Abstract
Objectives
We performed the two inhalation exposures, whole-body inhalation and nose-only inhalation, to investigate the pulmonary deposition and health effects of the two inhalation methods.
Methods
In both methods, we exposed rats to the same TiO2 nanoparticles at almost the same exposure concentration for 6 h and compared the deposited amounts of nanoparticles and histopathological changes in the lungs. Rats were exposed to rutile-type TiO2 nanoparticles generated by the spray-dry method for 6 h. The exposure concentration in the whole-body chamber was 4.10 ± 1.07 mg/m3, and that in nose-only chamber was 4.01 ± 1.11 mg/m3. The particle sizes were 230 and 180 nm, respectively. A control group was exposed to fresh air.
Results
The amounts of TiO2 deposited in the lungs as measured by ICP-AES after acid digestion just after the exposure were: 42.6 ± 3.5 μg in the whole-body exposure and 46.0 ± 7.7 μg in the nose-only exposure groups. The histopathological evaluation was the same in both exposure groups: no infiltration of inflammatory cells in the alveolar space and interstitium, and no fibrosis.
Conclusion
The two inhalation methods using the same material under the same exposure conditions resulted in the same particle deposition and histopathology in the lung.
Keywords: Whole-body inhalation, Nose-only inhalation, TiO2 nanoparticle, Deposition
Introduction
Inhalation studies are not frequently performed in assessments of the pulmonary effect of inhaled particles because of the difficulty of maintaining the system and the amount of time they require. But inhalation exposure has great advantages for evaluating human exposure because of the natural route of entry of the particles. There are two methods for performing inhalation exposure: whole-body exposure and nose-only exposure. The merits of nose-only exposure are that the exposure concentration can be high and there is less waste because of the small chamber volume, and it is possible to avoid entry of the particles by other routes and evaluate the particle effect by inhalation only.
The greatest concern when rats are kept immobile in the chamber is stress, and sometimes it is also difficult to control the humidity in the chamber when using the wet generation method of particles. In comparison with nose-only exposure, whole-body exposure adds the least stress other than that from the exposure material, making it more suitable for long and repeated inhalation. Still, a concern remains about whether contamination from other routes has an effect on the particle deposition and health. Therefore, in this study, to investigate the differences between the two methods in pulmonary deposition and health effects, we performed the nose-only inhalation study and whole-body inhalation study at almost the same exposure concentrations of TiO2 for the same period and compared the amounts deposited in the lung and the pathological changes.
In this study, we used TiO2 nanoparticles as the test material. TiO2 nanoparticle is insoluble and low toxic in addition to being widely used as colorants, sun-screenings or photo catalysts. Because the soluble materials clear fast from the lung by dissolution and the toxic materials accumulate in the lung by injuring the clearance mechanism, we used TiO2 nanoparticles in order to exclude these factors that affect the deposition amounts in lung. Besides the short time and high concentration exposure enables to obtain the deposition amounts without the need to consider a change of clearance rate and the accurate deposition fraction in lung.
Materials and methods
TiO2 nanoparticles
Nano-scale TiO2 (MT-150AW, TAYCA, Japan) has a crystalline structure and spindle shape. Its physicochemical properties are summarized in Table 1. TiO2 powder was suspended in ultra-pure water for nanoparticle generation.
Table 1.
Physicochemical properties of TiO2 in the experiment
| Physicochemical properties | TiO2 nanoparticle |
|---|---|
| Chemical formula | TiO2 |
| Product name and manufacturer | MT-150AW Tayca Co. Ltd. |
| Primary diameter | Short 12 nm Long 55 nm |
| Specific surface area (BET) | 121 m2/g |
| Shape | Spindle-shaped |
| Secondary particle diameter (DLS; number based) | 49.1 nm |
| Crystal structure | Rutile |
| Purity | 99.5 % |
| Bulk density | 4.17 g/cm2 |
| Solubility | Low |
Generation of TiO2 nanoparticles
The aerosol generating system used in this study consisted of a pressurized nebulizer (Nanomaster, JSR Corp., Tokyo, Japan) and a drying section [1, 2]. The TiO2 suspension used in these inhalation studies was observed by transmission electron microscope (TEM). The TEM specimens were prepared by dropping suspensions on TEM grids with carbon support films and drying them. The aerosols of TiO2 particles were also observed by TEM. The TEM specimens were prepared by electrically collecting the aerosols on TEM grids with carbon support films. TEM observation was performed with an EM922 (Carl Zeiss, Germany) at an accelerating voltage of 200 kV.
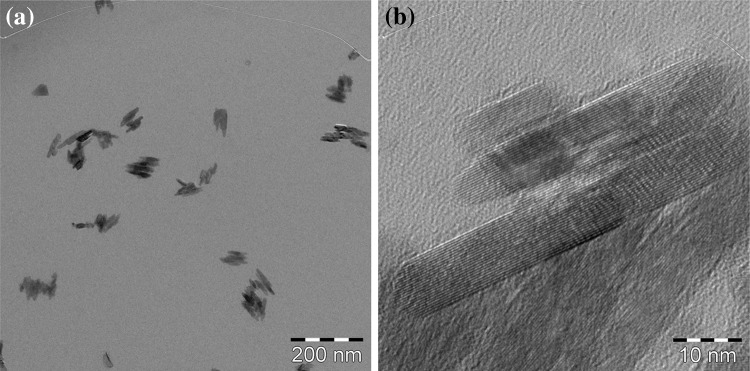
TEM images of the TiO2 suspensions are shown in Fig. 1a, b. The TiO2 particles are spindle-shaped, and the primary size is approximately 10 nm in width and 50 nm in length. The TiO2 particles make up aggregates with sizes of between 50 and 100 nm. As can be seen in the high-resolution images, the TiO2 particles have a clear crystalline form, and there is no damage caused by the preparation processes. These TiO2 suspensions were used for aerosol generation.
Fig. 1.
Low magnification (a) and high magnification (b) TEM images of the TiO2 suspensions used in this study
TiO2 suspensions were sprayed by the nebulizer with compressed air at an air flow rate of 40 L/min and with a suspension feeding rate of 0.8 mL/min. The sprayed droplets were passed through the drying section to evaporate the water in order to obtain aerosol particles. The resulting TiO2-nanoparticle aerosol was fed separately into a whole-body exposure chamber and a nose-only exposure chamber. For the whole-body exposure chamber, the aerosol flow was diluted with clean air at a flow rate of 60 L/min. The concentrations of the TiO2 suspensions were 5.0 mg/mL for the whole-body exposure chamber and 2.0 mg/mL for the nose-only exposure chamber. The particle size distribution of the aerosol was measured by a particle size spectrometer (model 1000XP WPS, MSP Corp., Shoreview, MN) that consisted of a differential mobility analyzer and a condensation particle counter (DMA–CPC) system. The aerosol particles were collected on copper grids by an electrostatic precipitator for TEM observation. The mass concentrations of the aerosols in the two chambers were measured several times by a gravimetrical method, i.e., the aerosol was admitted through fibrous filters, and the collected particles were weighed.
Animals
Fifteen Fischer 344 male rats (11 weeks old) were purchased from Charles River Laboratories International, Inc. (Japan). The animals were kept in the Laboratory Animal Research Center of the University of Occupational and Environmental Health for 1 week with access to free-feeding of commercial diet and water. All procedures and animal handling were done according to the guidelines described in the Japanese Guide for the Care and Use of Laboratory Animals as approved by the Animal Care and Use Committee, University of Occupational and Environmental Health, Japan.
Inhalation exposure
Rats were divided into three groups of five rats each for whole-body inhalation and for nose-only inhalation of TiO2 for 6 h, and for controls exposed to fresh air only.
The whole-body inhalation apparatus except the generator was previously reported [3]. The rats were kept in a cage (35 cm × 35 cm × 20 cm) inserted in the chambers during the exposure. The nose-only inhalation system was the SIS-R36B type (Shibata Kagaku Co. Japan). The rats were inserted in an acrylic tube individually during the exposure.
The TiO2 concentration in the chambers was measured every hour by weighing the filtered particles. The average mass concentration of the TiO2 aerosol was 4.01 ± 1.11 mg/m3 in the nose-only chamber and 4.10 ± 1.07 mg/m3 in the whole-body chamber, respectively. Figure 2 shows the particle size distributions in the aerosols inside the nose-only chamber and the whole-body chamber, as measured by a particle size spectrometer (MSP corp. USA). Almost the same particle size distributions were obtained repeatedly during the 6-h inhalation tests, indicating that a stable aerosol generation and supply were achieved. The geometric mean diameters (GMDs) of the aerosols were 230 and 180 nm in the whole-body and nose-only chambers, respectively.
Fig. 2.
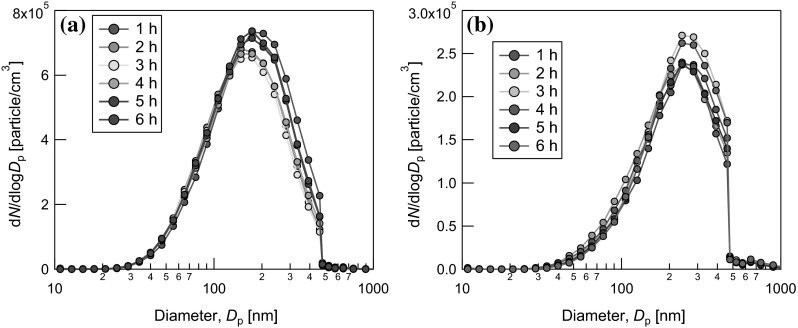
Particle size distributions of the aerosols inside a nose-only exposure chamber and b whole-body exposure chamber
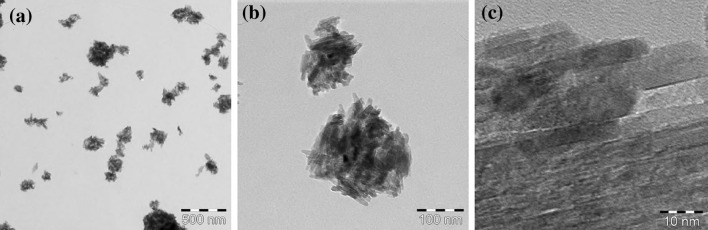
Figure 3a, b shows TEM images of the TiO2 aerosol in the nose-only inhalation chamber. The aerosol particles were aggregated and the size was between 20 and 300 nm. A high-resolution TEM image (Fig. 3c) shows a clear crystal lattice image, and there was no degradation of the TiO2 particles in the aerosol generation process.
Fig. 3.
Low magnification (a), medium magnification (b), and high magnification (c) TEM images of the TiO2 aerosols used in this study
All the rats were sacrificed by overdose of pentobarbital immediately after 6 h inhalation. The wet lung weights of each rat were measured, and each right lung was used for determining the amounts of TiO2, while each left lung was used for histopathological examination.
All procedures and animal handling were done according to the guidelines described in the Japanese Guide for the Care and Use of Laboratory Animals as approved by the Animal Care and Use Committee, University of Occupational and Environmental Health, Japan.
Determination of TiO2 in lungs
The TiO2 particles in each right lung were digested with lung tissues into the element with HNO3 H2SO4, (NH4)2SO4 and H2O2 by microwave digestion system (Ethos one, Milestone, Italy) under a high-temperature and high-pressure condition for 30 min, and the amounts of Ti ion in the digested solution were determined by inductive coupled plasma-atomic emission spectroscopy (SPS3500DD, SII NanoTechnology, Japan). The mass of TiO2 retained in each lung was calculated from the determined Ti amounts divided by the Ti content (59.9 %) of the TiO2 [4].
Histopathological examination
The left lungs were inflated and fixed by intratracheal infusion with 4 % paraformaldehyde at 25 cm H2O pressure for 1 night and then embedded in paraffin. Paraffin sections of 3 μm thickness were stained with hematoxylin and eosin (H&E).
Statistical analysis
Analysis of variance (ANOVA) and Dunnett’s test were applied where appropriate to determine individual differences using a computer statistical package (SPSS, SPSS Inc., Chicago, IL, USA).
Results and discussion
Lung burden of TiO2
The amounts of TiO2 in the lungs after the inhalation were 42.6 ± 3.5 μg in the whole-body inhalation group and 46.0 ± 7.7 μg in the nose-only inhalation group. There was no statistical difference between the two groups.
Deposition fractions
The deposition fractions of each group were calculated as follows:
where C is a average concentration during the exposure, mg/m3, T is a total exposure time, min, V is a estimated respiratory volume, mL/min (tidal volume: 2.1 mL, breaths: 102 times/min).
Therefore,
Body and wet lung weight
The body and wet lung weight and others in the three groups after the inhalation are summarized in Table 2.
Table 2.
Body and lung weights and the deposited amounts in each group
| Whole-body inhalation | Nose-only inhalation | Control | |
|---|---|---|---|
| Body weight before the exposure (g) | 236 ± 9 | 240 ± 2 | 240 ± 9 |
| Body weight after the exposure (g) | 238 ± 9 | 227 ± 7 | 242 ± 9 |
| Wet lung weight (g) | 0.895 ± 0.056 | 0.785 ± 0.034* | 0.901 ± 0.034 |
| Lung weight/body weight (%) | 0.376 ± 0.015 | 0.346 ± 0.010 | 0.373 ± 0.012 |
| Deposited TiO2 in lung (μg) | 42.6 ± 3.5 | 46.0 ± 7.7 |
* Statistical difference (P < 0.01) compared with control group
Before the exposure, the body weights were almost the same in the three groups. After the exposure, that in the nose-only inhalation group was less, but the difference was not significant. The wet lung weight in the nose-only inhalation group was significantly less compared with the control group, but not significantly different from that in the whole-body exposure.
Histopathological results
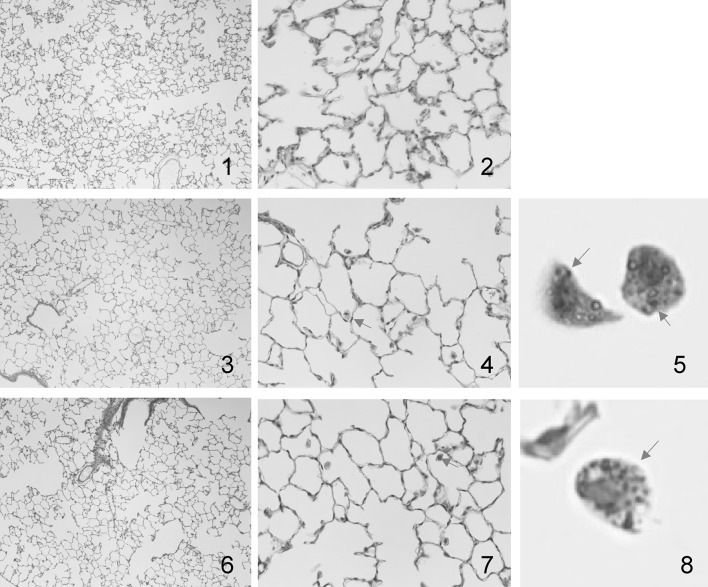
The histopathological results are summarized in Table 3. Typical images of the lung tissues are shown in Fig. 4. Infiltrations of inflammatory cells in the alveolar space and interstitium, fibrosis and tumorigenesis were not observed in any of the three groups. Macrophages engulfed pigment-like components in both whole-body and nose-only exposure (shown in Fig. 4, panels 5 and 8).
Table 3.
Pathological features in the rat lung following inhalation of TiO2 nanoparticles
| Whole-body inhalation (n = 5) | Nose-only inhalation (n = 5) | Control (n = 5) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathological feature in lung | − | ± | + | ++ | +++ | − | ± | + | ++ | +++ | − | ± | + | ++ | +++ |
| Macrophage appearance in alveolar space | 5 | 5 | 5 | ||||||||||||
| Diffuse | 5 | 5 | 5 | ||||||||||||
| Aggregate | 5 | 5 | 5 | ||||||||||||
| Pigment-like component | 5 | 5 | 5 | ||||||||||||
| Neutrophil infiltration in alveolar space | 5 | 5 | 5 | ||||||||||||
| Infiltration in interstitial area | 5 | 5 | 5 | ||||||||||||
| Fibrosis | 5 | 5 | 5 | ||||||||||||
Grade of changes: − none; ± minimum; + mild; ++ moderate; +++ remarked
Fig. 4.
Histopathological images of rat lung tissues in each group
The difference between the physicochemical properties of TiO2 nanoparticle in two inhalation experiments was evaluated. The crystallinity of the TiO2 particles in the chambers of whole-body and nose-only inhalation was confirmed, in spite of heating and spraying during their generation. Crystallinity is the most important factor in inhalation toxicity. It is well known that crystalline silica persists in the lung for a long period and induces fibrosis and tumors. More production of cytokines and gene expression related with inflammation were induced by crystalline silica than by amorphous silica [5, 6]. In the process of TiO2 generation it is possible to lose the crystallinity, but in the present study the confirmation of the crystallinity of the TiO2 in both inhalations was an important issue in comparing the health effects of the two methods.
With regard to the particle size as a physicochemical property, the average diameter in the chambers of the whole-body and nose-only inhalations were 230 and 180 nm, respectively. The difference in the GMDs in the chambers between the two methods may have been due to the different concentrations of the suspensions used. A higher concentration was required for the whole-body chamber since the aerosol flow had to be diluted by 2.5 times. The higher concentration caused the larger GMDs because the greater number of nanoparticles contained in each droplet tended to form larger agglomerates. The dilution also led to the lower concentration of aerosols in the whole-body exposure chamber. Kobayashi et al. reported in an instillation study that the inflammation tendency of TiO2 particles with different agglomeration sizes (18, 65, 300 nm, with a primary diameter of 5 nm) were almost the same [7]. The sizes of the TiO2 in the two chambers in our study were smaller than those reported above, so the different sizes may not have affected the toxicity.
In the present study, the deposition fractions in the whole-body and nose-only inhalations were 0.135 and 0.149, respectively. The deposition fractions calculated by an MPPD model (MPPD 2.11) using the same respiratory volume and exposure conditions were 0.111 for the whole-body inhalation and 0.130 for the nose-only inhalation, which were almost the same as our results. Our calculated deposition fractions were not considered to be particle clearance during exposure or in respiratory volume, which changes depending on body weight, therefore the deposition fraction might be a little bit altered when the other estimation [8, 9] of respiratory volume is adopted. Yeh et al. reported that the deposition fractions in whole-body and nose-only exposures of micron-size TiO2 particles were almost the same: 5.1 and 4.5 %, with a larger deposition of nano-size particles in the alveolar region compared with micron-size particles [10]. Our higher deposition fractions with nano-size particles, and the almost same deposition fraction in each of our experiments are in good agreement with Yeh’s results with micron-size particles.
The deposition amounts in the lung and the influences on the lung of the two inhalation methods in our study were similar, but Iwasaki et al. reported less LC50 and higher choline esterase activity in the blood in rats in a whole-body inhalation group compared to a nose-only inhalation group in the exposure to fenthion mist [11]. In contrast, studies of 3 insecticides (two mists and one solid) resulted in no significant difference in LD50 between whole-body and nose-only inhalation exposures [12]. Also, tobacco smoke exposure resulted in no significant difference in DNA adducts in the lung between whole-body and nose-only inhalation exposures [13]. A difference between the two inhalation methods is reported in mist exposures in these studies, but the two methods derived the same results for solid particles. The exposure material in our study was solid, therefore our results are consistent with previous studies in this point. In whole-body inhalation, there is a concern about the possibility of TiO2 nanoparticles entering into the rat digestive system via the oral route by grooming or other activities, and then systemic symptoms will appear. But when insoluble TiO2 nanoparticles are used, as in the present study, there will be almost no systemic effect when TiO2 happens to enter via the oral route. There were also no differences in the histopathological results of the same number of macrophages and pigment-like component considered to be TiO2. These similar histopathological results also indicated that the amounts of TiO2 in the lung were the same in both of the inhalation experiments.
The whole-body inhalation and the nose-only inhalation on the same experimental condition resulted in the similar deposited amounts and histopathological changes. But, the lung weights after the exposure in nose-only inhalation group showed significant decrease compared with that of the control group, although that was not significant compared with that in whole-body inhalation group. The difference in the lung–body weight ratio between in nose-only inhalation and in control group was not significant. It is not clear whether the difference in lung weight is caused by the method. It is necessary to perform further studies.
Conclusion
We performed a whole-body inhalation study and a nose-only inhalation study of the same TiO2 nanoparticles in almost the same experimental conditions and compared the particle deposition and histopathological changes in the lung. The two inhalation studies yielded almost the same results.
Acknowledgments
This work is part of the research program of “Development of innovative methodology for safety assessment of industrial nanomaterials” supported by Ministry of Economy, Trade and Industry (METI) of Japan.
Compliance with ethical standards
Conflict of interest
We have no conflict of interest.
References
- 1.Shimada M, Wang W-N, Okuyama K, Myojo T, Oyabu T, Morimoto Y, et al. Development and evaluation of an aerosol generation and supplying system for inhalation experiments of manufactured nanoparticles. Environ Sci Technol. 2009;43(14):5529–5534. doi: 10.1021/es9008773. [DOI] [PubMed] [Google Scholar]
- 2.Kubo M, Nakaoka A, Morimoto K, Shimada M, Horie M, Morimoto Y, et al. Aerosol generation by a spray-drying technique under coulomb explosion and rapid evaporation for the preparation of aerosol particles for inhalation tests. Aerosol Sci Technol. 2014;48(7):698–705. doi: 10.1080/02786826.2014.918930. [DOI] [Google Scholar]
- 3.Tanaka I, Akiyama T. A new dust generator for inhalation toxicity studies. Ann Occup Hyg. 1984;28:157–162. doi: 10.1093/annhyg/28.2.157. [DOI] [PubMed] [Google Scholar]
- 4.Oyabu T, Morimoto Y, Hirohashi M, Horie M, Kambara T, Byong Woo L, et al. Dose-dependent pulmonary response of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J Nanopart Res. 2013;15:1600. doi: 10.1007/s11051-013-1600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston CJ, Driscoll KE, Finkelstein JN, Baggs R, Michael MA, Reilly AO, et al. Pulmonary chemokine and mutagenic responses in rats after subchronic inhalation of amorphous and crystalline silica. Toxicol Sci. 2000;56:405–413. doi: 10.1093/toxsci/56.2.405. [DOI] [PubMed] [Google Scholar]
- 6.Parkins TN, Shukla A, Peeters PM, Steinbacher JL, Landry CC, Lathrop SA, et al. Differences in gene expression and cytokine production by crystalline vs. amorphous silica in human lung epithelial cells. Part Fiber Toxicol. 2012;9:6. doi: 10.1186/1743-8977-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi N, Naya M, Endoh S, Maru J, Yamamoto K, Nakanishi J. Comparative pulmonary toxicity study of nano-TiO2 particles of different sizes and agglomerations in rats: different short- and long-term post-instillation results. Toxicology. 2009;264:110–118. doi: 10.1016/j.tox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC. Measurement of the respiratory volumes of laboratory animals. Am J Physiol. 1947;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 2000;20(4):273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::AID-JAT657>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Yeh HC, Snipes MB, Eidson AF, Hobbs CH, Henry MC. Comparative evaluation of nose-only versus whole-body inhalation exposures for rats –aerosol characteristics and lung deposition. Inhal Toxicol. 1990;2:205–221. doi: 10.3109/08958379009145255. [DOI] [Google Scholar]
- 11.Iwasaki M, Yoshida M, Ikeda T, Tsuda S, Shirasu Y. Comparison of whole-body versus snout-only exposure in inhalation toxicity of Fenthion. Jpn J Vet Sci. 1988;50(1):23–30. doi: 10.1292/jvms1939.50.23. [DOI] [PubMed] [Google Scholar]
- 12.Sachsse K, Zbinden K, Ullmann L. Significance of mode of exposure in aerosol inhalation toxicity studies—head only versus whole body exposure. Arch Toxicol. 1980;4:305–311. doi: 10.1007/978-3-642-67729-8_62. [DOI] [PubMed] [Google Scholar]
- 13.Bond JA, Chen BT, Griffith WC, Mauderly JL. Inhaled cigarette smoke induces the formation of DNA adducts in lung of rats. Toxicol Appl Pharmacol. 1989;99:161–172. doi: 10.1016/0041-008X(89)90121-X. [DOI] [PubMed] [Google Scholar]