Key Clinical Message
Heparin‐induced thrombocytopenia (HIT) is a serious adverse reaction to heparin treatment with a high risk of thrombosis. Heparin must be discontinued immediately and replaced with alternative anticoagulants that do not interact with HIT antibodies. In this case, a lung cancer patient, diagnosed with HIT was successfully treated with apixaban.
Keywords: Apixaban, cancer, dalteparin, heparin‐induced thrombocytopenia
Introduction
Heparin‐induced thrombocytopenia (HIT) is characterized by thrombocytopenia and a high risk of thrombosis. HIT type I is a nonautoimmune, self‐limiting condition triggered by heparin‐influenced activation of platelets and only a mild decrease in platelet count. HIT type II is caused by antibodies against the heparin‐platelet factor 4‐complex and occurs in 0.2–5% of patients treated with heparin for 4 days or more. Heparin should immediately be discontinued and replaced by other anticoagulants such as argatroban, bivalirudin, fondaparinux, danaparoid, or lepirudin 1. However, these drugs are often not readily available; some has to be administered intravenously and the staff may have limited experience with their administration – overall making their use challenging. We therefore examined if a new oral anticoagulant (NOAC) could be used to safely manage HIT.
Case Story
Five months before admission, the patient (a 72‐year‐old woman) was diagnosed with pulmonary adenocarcinoma with a single cerebral metastasis; T2bN0M1. A month later, a cerebral metastasectomy was performed, and after 3 weeks, a 50 mm lung tumor was resected by thoracoscopic right lobectomy. Both interventions were without complications and the patient was in good performance after the surgeries. A month later, adjuvant chemotherapy (cisplatin and vinorelbine) was initiated. Two weeks after initiation of chemotherapy, a 5 mm cerebral relapse was visualized by MRI control scan, and a single dose of 18 Gy stereotactic radiation therapy was planned. Three weeks later – 3 days prior to the radiation therapy and 2 weeks after last dose of chemotherapy – the patient developed dyspnea. A spiral CT scan revealed pulmonary embolism (PE) and Fragmin® (dalteparin) 200 IE/kg equaling 12.500 IE daily was initiated. Twenty days after initiation of dalteparin, the platelet count was reduced to 84 × 109/L (Fig. 1A). The patient was suspected of HIT as she scored 5 on the 4 T's clinical score for the diagnosis of HIT (Fig. 1B) 2. This signals an intermediate probability of HIT 1 and considering a switch to an alternative anticoagulant is recommended 2. The following day, samples were collected for HIT testing. Due to absence of clinical signs of new thrombosis or progression of the PE, limited availability of fondaparinux (as well as the other alternative drugs used in HIT treatment) and since the patient was doing well and the HIT score was moderate, it was decided to continue dalteparin until HIT‐test results were available. The screening test for heparin‐PF4‐antibodies was highly positive (Rigshospitalet, Copenhagen, Denmark). Samples were sent for confirmation, and heparin‐induced platelet activation (HIPA) was positive in four of four samples (Institut Dessau, Dessau, Germany). Thus, both laboratory tests confirmed the diagnosis of HIT, and a shift from dalteparin was mandatory. We considered administration of fondaparinux as an alternative anticoagulant treatment, however, the correct dose was not immediately available from the hospital pharmacy and three subcutaneously injections then had to be given daily. Furthermore, the patient lived in the rural part of Denmark, far from the hospital, hence making administration and monitoring of the traditional drugs used to manage HIT very challenging. Treatment with an NOAC was appealing in this patient, and she was therefore informed of the diagnosis and treatment options and agreed to Eliquis® (apixaban) treatment, 5 mg twice daily. She had regular telephone interviews and blood samples, and 3 days after initiation of apixaban, she reported feeling remarkably better than while being treated with dalteparin. Platelet indices were measured, using Sysmex® XE‐5000. Platelet counts and parameters immediately improved, and D‐dimer decreased (Fig. 1A,C). No adverse reactions were reported. The HIT antibody test was repeated to confirm the diagnosis after 4 weeks and was still found positive. So far, the patient has been on apixaban for 3.5 months and evaluation is planned after 6 months of treatment.
Figure 1.
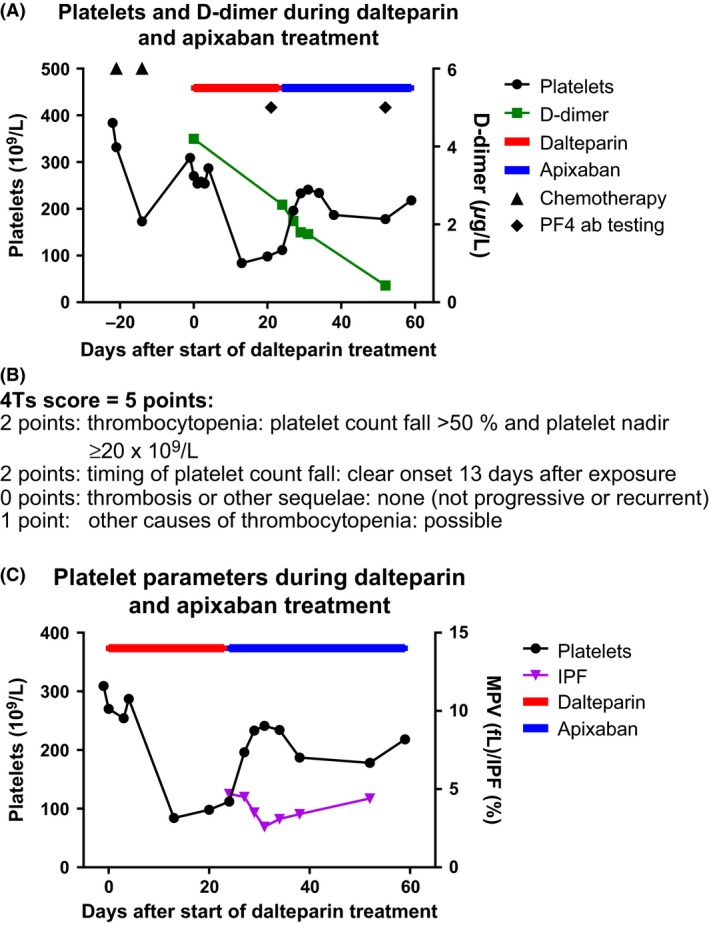
Platelet count and platelet parameters during dalteparin and apixaban treatment in a patient with HIT antibodies. The platelet count was initially normal and after chemotherapy, an expected drop in platelet count was seen, followed by a recovery phase. Between 5 and 13 days after administration of dalteparin, the platelet count decreased rapidly, and rose again after termination of the dalteparin and initiation of apixaban therapy (Panel A). D‐dimer was on a falling curve from initiation of anticoagulative therapy in general, though with a faster decrease after initiation of apixaban. (Panel B) The patients 4Ts score. (Panel C) The immature platelet fraction (IPF%) decreased after dalteparin was replaced with apixaban. The IPF% reflects the production of platelets in the bone marrow which can be related to increased peripheral destruction or consumption 6. In women a reference range for IPF% is 0.8–6.2% 7. In this patient, IPF% was not elevated, but maximum IPF% was measured just before start of apixaban, indicating a high turnover of platelets.
NOACs as monotherapy have not previously been reported in management of HIT, but the use has been speculated 3. A laboratory study showed that in contrast to heparin, apixaban did not activate platelet aggregation in the presence of HIT‐antibodies 4. We were aware, that the efficacy and safety of apixaban in patients with active cancer have not yet been established, but given that the patient was in good performance, had a normal eGFR and low risk of bleeding, it was decided to use apixaban 5 mg twice daily to manage the HIT and treat the PE. In a recent study 3, 22 patients with HIT treated with argatroban for 1.5 days followed by an NOAC, were retrospectively identified and followed for 1.5 years. Five patients continued on apixaban after argatroban. It was concluded that a short course of parenteral treatment with argatroban followed by an NOAC was safe and effective in normalization of platelet count and prevention of thrombosis. In a newly published review of NOAC as treatment for HIT (June, 2015), Miyares and Davis state that data are still sparse and insufficient to recommend clinical use, but apixaban is mentioned as a potential future anticoagulant for management of HIT 5.
Conclusion
We have safely used apixaban as monotherapy for the management of HIT. HIT is often seen in in‐patients who have multiple diseases, impaired renal function and a high risk of bleeding. These patients should follow the standard HIT treatment regimens. However, in the outpatients with low risk of bleeding and normal renal function, NOAC monotherapy seems to be both attractive and a safe alternative. However, before this can be generally recommended as standard treatment in HIT, the safety must be carefully evaluated in more patients.
Conflict of Interest
None declared.
Clinical Case Reports 2015; 3(12): 987–989
References
- 1. Lovecchio, F. 2014. Heparin‐induced thrombocytopenia. Clin. Toxicol. 52:579–583. [DOI] [PubMed] [Google Scholar]
- 2. Lo, G. K. , Juhl D., Warkentin T. E., Sigouin C. S., Eichler P., and Greinacher A.. 2006. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin‐induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 4:759–765. [DOI] [PubMed] [Google Scholar]
- 3. Sharifi, M. , Bay C., Vajo Z., Freeman W., Sharifi M., and Schwartz F.. 2015. New oral anticoagulants in the treatment of heparin‐ induced thrombocytopenia. Thromb. Res. 135:607–609. [DOI] [PubMed] [Google Scholar]
- 4. Walenga, J. M. , Prechel M., Hoppensteadt D., Escalante V., Chaudhry T., Jeske W. P., et al. 2013. Apixaban as an alternate oral anticoagulant for the management of patients with heparin‐induced thrombocytopenia. Clin. Appl. Thromb. Hemost. 19:482–487. [DOI] [PubMed] [Google Scholar]
- 5. Miyares, M. A. , and Davis K. A.. 2015. Direct‐Acting Oral Anticoagulants as Emerging Treatment Options for Heparin‐Induced Thrombocytopenia. Ann. Pharmacother. 49:735–739. [DOI] [PubMed] [Google Scholar]
- 6. Sysmex Xtra Online . 2012. The immature platelet fraction (IPF) – the first to know about megakaryocyte activity. Norderstedt, Germany: http://www.sysmex-europe.com/fileadmin/media/f100/Xtra/Xtra_article_IPF_The_first_to_know_abou__megakaryocyte_activity.pdf [Google Scholar]
- 7. Pekelharing, J. M. H. , Hauss O., de Jonge R., Lokhoff J., Sodikromo J., Spaans M., et al. 2010. Haematology reference intervals for established and novel parameters in healthy adults. Sysmex J. Int. 20:1–11. [Google Scholar]


