IRA B cells: origin and functions
Keywords: B1a cells, GM-CSF, IgM, IL-3, innate immunity, IRA B cells
Abstract
Innate response activator (IRA) B cells are a subset of B-1a derived B cells that produce the growth factors granulocyte macrophage colony stimulating factor and IL-3. In mouse models of sepsis and pneumonia, B-1a B cells residing in serosal sites recognize bacteria, migrate to the spleen or lung, and differentiate to IRA B cells that then contribute to the host response by amplifying inflammation and producing polyreactive IgM. In atherosclerosis, IRA B cells accumulate in the spleen, where they promote extramedullary hematopoiesis and activate classical dendritic cells. In this review, we focus on the ontogeny and function of IRA B cells in acute and chronic inflammation.
Introduction
When Cooper, Peterson, and Moore reported the discovery of B cells 50 years ago (1, 2), they not only settled a polarizing and heated debate on the nature of the immune system but also ushered in a new investigative era. Identifying an antibody-producing lymphocyte, distinct from T cells, that develops in a unique environment (chicken Bursa of Fabricius; human bone marrow), was the scaffold upon which the current knowledge of humoral and cellular immunity, as we know it today, was built. Over the years, landmark observations, ranging from deciphering B-cell ontogeny (3, 4) and organization within germinal centers of secondary lymphoid organs (5, 6) to characterizing immunoglobulin structure (7, 8), V(D)J recombination (9–11), and affinity maturation (12–14), have concretely revealed the importance of B lymphocytes within the ever-expanding immune cell family.
Over time, we have come to appreciate that B-cell heterogeneity is a function of both vertically integrated ontogenic hierarchy (pro-B cells give rise to T1 B cells, then to T2 B cells, etc.) and environmentally-elicited horizontal diversification (memory cells, plasmablasts, and plasma cells) (15). At the population level, arguably the most intriguing discovery was the Herzenberg laboratory’s identification of a seemingly separate B-cell lineage, enriched in serosal spaces and dedicated to the production of natural antibodies (16). Years before we knew how Toll-like receptors (TLRs) bridge the gap between innate and adaptive immunity, and decades before we appreciated that natural killer cells belong to a large innate lymphoid cell (ILC) family, here were B cells that despite definitively being ‘adaptive immune’ cells were apparently not connected to adaptive immunity. We refer to these cells as B-1 B cells. As opposed to the more familiar B-2 B cells that circulate and settle in B-cell follicles, B-1 B cells’ spatiotemporal characteristics remain somewhat obscure. In this review, we will focus on innate response activator (IRA) B cells, a recently recognized member of the B-1 B-cell family.
IRA B cells’ phenotype and ontogeny
What are they?
B cells may be exclusive antibody producers, but antibodies are not the only product of B cells. Over the last 15 years, a number of laboratories demonstrated that B cells can produce interleukin 10 (IL-10), a cytokine typically considered a T-cell product and most famous for regulating (or suppressing) inflammation (17–20). IL-10-producing B cells, termed B10 cells, are phenotypically heterogeneous and broadly present in health and disease (see review by Tedder et al. in this issue). B cells have also been shown to generate many other products in vitro, in vivo, or both. In addition to IL-10, B cells can produce IL-6 (21), Mcp-1/Ccl2 (22, 23), Ccl3 (23, 24), Ccl4 (23), Ccl5/RANTES (23), Ccl7 (25), Ccl11 (23) and Ccl22 (26).
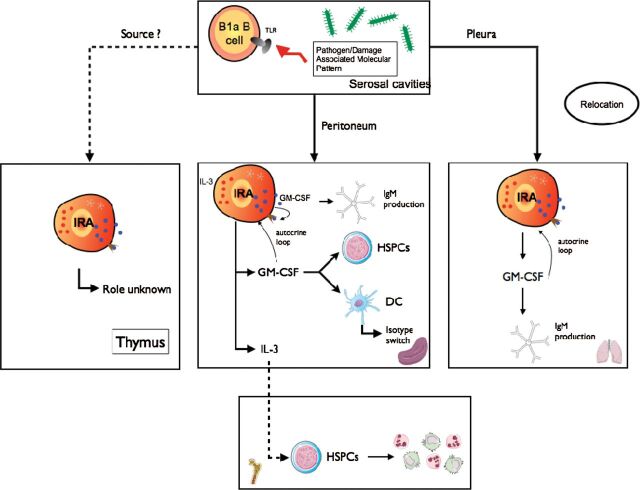
In exploring mechanisms that drive extramedullary hematopoiesis, which is the leukocyte production in locations outside the bone marrow, Rauch et al. (27) tested for the presence of granulocyte macrophage colony stimulating factor (GM-CSF) in the spleen after repeated LPS delivery to the peritoneum. GM-CSF was first observed in the 1960s, at the Walter and Eliza Hall Institute, as a product of kidney feeder cells that stimulated production of myelocytes and granulocytes, but not erythrocytes (28). The factor was named and cloned, and the mouse knockout was eventually generated in 1994 by two independent groups (29–31). The knockout was likely a bit of a disappointment: aside from progressive lung proteinosis, which was later shown to result from impaired surfactant clearance by alveolar macrophages, the mice were otherwise healthy, with a seemingly normal leukocyte diversity. In other words, GM-CSF was not as important to steady-state hematopoiesis as M-CSF, the closely related growth factor essential to monocytes and their descendants (32). Nevertheless, over the years many investigators have uncovered a variety of important functions by which GM-CSF influences the host response; in aggregate, GM-CSF functions are related to myeloid leukocyte activation, differentiation and proliferation (33). These myriad myeloid leukocyte functions impelled Rauch et al. (27) to determine the source of GM-CSF in the spleen. Using intracellular antibody staining and flow cytometry, the investigators detected a distinct population staining positive for GM-CSF comprising 1–4% of the B-cell population in the spleen 4 days after LPS administration. On their surfaces, these cells expressed CD19, B220, IgM, MHCII, CD5, CD43, CD93, CD138, VLA4, CD284 at relatively high levels. The cells were dimly positive for IgD, CD23, CD21 but negative for CD11b, CD3, Ly-6G, Ly-6C, NK1.1, CD49b, Ter119, CD4, CD8, CD11c. They were, in short, B cells, identifiable by immunofluorescence and flow cytometry and capable of producing abundant quantities of IgM. Because they produced a growth factor known to activate innate leukocytes, they were named IRA B cells (Fig. 1).
Fig. 1.
The origin and function of IRA B cells. B1a B cells, following LPS–TLR4 interaction, relocate from the peritoneum or the pleural space to spleen or lung and develop into IRA B cells. IRA B cells produce GM-CSF that enhances IgM secretion, via an autocrine loop, activate DCs and boost proliferation of HSPCs. IRA B cells also produce IL-3 which promotes neutrophil and monocyte production.
How do they arise?
To investigate IRA B-cell origins, Rauch et al. considered the clues: IRA B cells appeared in the splenic red pulp after LPS injection and expressed markers that closely resembled B-1 B cells, immature cells or marginal zone B cells. After performing adoptive fate mapping and parabiosis experiments; using mice lacking B cells, TLR4, Myd88, TIR-domain-containing adapter-inducing interferon-β (TRIF) or BAFF receptor (B-cell activating factor receptor); and blocking VLA4, the researchers concluded that IRA B cells derive from peritoneal B-1a B cells that relocate from the peritoneum to the spleen after recognizing LPS (the typical pathogen-associated molecular pattern) with TLR4, which signaled toward Myd88 but not TRIF. In the absence of B cells or BAFF receptor, IRA B cells did not arise; after blocking VLA4, they failed to colonize the spleen. Transcriptome analysis of IRA B cells and comparison to other B cell subsets revealed that IRA B cells are unique, though most closely aligned with plasma cells. The similarity to plasma cells was not particularly surprising given IRA B cells’ high expression of CD138, Xbp1, and IgM. Situating IRA B cells within the B-1 family provided valuable context: B-1 cells are enriched in serosal sites, can self-renew, and appear early during embryonic life (8.5 days in mice) (34) but rely on the spleen for renewal and seeding in body cavities (35, 36). B-1 cells also produce IgM antibodies but neither settle in germinal centers nor participate in somatic hypermutation—they are innate-like. As products of B-1 cells, IRA B cells can therefore be conceptualized both as innate responders (B cells belonging to the innate B-cell family) and response activators (producers of a factor known to activate innate myeloid cells).
IRA B cells’ function
Are they important?
Identifying a GM-CSF-producing B cell that appears after LPS may be interesting per se, but evaluating that cell’s importance to the host response is far more significant. Rauch et al. tested IRA B cells’ function by generating mixed chimeric mice whose B cells lacked the capacity to produce GM-CSF and subjecting the animals to a model of polymicrobial sepsis induced by cecal ligation and puncture (CLP), a life-threatening condition. Sometimes referred to as ‘blood poisoning’, sepsis claims up to half a million lives in USA every year (37, 38). Its pathophysiology has confounded scientists and physicians, and several recent failed clinical trials have only underlined how incompletely we understand the condition (39). Rauch et al. discovered that mice with a B-cell-restricted GM-CSF deficiency died earlier and in larger numbers than controls, suggesting that IRA B cells are protective in sepsis. Specifically, in the absence of B-cell-derived GM-CSF, the animals developed pronounced inflammation, a cytokine storm, and more severe bacteremia, which led to septic shock, multi-organ failure and death. IRA B-cell-derived GM-CSF somehow staved off infection and curbed inflammation.
Following the identification of IRA B cells’ effect on sepsis, Weber et al. (40) wished to elucidate how B-cell-derived GM-CSF might be protective. Noting that IRA B cells produce IgM and express the β common chain of the GM-CSF receptor (CD131) that is a part of the GM-CSF, IL-3 and IL-5 receptors, the investigators speculated that B-cell-derived GM-CSF controls IgM in an autocrine loop. Indeed, unlike GM-CSF-deficient and CD131-deficient B-1a B cells, wild-type B-1a B cells stimulated in vitro with LPS produced IgM and GM-CSF. Moreover, the addition of GM-CSF partially restored IgM production in GM-CSF-deficient cells. The data suggested that GM-CSF production by IRA B cells protects the host by generating polyreactive IgM that innately recognizes bacterial components and marks them for phagocyte-mediated elimination; without IgM, bacteria have more leeway to infect, proliferate, breach barriers and wreak inflammatory havoc.
If IRA B cells’ GM-CSF crucially protects against polymicrobial sepsis or pneumonia, then strategies that increase the number of these cells, prevent IRA B-cell loss, amplify GM-CSF secretion or boost the IgM response could represent novel axis of treatment.
Where else do they arise?
The observation that IRA B cells differentiate from B-1a B cells in the peritoneum, which is a serosal location, led to the hypothesis that IRA B cells might also develop in other serosal sites, such as the pleural space (41, 42). Using a model of airway infection, Weber et al. (40) showed that B-1a B cells residing in the pleural space mobilize to the lung where they produce IgM via autocrine GM-CSF signaling. Although the study did not prove that pleural B-1a B cells migrate directly across the mesothelium into the lung, cell tracking studies using intrapleural GFP+ B-cell transfer revealed cell accumulation in the pleural space and lungs, but not blood, a result that supports a direct route independent of blood vessels. The study concluded that a protective leukocyte population resides outside the lungs and rapidly mobilizes after lung infection. It is unknown whether IRA B cells can mobilize from other locations, such as the pericardial space.
Are IRA B cells exclusively involved in infection?
While exploring IRA B cellular function in contexts other than infection, Hilgendorf et al. (43) observed that IRA B cells accumulate in secondary lymphoid organs of mice with atherosclerosis, the chronic lipid-driven inflammatory disease characterized by the gradual accrual of lipoproteins and leukocytes in the vessel wall (44–46). As the underlying condition behind myocardial infarction and stroke, atherosclerosis remains by far the most lethal disease worldwide, despite the success of statins, ACE inhibitors and other drugs (47). It is unclear what triggers the appearance of IRA B cells in atherosclerosis—Hilgendorf et al. observed IRA B cells in atherosclerotic humans as well as Ldlr –/– and Apoe –/– mice (used as a model of atherosclerosis)—although it is likely that either scavenger receptors or the B-cell receptor (BCR) on B-1a B cells recognize oxidation-specific epitopes (48, 49) in ways similar to how bacterial components such as LPS trigger TLR4-mediated IRA B-cell differentiation. Once settled in the spleen, IRA B cells may promote extramedullary hematopoiesis (50), the process by which the bone marrow outsources leukocyte production to the spleen (51). Wang et al. reconstituted lethally irradiated mice lacking the LDL receptor (Ldlr –/– ) with ApoE knock-out (Apoe –/– ) or Apoe –/– CD131–/– bone marrow, and observed that mice lacking CD131 had reduced myelopoiesis and reduced proliferation of hematopoietic stem and progenitor cells (HSPCs) in the spleen. We have previously shown that the spleen is monocyte reservoir and a major site of extramedullary hematopoiesis during chronic inflammation (51, 52). Accordingly, Apoe –/– CD131–/– mice had reduced numbers of neutrophils and monocytes in the blood compared with Apoe –/– mice because of reduced medullary and extramedullary hematopoiesis, resulting in fewer cells that infiltrated the plaque.
In early atherosclerosis, when extramedullary hematopoiesis is not yet dominant, IRA B cells generate and activate splenic classical dendritic cells (DCs), which then produce IL-12, thus favoring an atherosclerosis-aggravating IFNγ-dominant TH1 environment. Consequently, in the absence of GM-CSF-producing B cells, TH1-type immunity is diminished, antibodies recognizing oxidation-specific epitopes harbor TH2-associated Fc regions, and atherosclerotic lesions are smaller (43).
IRA B cells may therefore be targeted to treat atherosclerosis at an early phase to diminish TH1 type immunity and both at the early and the later phases to decrease myeloid cell production in the bone marrow and spleen.
What else do IRA B cells produce?
The gene encoding GM-CSF, located on chromosome 11 in the mouse and chromosome 5 in the human, is adjacent to the gene encoding IL-3, a cytokine identified in mice in 1981 (53, 54) and in humans in 1986 (55). Because IL-3 is important to leukocyte production, proliferation, and survival (56), and because of its proximity on the genome to GM-CSF, Weber Chousterman, He et al. (57) asked whether IRA B cells likewise produce IL-3. The answer was yes: IRA B cells are major sources of IL-3 in humans and mice with sepsis. To determine whether IL-3 is important in the host response to bacterial infection, Weber, Chousterman, He et al. subjected Il3 –/– mice to CLP. Compared with wild-type mice, Il3 –/– mice were protected from sepsis, and the differences between the groups remained significant even when they were given antibiotics. Mechanistically, IL-3 promoted the production of inflammatory monocytes and neutrophils, which are the cell sources of the cytokine storm associated with severe sepsis and septic shock that causes organ damage and death. Thus, IRA B cells and their IL-3 product are upstream sentinels and amplifiers of acute inflammation. Significantly, a clinical trial on nearly 100 septic patients revealed that high IL-3 levels in plasma associated with high mortality even after investigators adjusted for various prognostic indicators. The data collectively show that IRA B cells can be both protective and detrimental in sepsis depending on the cytokine they produce. Although GM-CSF protects against sepsis by producing neutralizing antibodies with broad specificities, IL-3 can dangerously over-produce inflammatory cells. Thus, diminishing IL-3 production while conserving GM-CSF synthesis may be essential when dealing with treatments focused on the positive and detrimental contributions of IL-3 on the pathogenesis of this disease.
Conclusions and ongoing questions
As producers of potent growth factors, IRA B cells significantly contribute to immunity and inflammation. Moving forward, many questions remain. Why are B cells the major producers of GM-CSF and IL-3 in bacterial infection? For example, in the steady state, intestinal type 3 ILCs (58) and lung epithelial cells (59) produce GM-CSF. Clearly neither GM-CSF nor IL-3 are exclusive to B cells, yet, under some conditions, B cells become these growth factors’ major sources. Could the BCR play a role in driving growth factor transcription, perhaps by integrating signals with those downstream of pattern recognition receptors? Moreover, what is the ultimate fate of IRA B cells? Are they nothing more than short-lived plasmablasts, or do they have functions beyond those first several days, perhaps related to memory or trained immunity? Can they eventually class-switch? Do they produce other growth factors? Do they have a role in autoimmune disease such as arthritis or multiple sclerosis? These and other questions will be critical over the next several years for anyone interested in deciphering the scope of this B-1 subset.
Funding
This work was supported by grants R01HL095612, R56AI104695, and Howard M. Goodman Fellowship (to F.K.S.); Société Française d’Anesthésie et de Réanimation (SFAR), and Philippe Foundation (to B.G.C.).
Acknowledgements
The authors declare they have no conflict of interest.
References
- 1. Cooper M. D., Peterson R. D., Good R. A. 1965. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature 205:143. [DOI] [PubMed] [Google Scholar]
- 2. Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. 1966. The functions of the thymus system and the bursa system in the chicken. J. Exp. Med. 123:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy R. R., Kincade P. W., Dorshkind K. 2007. The protean nature of cells in the B lymphocyte lineage. Immunity. 26:703. [DOI] [PubMed] [Google Scholar]
- 4. LeBien T. W. 2000. Fates of human B-cell precursors. Blood 96:9. [PubMed] [Google Scholar]
- 5. Jacob J., Kelsoe G., Rajewsky K., Weiss U. 1991. Intraclonal generation of antibody mutants in germinal centres. Nature 354:389. [DOI] [PubMed] [Google Scholar]
- 6. Kelsoe G. 1996. Life and death in germinal centers (redux). Immunity 4:107. [DOI] [PubMed] [Google Scholar]
- 7. Edelman G. M., Gally J. A. 1964. A model for the 7S antibody molecule. Proc. Natl Acad. Sci. U. S. A. 51:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porter R. R. 1963. Chemical structure of gamma-globulin and antibodies. Br. Med. Bull. 19:197. [DOI] [PubMed] [Google Scholar]
- 9. Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. 1978. A complete immunoglobulin gene is created by somatic recombination. Cell 15:1. [DOI] [PubMed] [Google Scholar]
- 10. Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. 1990. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248:1517. [DOI] [PubMed] [Google Scholar]
- 11. Schatz D. G., Oettinger M. A., Baltimore D. 1989. The V(D)J recombination activating gene, RAG-1 Cell. 59:1035. [DOI] [PubMed] [Google Scholar]
- 12. Fidler J. M. 1979. The induction of hapten-specific immunological tolerance and immunity in B lymphocytes. VI. Differential tolerance susceptibility in adult spleen as a function of B-cell maturation level. J. Exp. Med. 150:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herzenberg L. A., Black S. J., Tokuhisa T., Herzenberg L. A. 1980. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J. Exp. Med. 151:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takemori T., Tada T. 1974. Selective roles of thymus-derived lymphocytes in the antibody response. II. Preferential suppression of high-affinity antibody-forming cells by carrier-primed suppressor T cells. J. Exp. Med. 140:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11:34. [DOI] [PubMed] [Google Scholar]
- 16. Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 157:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fillatreau S., Sweenie C. H., McGeachy M. J., Gray D., Anderton S. M. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944. [DOI] [PubMed] [Google Scholar]
- 18. Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R. S., Bhan A. K. 2002. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219. [DOI] [PubMed] [Google Scholar]
- 19. Mizoguchi E., Mizoguchi A., Preffer F. I., Bhan A. K. 2000. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int. Immunol. 12:597. [DOI] [PubMed] [Google Scholar]
- 20. Yanaba K., Bouaziz J. D., Haas K. M., Poe J. C., Fujimoto M., Tedder T. F. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28:639. [DOI] [PubMed] [Google Scholar]
- 21. Barr T. A., Shen P., Brown S., et al. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 209:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flaishon L., Becker-Herman S., Hart G., Levo Y., Kuziel W. A., Shachar I. 2004. Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration. Blood 104:933. [DOI] [PubMed] [Google Scholar]
- 23. Honczarenko M., Le Y., Glodek A. M., et al. 2002. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood 100:2321. [DOI] [PubMed] [Google Scholar]
- 24. Delogu A., Schebesta A., Sun Q., Aschenbrenner K., Perlot T., Busslinger M. 2006. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity 24:269. [DOI] [PubMed] [Google Scholar]
- 25. Zouggari Y., Ait-Oufella H., Bonnin P., et al. 2013. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaniel C., Pardali E., Sallusto F., et al. 1998. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J. Exp. Med. 188:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rauch P. J., Chudnovskiy A., Robbins C. S., et al. 2012. Innate response activator B cells protect against microbial sepsis. Science 335:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bradley T. R., Metcalf D. 1966. The growth of mouse bone marrow cells in vitro. Aust. J. Exp. Biol. Med. Sci. 44:287. [DOI] [PubMed] [Google Scholar]
- 29. Dranoff G., Crawford A. D., Sadelain M., et al. 1994. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264:713. [DOI] [PubMed] [Google Scholar]
- 30. Stanley E., Lieschke G. J., Grail D., et al. 1994. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl Acad. Sci. U. S. A. 91:5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dranoff G., Mulligan R. C. 1994. Activities of granulocyte-macrophage colony-stimulating factor revealed by gene transfer and gene knockout studies. Stem Cells. 12(Suppl. 1):173. [PubMed] [Google Scholar]
- 32. Hamilton J. A. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533. [DOI] [PubMed] [Google Scholar]
- 33. Hamilton J. A., Achuthan A. 2012. Colony stimulating factors and myeloid cell biology in health and disease. Trends. Immunol 34:81. [DOI] [PubMed] [Google Scholar]
- 34. Godin I. E., Garcia-Porrero J. A., Coutinho A., Dieterlen-Lièvre F., Marcos M. A. 1993. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 364:67. [DOI] [PubMed] [Google Scholar]
- 35. Wardemann H., Boehm T., Dear N., Carsetti R. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ansel K. M., Harris R. B., Cyster J. G. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 16:67. [DOI] [PubMed] [Google Scholar]
- 37. Angus D. C., van der Poll T. 2013. Severe sepsis and septic shock. N. Engl. J. Med. 369:2063. [DOI] [PubMed] [Google Scholar]
- 38. Martin G. S., Mannino D. M., Eaton S., Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546. [DOI] [PubMed] [Google Scholar]
- 39. Dolgin E. 2012. Trial failure prompts soul-searching for critical-care specialists. Nat. Med. 18:1000. [DOI] [PubMed] [Google Scholar]
- 40. Weber G. F., Chousterman B. G., Hilgendorf I., et al. 2014. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 211:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jantz M. A., Antony V. B. 2008. Pathophysiology of the pleura. Respiration 75:121. [DOI] [PubMed] [Google Scholar]
- 42. Teng R., Johkura K., Ogiwara N., et al. 2003. Morphological analysis of leucocyte transmigration in the pleural cavity. J. Anat. 203:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hilgendorf I., Theurl I., Gerhardt L. M., et al. 2014. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129:1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Libby P., Ridker P. M., Hansson G. K. 2009. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54:2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weber C., Noels H. 2011. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17:1410. [DOI] [PubMed] [Google Scholar]
- 46. Swirski F. K., Nahrendorf M. 2013. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bloom D. E., Cafiero E. T., Jane-Llopis E., et al. 2012. The Global Economic Burden of Noncommunicable Diseases. Record no 90. Program on the Global Demography of Aging. World Economic Forum, Geneva.
- 48. Binder C. J. 2012. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv. Exp. Med. Biol. 750:2. [DOI] [PubMed] [Google Scholar]
- 49. Tsiantoulas D., Gruber S., Binder C. J. 2012. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front. Immunol. 3:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang M., Subramanian M., Abramowicz S., et al. 2014. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler. Thromb. Vasc. Biol. 34:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robbins C. S., Chudnovskiy A., Rauch P. J., et al. 2012. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swirski F. K., Nahrendorf M., Etzrodt M., et al. 2009. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hapel A. J., Lee J. C., Farrar W. L., Ihle J. N. 1981. Establishment of continuous cultures of thy1.2+, Lyt1+, 2-T cells with purified interleukin 3. Cell 25:179. [DOI] [PubMed] [Google Scholar]
- 54. Ihle J. N., Pepersack L., Rebar L. 1981. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J. Immunol. 126:2184. [PubMed] [Google Scholar]
- 55. Yang Y. C., Ciarletta A. B., Temple P. A., et al. 1986. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell 47:3. [DOI] [PubMed] [Google Scholar]
- 56. Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. 1990. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343:76. [DOI] [PubMed] [Google Scholar]
- 57. Weber G. F., Chousterman B. G., He S., et al. 2015. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mortha A., Chudnovskiy A., Hashimoto D., et al. 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huffman J. A., Hull W. M., Dranoff G., Mulligan R. C., Whitsett J. A. 1996. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J. Clin. Invest. 97:649. [DOI] [PMC free article] [PubMed] [Google Scholar]