Yudkin et al.’ fetuses-at-risk (FAR) approach1 for estimating gestational age-specific stillbirth rates languished for years before being widely accepted. More recently, this formulation has been extended to pregnancy-related postnatal events, with proponents claiming that the incidence of birth, growth restriction and perinatal death are key phenomena in perinatology.2 However, epidemiologists have been reluctant to debate and develop these concepts, leaving clinicians to pursue applications without adequate epidemiologic input.3 I was therefore pleasantly surprised when Professor Cande Ananth invited commentaries on a manuscript criticising the extended FAR model. Hopefully, such discussion will help the perinatal community to accept or reject the extended FAR approach with greater immediacy than was shown to Yudkin et al.'s original proposition.
The critique
Dr. Olga Basso argues that the extended FAR formulation is compromised because rates of postnatal events are influenced by the frequency of livebirth.4 The approach is appropriate for calculating rates of antepartum stillbirth (which involve a single event), but fails when applied to postnatal phenomena (which involve two events, namely, birth and a postnatal event). A 100% risk of death after birth due to a factor that does not influence gestational duration leads to a low rate of death at preterm gestation, and to a high rate at term when birth frequency is high. The example of renal agenesis is offered as a case in point.4
Logical vs. biological and epidemiological inference
Although I was intuitively aware that the incidence of birth influences the incidence of pregnancy-related postnatal outcomes, I found Dr. Basso's paper helpful as it formalises and clarifies this relationship. On the other hand, Dr. Basso's critique is theory-laden, with the traditional perinatal perspective colouring her evaluation of the extended FAR approach. More importantly, various aspects of her outlook, although logical, are less than compelling from a biological and epidemiological standpoint.
Incidence patterns of pregnancy-related postnatal events
Despite being influenced by birth rates, pregnancy-related postnatal phenomena exhibit diverse incidence trajectories.5 Rates of retinopathy peak at 25–26 weeks and then fall even as birth rates increase (Figure 1a). On the other hand, rates of meconium aspiration syndrome rise sharply after 39 weeks gestation. The incidence of respiratory distress syndrome shows an intriguing bimodal pattern, which first peaks at 34–36 weeks, declines until 39 weeks, and then rises to a second peak at post-term gestation. This bimodal distribution, and the heterogeneity of early and late gestation respiratory distress syndrome in terms of risk factors and morbidity correlates, suggests that the syndrome includes at least two distinct disease entities.6
Figure 1.
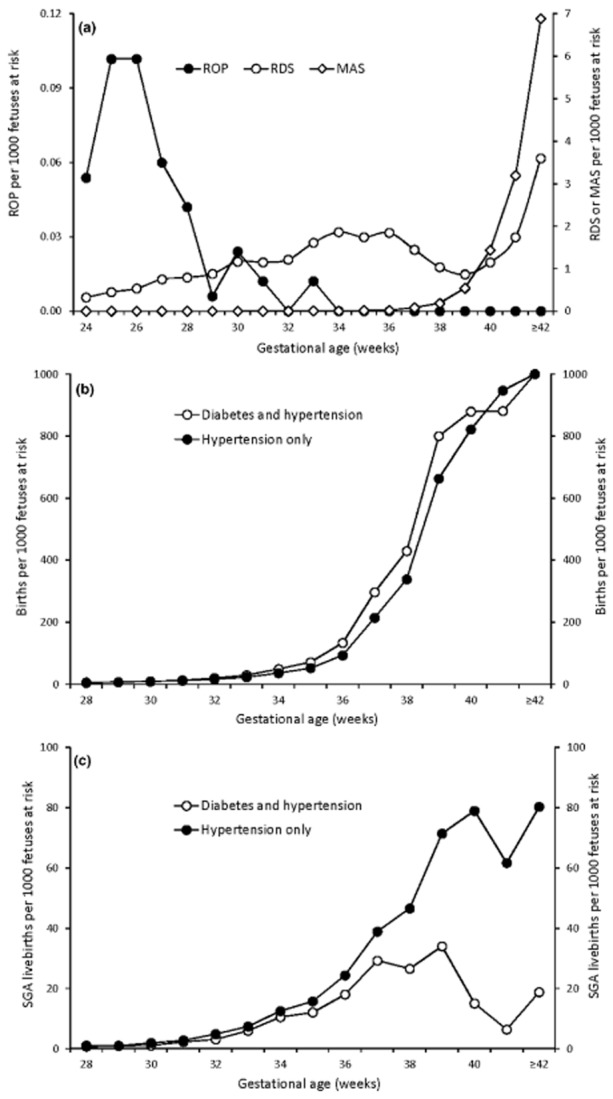
Gestational age-specific rates of pregnancy-related postnatal phenomena showing diverse incidence trajectories. Gestational age-specific incidence rates of retinopathy of prematurity (ROP), meconium aspiration syndrome (MAS) and respiratory distress syndrome (RDS), Nova Scotia 1988–20075 (a), and gestational age-specific incidence rates of livebirth (b) and small-for-gestational age (SGA) livebirth (c) among women with hypertension and diabetes mellitus, and among women with hypertension only, United States, 2011–13.
Another example of a pregnancy-related postnatal event whose pattern is not overwhelmed by birth rates is seen in contrasts of women with hypertension and diabetes vs. women with hypertension alone. Although the incidence of birth is higher among women with both complications (Figure 1b), incidence rates of small-for-gestational age (SGA) livebirth are higher among women with hypertension alone (Figure 1c).
Logical vs. biological reasoning
Bilateral renal agenesis is a rare condition that leads to severe oligohydramnios and anhydramnios.7 The assumption that this anomaly has no effect on gestational duration and the assertion that the incidence pattern of neonatal death from renal agenesis follows the incidence pattern of birth are both incorrect7 (Figure 2a).
Figure 2.
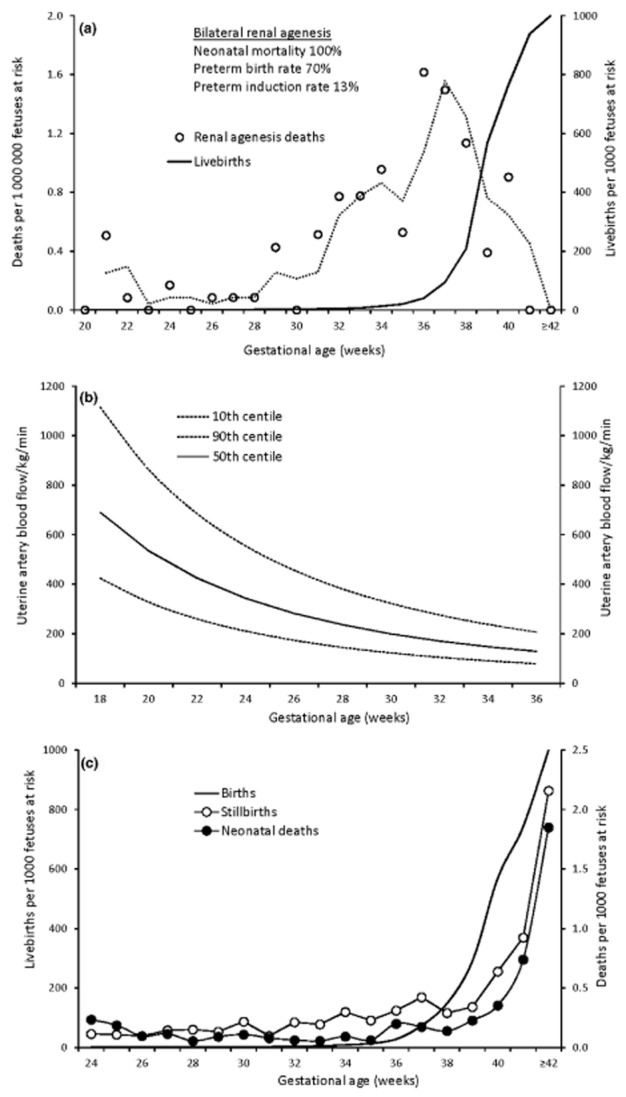
Miscellaneous gestational age-specific incidence patterns illustrating the natural history of pregnancy. Gestational age-specific rates of neonatal death from bilateral renal agenesis (dotted line shows the moving average) and gestational age-specific livebirth rates, United States, 2011–13 (a), uterine artery blood flow per kilogram fetal weight per minute by gestational age8 (mL/kg/min) (b), and gestational age-specific rates of birth, stillbirth and neonatal death, Nova Scotia, 1988–20075 (c).
Dr. Basso's critique also fails to distinguish between pregnancy-related postnatal events (e.g. cerebral palsy) and postnatal events that are not pregnancy-related (e.g. childhood diabetes). Pregnancy-related postnatal events, i.e. postnatal events that have their origins in pregnancy, justify the latent period invoked in extended FAR models, whereas postnatal events that are not pregnancy-related (e.g. neonatal tetanus, tonsillitis, impetigo) do not require the extended FAR approach.
Such a logical-biological dichotomy is also evident in the claim that the FAR approach is appropriate for antepartum stillbirth but not neonatal death. The argument that antepartum stillbirth represents one event, while neonatal death represents two events, is belied by the fact that stillbirth in fact represents two events (i.e. fetal death and subsequent birth) that are typically separated by days or weeks.9
Logical vs.epidemiological reasoning
Epidemiologic principles are the epistemological tools required for understanding causality in medicine, with unconfounded associations based on incidence rates serving as the foundation for causal inference.10 The extended FAR model is a causal model insofar as it treats gestational age as survival time and permits the estimation of incidence rates across the fetal–infant continuum. In contrast, the traditional perinatal model treats gestational age as a determinant and segregates in utero and postnatal phenomena. This model works well for prognostic purposes but fails as a causal model on several counts.2 Perhaps the most subtle failure of the traditional model is the assumption that neonatal mortality rates represent a cumulative incidence estimated over 28 days of follow up. Because a majority of neonatal deaths occurs in the first hour or day after birth, and because most such infants are critically ill at birth, the traditional neonatal mortality rate is better viewed as a point prevalence similar to the stillbirth rate. Thus stillbirth rates express the proportion of fetuses who are dead at birth, whereas conceptually, neonatal death rates express the proportion of infants who are dying at birth. The paradoxical crossover seen in traditional gestational age-specific contrasts of stillbirth and neonatal death rates by maternal smoking, race, plurality, etc.,2,5 and the parallel incidence patterns of stillbirth and neonatal death seen under the FAR formulation2,5 are testament to the similarity of these two seemingly different rate constructs.
The argument that fetuses cannot constitute the denominator for pregnancy-related postnatal events because only livebirths experience postnatal events is logically seductive but specious. It is entirely reasonable for a woman with severe pre-eclampsia to enquire about her fetus's potential risk of stillbirth, neonatal death or cerebral palsy. Cancer epidemiologists have long estimated age-specific rates of breast cancer mortality without any restriction that such rates be confined to women with breast cancer.
The influence of birth rates on pregnancy-related postnatal events notwithstanding, the antecedents and other epidemiologic characteristics of such outcomes can be assessed by studying relative patterns (e.g. incidence rates of meconium aspiration syndrome by smoking or socioeconomic status). Quantification of the effect of any determinant on a pregnancy-related postnatal outcome will require assessments of direct effects and effects mediated by differential birth rates.
The extended FAR model
New insights, explanatory power and a consilience of inductions make the extended FAR approach a coherent and appealing theory.
Support from biomedical studies
Animal and human studies show that the ability of the uteroplacental system to support the pregnancy declines in late gestation as progressive reductions in fetal weight-normalised uterine and umbilical artery blood flow result in decreased oxygen availability8,11 (Figure 2b). Sheep studies12 show that these vascular and metabolic changes are accompanied by a decrease in fetal movements and increasing periods of quiescence in late gestation (‘the quiescence of the lambs’). The decline in uteroplacental function is likely responsible for initiating birth and also for the increased incidence of pregnancy complications, growth restriction, fetal death and neonatal mortality/morbidity in late gestation.2,5 The increasing incidence of stillbirth and neonatal death with advancing gestation (Figure 2c) is a feature of the natural history of pregnancy and a manifestation of processes that also increase other diseases of late gestation (such as respiratory depression at birth, seizures and other features of neonatal encephalopathy).2
Relationship between labour induction and caesarean delivery
Although clinical observation suggests that labour induction leads to an increase in caesarean delivery rates, randomised trials13 show that labour induction in fact reduces caesarean delivery rates. Trial findings are consistent with the extended FAR model, which shows that caesarean delivery rates increase with increasing gestation, implying that early delivery through labour induction will lead to a reduction in caesarean delivery.14 The fallacious clinical conclusion occurs because clinicians mentally compare caesarean rates among women undergoing labour induction with caesarean rates among women in spontaneous labour at the same gestation (instead of caesarean rates among women not in labour who are expectantly managed at that gestation).15
Temporal reductions in SGA livebirth
The rising pattern of SGA livebirth with advancing gestation observed under the extended FAR model implies that increases in iatrogenic early delivery will result in declines in overall SGA rates. This proposition is supported by evidence from the Disproportionate Intrauterine Growth Intervention Trial At Term (DIGITAT) trial, which showed that labour induction results in earlier delivery and in a two- to threefold lower rate of SGA compared with expectant management.16 Kitagawa decomposition analyses based on the extended FAR formulation also show that recent temporal reductions in SGA rates were primarily due to increases in iatrogenic early delivery.17
Patterns of gestational age-specific SGA and perinatal mortality
Under the traditional perinatal model, rates of SGA are assumed to be constant across gestation, and this is at odds with the simultaneous exponential decline in perinatal mortality. However, rising patterns of SGA livebirth are congruent with rising rates of perinatal death observed with increasing gestation under the extended FAR model.5
Non-proportional hazards
The incidence of SGA livebirth and perinatal death increases towards term gestation, and contrasts by maternal smoking status and other determinants show that hazard ratios also increase at late gestation.2,18 The increase in rates of SGA and perinatal death towards term is consistent with the observed decline in uteroplacental function, while increasing hazard ratios suggest synergistic modification of the effect of declining uteroplacental function on adverse perinatal outcomes by harmful influences such as maternal smoking.
Framework for obstetric theory
Recent increases in medically indicated iatrogenic early delivery and consequent reductions in perinatal mortality are inconsistent with the traditional perinatal model in which perinatal mortality declines exponentially with advancing gestation. However, the association between iatrogenic early delivery and perinatal mortality is consistent with the extended FAR formulation, which shows that perinatal mortality rates increase with increasing gestation.14
Intersecting perinatal mortality curves
The extended FAR model's resolution of the paradox of intersecting perinatal mortality curves is compelling because it is parsimonious and because a single explanation resolves both the stillbirth and neonatal mortality crossover across diverse contrasts.19
Heterogeneity of respiratory distress syndrome
As with the bimodal pattern of Hodgkin's disease (which represents two different aetiologic processes20), the bimodal pattern of respiratory distress syndrome seen under the extended FAR formulation highlights the heterogeneity of respiratory distress syndrome.6
A note on competing risks
The paradigm for addressing the competing risks of severe neonatal morbidity and neonatal mortality in randomised trials involves the use of a composite outcome. Thus trials examining the effect of an intervention on bronchopulmonary dysplasia among very preterm infants resolve the competing risk problem by using composite bronchopulmonary dysplasia or death as the outcome. This avoids competing risk issues and also addresses validity (by permitting an intent-to-treat analysis) and clinical/social concerns (by addressing both relevant outcomes). Use of a composite outcome within the extended FAR framework also resolves other competing risk problems such as those between spontaneous and iatrogenic preterm birth, and between stillbirth and neonatal death.
A unified and parsimonious theory of perinatology
The extended FAR approach expands Yudkin et al.'s1 perspective and permits the modelling of both antenatal and postnatal phenomena that have their origins in pregnancy. This formulation therefore represents a unified and parsimonious epidemiologic model for relevant events and states from conception to the postnatal period.
Conclusion
Dr. Basso's paper is a long overdue expression of the doubts surrounding the extended FAR model. Her honest and courageous criticism provides insight into the model but does little to discredit the approach. More generally, the extended FAR formulation presents an uncomfortable challenge to the perinatal community because it provides a perspective very much at odds with the traditional paradigm, while simultaneously resolving many conundrums and paradoxes, and providing a coherent theoretical framework for justifying obstetric intervention. The speed and scientific rigour with which the perinatal community evaluates these fundamental issues will reveal the extent to which the science of perinatology is articulate and mature.
Acknowledgments
Dr. Joseph's work is supported by the Child and Family Research Institute and by a Chair award from the Canadian Institutes of Health Research (APR-126338).
References
- Yudkin PL, Wood L, Redman CWG. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1:1192–1194. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- Joseph KS. Incidence-based measures of birth, growth restriction and death can free perinatal epidemiology from erroneous concepts of risk. Journal of Clinical Epidemiology. 2004;57:889–897. doi: 10.1016/j.jclinepi.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Page JM, Pilliod RA, Snowden JM, Caughey AB. The risk of stillbirth and infant death by each additional week of expectant management in twin pregnancies. American Journal of Obstetrics and Gynecology. 2015;212:630.e1–630.e7. doi: 10.1016/j.ajog.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Basso O. Implications of using a fetuses-at-risk approach when fetuses are not at risk. Paediatric and Perinatal Epidemiology. 2016;30:3–10. doi: 10.1111/ppe.12254. [DOI] [PubMed] [Google Scholar]
- Joseph KS. The natural history of pregnancy: diseases of early and late gestation. BJOG: An International Journal of Obstetrics and Gynaecology. 2011;118:1617–1629. doi: 10.1111/j.1471-0528.2011.03128.x. [DOI] [PubMed] [Google Scholar]
- Mehrabadi A, Lisonkova S, Joseph KS. 2015. Heterogeneity of respiratory distress syndrome: risk factors and morbidity associated with early and late gestation disease. Annual Meeting of the Fetal and Neonatal Physiology Society. Vancouver, Canada. August. Abstract O35.
- Spiro JE, Konrad M, Rieger-Fackeldey E, Masjosthusmann K, Amler S, Klockenbusch W, et al. Renal oligo- and anhydramnios: cause, course and outcome – a single-center study. Archives of Gynecology and Obstetrics. 2015;292:327–336. doi: 10.1007/s00404-015-3648-7. [DOI] [PubMed] [Google Scholar]
- Rigano S, Ferrazzi E, Boito S, Pennati G, Padoan A, Galan H. Blood flow volume of uterine arteries in human pregnancies determined using 3D and bi-dimensional imaging, angio-Doppler, and fluid-dynamic modeling. Placenta. 2010;31:37–43. doi: 10.1016/j.placenta.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Joseph KS, Kinniburgh B, Hutcheon JA, Mehrabadi A, Dahlgren L, Basso M, et al. Rationalizing definitions and procedures for optimizing clinical care and public health in fetal death and stillbirth. Obstetrics & Gynecology. 2015;125:784–788. doi: 10.1097/AOG.0000000000000717. [DOI] [PubMed] [Google Scholar]
- Miettinen OS. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. Toronto: John Wiley & Sons; 1985. [Google Scholar]
- Rurak D, Bessette NW. Changes in fetal lamb arterial blood gas and acid-base status with advancing gestation. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 2013;304:R908–R916. doi: 10.1152/ajpregu.00430.2012. [DOI] [PubMed] [Google Scholar]
- Rurak D, Wittman B. Real-time ultrasound assessment of body and breathing movements and abdominal diameter in fetal lambs from 55 days of gestation to term. Reproductive Sciences. 2013;20:414–425. doi: 10.1177/1933719112459229. [DOI] [PubMed] [Google Scholar]
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews. 2012;(6) doi: 10.1002/14651858.CD004945.pub3. CD004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph KS. Theory of obstetrics: an epidemiologic framework for justifying medically indicated early delivery. BioMed Central Pregnancy and Childbirth. 2007;7:4. doi: 10.1186/1471-2393-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE, Caughey AB. Induction of labor and cesarean: what is the true relationship? Clinical Obstetrics and Gynecology. 2015;58:269–281. doi: 10.1097/GRF.0000000000000112. [DOI] [PubMed] [Google Scholar]
- Boers KE, Vijgen SM, Bijlenga D, van der Post JA, Bekedam DJ, Kwee A, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT) British Medical Journal. 2010;341:c7087. doi: 10.1136/bmj.c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A, Lisonkova S, Joseph KS. The association between temporal changes in the use of obstetrical interventions and small- for-gestational age births. BioMed Central Pregnancy and Childbirth. 2015;15:233. doi: 10.1186/s12884-015-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RW, Joseph KS, Ananth CV, Grondines J, Abrahamowicz M, Kramer MS. A proportional hazards model with time-dependent covariates and time-varying effects for analysis of fetal and infant death. American Journal of Epidemiology. 2004;160:199–206. doi: 10.1093/aje/kwh201. [DOI] [PubMed] [Google Scholar]
- Joseph KS, Liu S, Demissie K, Wen SW, Platt RW, Ananth CV, et al. A parsimonious explanation for intersecting perinatal mortality curves: understanding the effect of plurality and of parity. BioMed Central Pregnancy and Childbirth. 2003;3:3. doi: 10.1186/1471-2393-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. Joural of Internal Medicine. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]


