were frequently male and presented with PP or pathologic pubertal progress at an age between childhood and adolescence; therefore, abnormal pubertal development warrants medical attention even beyond the defined age of PP.
Consequently, a final diagnosis of idiopathic PP should not be made before assessment of the hypothalamo-pituitary-gonadal axis followed by appropriate laboratory tests (e.g., hCG, adrenal DHEA, and androstenedione). When results are equivocal, close follow-up, repeat studies, and an interdisciplinary approach are mandatory.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: P30CA021765; Grant sponsor: American Lebanese Syrian Associated Charities (ALSAC)
We thank Sharon Naron for editing the manuscript.
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Bramswig J, Dubbers A. Disorders of pubertal development. Deutsches Arzteblatt Int. 2009;106:295–303. doi: 10.3238/arztebl.2009.0295. quiz 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfield RL, Bachrach LK, Chernausek SD, et al. Current age of onset of puberty. Pediatrics. 2000;106:622–623. doi: 10.1542/peds.106.3.622. [DOI] [PubMed] [Google Scholar]
- 4.Eugster EA. Peripheral precocious puberty: Causes and current management. Horm Res. 2009;71:64–67. doi: 10.1159/000178041. [DOI] [PubMed] [Google Scholar]
- 5.Prete G, Couto-Silva AC, Trivin C, et al. Idiopathic central precocious puberty in girls: Presentation factors. BMC Pediatr. 2008;8:27. doi: 10.1186/1471-2431-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358:2366–2377. doi: 10.1056/NEJMcp0800459. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen SS, Aksglaede L, Mouritsen A, et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab. 2011;96:1393–1401. doi: 10.1210/jc.2010-2745. [DOI] [PubMed] [Google Scholar]
- 8.Kaplowitz P. Clinical characteristics of 104 children referred for evaluation of precocious puberty. J Clin Endocrinol Metab. 2004;89:3644–3650. doi: 10.1210/jc.2003-031532. [DOI] [PubMed] [Google Scholar]
- 9.Bridges NA, Christopher JA, Hindmarsh PC, et al. Sexual precocity: Sex incidence and aetiology. Arch Dis Child. 1994;70:116–118. doi: 10.1136/adc.70.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Graaf JH, Tamminga RY, Kamps WA. Paraneoplastic manifestations in children. Eur J Pediatr. 1994;153:784–791. doi: 10.1007/BF01972883. [DOI] [PubMed] [Google Scholar]
- 11.Arshad RR, Woo SY, Abbassi V, et al. Virilizing hepatoblastoma: Precocious sexual development and partial response of pulmonary metastases to cis-platinum. CA Cancer J Clin. 1982;32:293–300. doi: 10.3322/canjclin.32.5.293. [DOI] [PubMed] [Google Scholar]
- 12.Navarro C, Corretger JM, Sancho A, et al. Paraneoplasic precocious puberty. Report of a new case with hepatoblastoma and review of the literature. Cancer. 1985;56:1725–1729. doi: 10.1002/1097-0142(19851001)56:7<1725::aid-cncr2820560743>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Muir A. Precocious puberty. Pediatr Rev. 2006;27:373–381. doi: 10.1542/pir.27-10-373. [DOI] [PubMed] [Google Scholar]
- 14.Zwiebel WJ, Murray KA. Imaging assessment of pubertal disorders. Semin Ultrasound CT MR. 1995;16:296–303. doi: 10.1016/0887-2171(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 15.Perilongo G, Rigon F, Murgia A. Oncologic causes of precocious puberty. Pediatr Hematol Oncol. 1989;6:331–340. doi: 10.3109/08880018909034304. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawara A, Ikeda K, Tsuneyoshi M, et al. Hepatoblastoma producing both alpha-fetoprotein and human chorionic gonadotropin. Clinicopathologic analysis of four cases and a review of the literature. Cancer. 1985;56:1636–1642. doi: 10.1002/1097-0142(19851001)56:7<1636::aid-cncr2820560729>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Pierson M, Gilgenkrantz S, Saborio M, et al. Klinefelter’s syndrome. Trilogy of Fallot. Teratoma of the mediastinum and early puberty. Pediatrie. 1975;30:185–192. [PubMed] [Google Scholar]
- 18.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 19.Pomarede R, Finidori J, Czernichow P, et al. Germinoma in a boy with precocious puberty: Evidence of hCG secretion by the tumoral cells. Child’s Brain. 1984;11:298–303. doi: 10.1159/000120190. [DOI] [PubMed] [Google Scholar]
- 20.Calaminus G, Andreussi L, Garre ML, et al. Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT) Klin Padiatr. 1997;209:222–227. doi: 10.1055/s-2008-1043954. [DOI] [PubMed] [Google Scholar]
- 21.Kubo O, Yamasaki N, Kamijo Y, et al. Human chorionic gonadotropin produced by ectopic pinealoma in a girl with precocious puberty. Case report. J Neurosurg. 1977;47:101–105. doi: 10.3171/jns.1977.47.1.0101. [DOI] [PubMed] [Google Scholar]
- 22.O’Marcaigh AS, Ledger GA, Roche PC, et al. Aromatase expression in human germinomas with possible biological effects. J Clin Endocrinol Metab. 1995;80:3763–3766. doi: 10.1210/jcem.80.12.8530631. [DOI] [PubMed] [Google Scholar]
- 23.Raafat F, Klys H, Rylance G. Juvenile granulosa cell tumor. Pediatr Pathol. 1990;10:617–623. doi: 10.3109/15513819009067150. [DOI] [PubMed] [Google Scholar]
- 24.Cecchetto G, Alaggio R, Bisogno G, et al. Sex cord-stromal tumors of the testis in children. A clinicopathologic report from the Italian TREP project. J Pediatr Surg. 2010;45:1868–1873. doi: 10.1016/j.jpedsurg.2010.02.120. [DOI] [PubMed] [Google Scholar]
- 25.Cecchetto G, Ferrari A, Bernini G, et al. Sex cord stromal tumors of the ovary in children: A clinicopathological report from the Italian TREP project. Pediatr Blood Cancer. 2011;56:1062–1067. doi: 10.1002/pbc.22918. [DOI] [PubMed] [Google Scholar]
- 26.Kukuvitis A, Matte C, Polychronakos C. Central precocious puberty following feminizing right ovarian granulosa cell tumor. Horm Res. 1995;44:268–270. doi: 10.1159/000184639. [DOI] [PubMed] [Google Scholar]
- 27.Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 28.Stephen MD, Zage PE, Waguespack SG. Gonadotropin-dependent precocious puberty: Neoplastic causes and endocrine considerations. Int J Pediatr Endocrinol. 2011;2011:184502. doi: 10.1155/2011/184502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viano JC, Herrera EJ, Suarez JC. Cerebellar astrocytomas: A 24-year experience. Childs Nerv Syst. 2001;17:607–610. doi: 10.1007/s003810100479. discussion 611. [DOI] [PubMed] [Google Scholar]
- 30.Bajpai A, Sharma J, Kabra M, et al. Precocious puberty: Clinical and endocrine profile and factors indicating neurogenic precocity in Indian children. J Pediatr Endocrinol Metab. 2002;15:1173–1181. doi: 10.1515/jpem.2002.15.8.1173. [DOI] [PubMed] [Google Scholar]