Abstract
Objective
To investigate what influences patients' health care decisions and what the implications are for the provision of information on the quality of health care providers to patients.
Data Sources/Study Setting
Dutch patient samples between November 2006 and February 2007.
Study Design
Discrete choice experiments were conducted in three patient groups to explore what influences choice for health care providers.
Data Collection
Data were obtained from 616 patients with knee arthrosis, 368 patients with chronic depression, and 421 representatives of patients with Alzheimer's disease.
Principal Findings
The three patients groups chose health care providers on a different basis. The most valued attributes were effectiveness and safety (knee arthrosis); continuity of care and relationship with the therapist (chronic depression); and expertise (Alzheimer's disease). Preferences differed between subgroups, mainly in relation to patients' choice profiles, severity of disease, and some background characteristics.
Conclusions
This study showed that there is substantial room for (quality) information about health care providers in patients' decision processes. This information should be tailor‐made, targeting specific patient segments, because different actors and factors play a part in their search and selection process.
Keywords: Quality indicators, discrete choice experiment, patient preferences, health services research, quality assurance
During the last decade, health care reforms in many countries have aimed at giving a central role to the health care “consumer” (Ministry of Health Welfare and Sports 2002; Department of Health 2004; Thomson and Dixon 2006) through a focus on patient choice (Appleby, Harrison, and Devlin 2003; Burge et al. 2005; Steer 2006; Dixon, Robertson, and Bal 2010a), patient empowerment (Wensing 2000; Victoor et al. 2012a; Fredriksson 2013), and decision support (Hibbard, Slovic, and Jewett 1997; Hibbard and Peters 2003; Hibbard, Greene, and Daniel 2010). Providing the consumer with comparative (quality) information about health care providers is an essential aspect of these attempts. At the root of these efforts is the assumption that patients would act upon this information as rational health care consumers. The evidence base for this assumption is, however, still weak (Dixon et al. 2010b; Victoor et al. 2012b; Ketelaar, Faber et al. 2014), which begs the question of whether the resources needed to implement these policies represent money well spent.
Literature shows that patients' choices are more or less influenced by (infra)structural aspects of health care quality (the availability of providers, the accessibility of the providers, the type and size of the providers, the availability/experience/quality of the staff, the organization of health care, the cost of treatment, and sociodemographic factors of the individual doctors), as well as by process (interpersonal factors, availability of information, continuity of treatment, waiting time, and the quality of treatment) and by outcomes (Victoor et al. 2012a,b). The problem with many studies, however, is threefold. First, there are, for example, studies that just asked patients to rate or score factors that may influence their choice of provider according to relevance (Harris 2003; Cheng and Song 2004; Fung et al. 2005) or qualitative studies based on interviews or focus groups where the influence of comparative performance information was discussed (Ketelaar, Faber et al. 2014). To identify the relative weight of (f)actors influencing patients' decision processes, however, these results are of limited use, as the studies in question do not identify trade‐offs between factors and hardly differentiate between factors, a problem that is worsened by the fact that many patients find it difficult to prioritize using scales (Devellis 2006). Second, the majority of these studies either do not differentiate between patient groups or between individuals within a group of patients (Marshall et al. 2000) or only focus on one specific health care setting: “Many studies do not explicitly address the issue that their findings may depend on the specific decision making context, for example, that they focus on a hospital or GP, that they asked for patients' preferences or the attributes they based their decision on” (Victoor et al. 2012a,b, p. 12). What is needed is an approach that reveals the relative importance of factors and actors that influence patients' decisions and that takes into account the fact that patients' preferences may change over time and may differ between segments or groups. Third, the available evidence is not only incomplete, it also lacks the ability to verify the—frequently contested—“rational health care consumer” assumption (Marshall, Hiscock, and Sibbald 2002; McDonald et al. 2007). The idea that patients do not actively choose providers but just go to the nearest one is widely shared (Salisbury 1989; Varkevisser, van der Geest, and Schut 2009; Exworthy and Peckham 2010). At best, patients seem to be guided by their own earlier experiences with care providers (Schwartz, Woloshin, and Birkmeyer 2005; Marang‐Van‐De Mheen et al. 2010), or those of families and friends (Marshall, Hiscock, and Sibbald 2002; Lux et al. 2011), or they trust and follow their general practitioner's advice (Grumbach et al. 1999; Dijs‐Elsinga et al. 2010). In so far as patients are interested in differences between providers at all, they tend to focus on service and relational quality aspects, which they can observe and judge for themselves (Linder‐Pelz 1982; Fotaki et al. 2008; Faber et al. 2009). From this perspective, providing quality information would not empower patients to make better choices (Marshall et al. 2000; Schneider and Lieberman 2001).
Given these limitations and debates, the present study sought to investigate which actors and factors influence patients' health care decisions, how these preferences differ between and within patient groups, and what the implications are for providing information on the quality of health care providers to patients. To gain a better insight into the relative importance of these aspects of health care for different patients, we conducted discrete choice experiments (DCE) in three patient groups, namely patients with knee arthrosis, chronic depression, and Alzheimer's disease. These are high‐volume health conditions in most countries, which at the same time represent three typical health care settings for patient decision making and provider choice.
Methods
Study Sample
Patients with knee arthrosis were recruited in January and February 2007 on the basis of being on a waiting list for knee arthroplasty or ostheotomy or having undergone such an operation in 2006. Patients were recruited via the orthopedic departments of two academic and four general hospitals and via the website and call center of the Dutch Association of Orthopaedic Patients (SPO). In addition, patients who had participated in a preliminary study (see below) were approached. Paper questionnaires were sent by mail to 806 patients who had indicated their willingness to participate.
We initially attempted to recruit patients with chronic depression via mental health care providers, but this appeared difficult, largely because of the closed character of these institutions and the patient‐doctor relationship. We therefore decided to recruit patients with chronic depression from the general population. Our recruitment strategy involved sending an invitation to administer a questionnaire to members of an existing Internet panel (consisting of Dutch civilians age 18 years and older; maintained by Survey Sampling International), who had reported suffering from depression. To confirm their self‐reported diagnosis, the questionnaire included questions to establish whether a respondent met the DSM‐IV‐TR‐criteria for Dysthymic Disorder, the mildest form of depression. In addition, the severity of the depression was assessed using the Beck Depression Inventory‐II scale (American Psychiatric Association 2000). In January 2007, a total of 3,500 panel members were invited by email to participate in the study and to complete the web‐based questionnaire.
Representatives of patients with Alzheimer's disease were recruited in two different ways. First, a number of nursing homes, residential homes for elderly, and ambulatory mental health care services were asked to invite their clients to participate in our study. Second, we advertised in a popular weekly magazine for middle‐aged women that featured a special issue on Alzheimer's disease in November 2006. A total of 550 representatives expressed their willingness to participate in the study and were sent a paper questionnaire.
The sampling protocols for all three DCEs were authorized by the medical ethical committee of the Erasmus MC Rotterdam.
Preliminary Study
Prior to the current study, the search and selection processes for a health care provider of the above patient groups were investigated in depth. First, we conducted semistructured interviews with 23 purposefully sampled patients with knee arthrosis, 15 patients with chronic depression, and 15 patients with Alzheimer's disease and/or their representatives. Following a grounded theory approach in the phases of both data collection and analysis (Patton 2002), we derived three long lists of actors and factors that may play a part in the search for and selection of a health care provider. Next, we used Q‐methodology (Brown 1980; Cross 2005; Watts and Stenner 2005) to identify choice profiles in all three patient groups, based on differences and similarities in the importance these patients attached to a structured sample of the actors and factors identified through the interviews (Groenewoud, Kreuger, and Van Exel 2007). A total of 45 patients with knee arthrosis, 44 patients with chronic depression, and 41 patients with Alzheimer's disease and/or their representatives participated in this Q‐methodological study, from which two main choice profiles among patients emerged: an outcome‐focused and a trust‐focused profile, representing different rankings of the actors and factors that influence patients' decision processes. The results of these two preliminary studies supported the current study by giving us a deeper insight into patients' attitudes toward health care choice in general, as well as by generating a preselection of actors and factors that patients with different choice profiles find important. Here, we follow current insights in literature, that show how attributes and levels should be derived from prior qualitative research (Coast and Horrocks 2007).
Current Study: Three Discrete Choice Experiments
To explore the relative importance of these actors and factors in health care decisions, we conducted a DCE in each patient group. DCE is a method for quantifying consumer preferences for commodities or services by analyzing their choices in hypothetical choice situations. The method is based on random utility theory (McFadden 1974) and Lancaster's economic theory of value (Lancaster 1966). It is built on the assumptions that health care interventions, services, or policies can be described by their characteristics (called attributes), and that a person's valuation depends on the levels of these characteristics (Ryan 2004; Ryan, Gerard, and Amaya‐Amaya 2008). DCEs are widely used in health services research (e.g., Gyrd‐Hansen and Sogaard 2001; Hall et al. 2006).
Attributes and Levels
In the preliminary study, we found that the attribute set should cover aspects concerning the structure, process, and outcome of health care. For instance, the preliminary study showed that accessibility of care and expertise/competence of staff were considered important structure factors for choice of care provider, advice/referral, waiting time, and patient‐centeredness process factors, and effectiveness and safety outcome factors. In other words, the attribute set should cover characteristics of the health care provider, the health services provided, and the different actors involved in the decision making process (i.e., the social context of decision making). For example, we defined “Provider was recommended by…” as an attribute and “your general practitioner” as one of its levels.
The definition of each attribute and its levels varied across disease groups, depending on disease characteristics and the priorities indicated by the patient groups (Groenewoud, Kreuger, and Van Exel 2007). Using the interview material and the results of the Q‐methodological study as a starting point, the authors condensed the set of potential candidate attributes to a more manageable set of attributes. This was done in several “waves” by two of the authors, who separately filtered double or overlapping attributes. Then, a comparison was made after which this procedure was repeated. This resulted in a design with 10 attributes for the DCEs among patients with knee arthrosis and chronic depression, and 11 attributes for the DCE among representatives of patients with Alzheimer's disease. Three levels were defined for each attribute, in ascending order from worst to best. As far as possible, the levels were based on real health care performance data—for example, waiting times and risk of infections—to present respondents with situations as representative as possible of what may occur in “real practice.” The design was pilot‐tested with a selection of the patients who had been interviewed in the preliminary study. The three final sets of attributes and levels are presented in Tables 1, 2, and 3.
Table 1.
Main Model: Knee Arthrosis
| Quality Domain | Attributes and Levels | Coeff | SE | [95% CI] |
|---|---|---|---|---|
| Accessibility | Travel distance | |||
| 150 km | Reference value | |||
| 50 km | 0.367a | 0.050 | 0.268–0.465 | |
| 10 km | 0.785a | 0.057 | 0.673–0.897 | |
| Expertise/competence | No. of knee operations per month | |||
| 2 | Reference value | |||
| 8 | 0.222a | 0.053 | 0.118–0.327 | |
| 10 | 0.240a | 0.051 | 0.139–0.341 | |
| Type of hospital | ||||
| General | Reference value | |||
| University | −0.025 | 0.053 | −0.129 to 0.079 | |
| Orthopedic | 0.075 | 0.052 | −0.026 to 0.176 | |
| Advice and referral | Provider recommended by… | |||
| Family | Reference value | |||
| Patient organization | 0.020 | 0.051 | −0.080 to 0.119 | |
| General practitioner | 0.197a | 0.051 | 0.096–0.297 | |
| Timelineness | Waiting time (weeks) | |||
| 20 | Reference value | |||
| 8 | 0.112a | 0.054 | 0.006–0.218 | |
| 2 | 0.139a | 0.050 | 0.041–0.238 | |
| Care process | Information is given to you… | |||
| Before treatment, written | Reference value | |||
| Before treatment, written and oral | 0.102a | 0.052 | 0.000–0.203 | |
| Continuously, written and oral | 0.121a | 0.050 | 0.022–0.220 | |
| Patient‐centeredness | Prior exp. with hospital | |||
| Not very good | Reference value | |||
| Never been there before | 0.195a | 0.053 | 0.092–0.298 | |
| Good | 0.624a | 0.057 | 0.512–0.736 | |
| Prior exp. with medical specialist | ||||
| Did not match very well | Reference value | |||
| Never been there before | 0.056 | 0.058 | −0.058 to 0.169 | |
| Matched well | 0.373a | 0.049 | 0.277–0.469 | |
| Effectiveness/Safety | Average before‐after degree of knee bending | |||
| 30° | Reference value | |||
| 90° | 1.068a | 0.052 | 0.965–1.170 | |
| 120° | 1.502a | 0.058 | 1.389–1.615 | |
| Risk of wound infections (%) | ||||
| 5 | Reference value | |||
| 2,5 | 0.327a | 0.050 | 0.230–0.424 | |
| 1 | 0.527a | 0.050 | 0.429–0.624 | |
p < .05.
Table 2.
Main Model: Chronic Depression
| Quality Domain | Attributes and Levels | Coeff | SE | [95% CI] |
|---|---|---|---|---|
| Accessibility | Costs per consultation (€) | |||
| 80 | Reference value | |||
| 15 | 0.689a | 0.062 | 0.568–0.809 | |
| 0 | 0.884a | 0.065 | 0.756–1.012 | |
| Expertise/competence | Expertise, exp., specialization | |||
| Social‐psychiatric nurse | Reference value | |||
| Medical doctor | −0.215a | 0.065 | −0.343 to −0.088 | |
| Psychiatrist | 0.137a | 0.062 | 0.015–0.259 | |
| Vision on treatment | ||||
| No clear vision | Reference value | |||
| Vision does not match with client | −0.088 | 0.060 | −0.204 to 0.029 | |
| Vision matches with client | 0.513a | 0.063 | 0.389–0.636 | |
| Timelineness | Waiting time (weeks) | |||
| 24 | Reference value | |||
| 12 | 0.158a | 0.066 | 0.028–0.289 | |
| 2 to 3 | 0.411a | 0.064 | 0.286–0.536 | |
| Care process | Intake and care plan | |||
| Limited intake, no care plan | Reference value | |||
| Extensive intake, no clear care plan | 0.078 | 0.065 | −0.049 to 0.205 | |
| Extensive intake with clear care plan | 0.332a | 0.060 | 0.214–0.450 | |
| Patient‐centeredness | % of clients satisfied with interpersonal treatment | |||
| 25 | Reference value | |||
| 50 | 0.253a | 0.063 | 0.129–0.377 | |
| 80 | 0.437a | 0.067 | 0.306–0.569 | |
| Relationship with therapist | ||||
| No contact before; no relationship yet | Reference value | |||
| Prior contact; not a very good relationship | −0.640a | 0.064 | −0.765 to −0.515 | |
| Prior contact; good relationship | 0.230a | 0.064 | 0.105–0.355 | |
| Continuity of care | ||||
| Change in treating professional | Reference value | |||
| Fixed team of professionals | 0.648a | 0.065 | 0.521–0.774 | |
| Always same professional | 0.769a | 0.064 | 0.644–0.894 | |
| Participation | ||||
| Hardly any possibilities for participation | Reference value | |||
| Participation; professional in control | 0.503a | 0.063 | 0.380–0.627 | |
| Participation; client in control | 0.640a | 0.065 | 0.513–0.767 | |
| Effectiveness/safety | Expected result: % people reporting good results | |||
| 20 | Reference value | |||
| 50 | 0.324a | 0.061 | 0.204–0.444 | |
| 80 | 0.581a | 0.063 | 0.457–0.705 | |
p < .05.
Table 3.
Main Model: Alzheimer's Disease
| Quality Domain | Attributes and Levels | Coeff | SE | [95% CI] |
|---|---|---|---|---|
| Accessibility | Travel distance | |||
| 60 km | Reference value | |||
| 20 km | 0.659a | 0.063 | 0.535–0.784 | |
| 5 km | 1.086a | 0.066 | 0.957–1.215 | |
| Expertise/competence | Expertise of the institution regarding Alzheimer's disease | |||
| Not specialized | Reference value | |||
| A specialized ward/unit | 0.556a | 0.065 | 0.428–0.684 | |
| Institution is specialized | 0.649a | 0.061 | 0.530–0.768 | |
| Advice and referral | Provider recommended by… | |||
| No one in particular | Reference value | |||
| Family or friends | 0.046 | 0.066 | −0.083 to 0.175 | |
| GP or medical specialist | 0.155a | 0.064 | 0.030–0.280 | |
| Timelineness | Waiting time (months) | |||
| 12 | Reference value | |||
| 8 | 0.130a | 0.065 | 0.004–0.257 | |
| 4 | 0.359a | 0.061 | 0.240–0.478 | |
| Care process | Hours of personal care/week | |||
| 4 | Reference value | |||
| 10 | 0.484a | 0.067 | 0.353–0.615 | |
| 16 | 0.736a | 0.057 | 0.624–0.849 | |
| Patient‐centeredness | Percentage of residents experiencing good interpersonal treatment | |||
| 25 | Reference value | |||
| 50 | 0.376a | 0.064 | 0.251–0.502 | |
| 75 | 0.666a | 0.062 | 0.544–0.788 | |
| Percentage of representatives satisfied with communication with staff | ||||
| 50 | Reference value | |||
| 70 | 0.091 | 0.063 | −0.031 to 0.214 | |
| 90 | 0.251a | 0.064 | 0.126–0.376 | |
| Deliver care as agreed | ||||
| Seldom | Reference value | |||
| Sometimes | 0.137a | 0.063 | 0.013–0.261 | |
| Always | 0.514a | 0.061 | 0.393–0.634 | |
| Effectiveness/safety | Percentage of residents feeling safe and comfortable | |||
| 50 | Reference value | |||
| 70 | 0.542a | 0.065 | 0.415–0.669 | |
| 90 | 0.883a | 0.063 | 0.760–1.006 | |
| Risk of pressure ulcers | ||||
| 20% | Reference value | |||
| 10% | 0.180a | 0.067 | 0.050–0.311 | |
| 2% | 0.508a | 0.056 | 0.399–0.618 | |
| No. of personnel per 15 residents | ||||
| 1 | Reference value | |||
| 2 | 0.346a | 0.065 | 0.219–0.473 | |
| 3 | 0.629a | 0.062 | 0.507–0.751 | |
p < .05.
Experimental Design
In the DCE, we employed an orthogonal, fractional factorial design consisting of 27 choice sets, which were generated following the strategy outlined by Street, Burgess, and Louviere (2005). We selected an orthogonal main effects plan (o.a.27.13.3.2) from a directory of orthogonal arrays (Sloane 2010), removing two or three columns to get a starting design with the right number of attributes for each DCE. This main effects plan represented the scenarios that appeared as the first option in the choice sets, and then we made systematic level changes (changing the 0s to 1s, the 1s to 2s and the 2s to 0s) to represent the profiles that appear as the second option in the choice sets. Because evaluating 27 scenarios may lead to respondent fatigue (Ubach et al. 2003), we blocked the design into three sets of nine scenarios. Each pair of scenarios was presented in forced‐choice response mode (see Figure 1). Together with the questionnaire, participants received an explanation of the meaning of each attribute and its levels.
Figure 1.
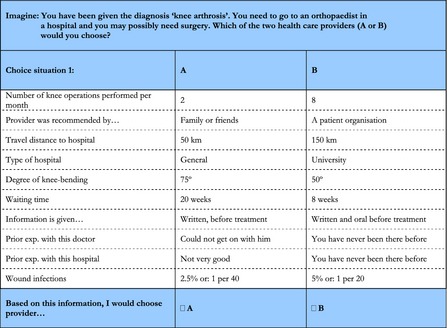
A Pair of Scenarios
Survey Section of the Questionnaire
In addition to the evaluation of nine DCE scenarios, the questionnaire asked respondents about a number of personal, disease‐related, and health care‐related characteristics that emerged as potentially relevant during the preliminary study but were not selected as attributes for the DCE. Furthermore, respondents were presented with statements about making choices in health care to distinguish between people with passive and active choice behavior, and with short descriptions of the outcome‐ and trust‐focused choice profiles (Groenewoud, Kreuger, and Van Exel 2007) to distinguish between patients with different approaches to the choice of care provider.
Analysis
We analyzed the discrete choice data by means of a conditional logit (McFadden 1974), using Stata 11 (StataCorp, College Station, TX, US). Given the assumption of compensatory decision making, individuals were assumed to consider all the attributes in the choice set, and to trade between them. The conditional logit model assumes that an individual i′s utility of making choice j—represented as U ij—is composed of an observable and an unobservable component:
| (1) |
where X ij as the observable stochastic component defined by the vector of choice attributes J = 1,…,j (and β the vector of attribute parameters to be estimated) and ε ij as the unobservable random error component which captures elements of U ij that are not represented in X ij. Because U ij is unknown, it is assumed that when individual i chooses alternative j, U ij is the maximum of the utilities for all the J alternatives, and the probability that alternative j will be chosen is:
| (2) |
The DC models we estimated included main‐effect terms for all attributes. All attributes were treated as categorical variables, and thus transformed into dummies for modeling. Dummy coding was applied in the analyses. Parameter estimates were not adjusted for having obtained multiple observations per respondent.
Separate models were specified for each of the three disease groups as well as for subgroups with divergent characteristics within each disease group. We compare the sign and the magnitude of coefficients across the three disease group models to identify the factors or actors which each patient group draws upon when choosing their health care provider. It is not possible to directly compare the magnitude of coefficient estimates between the main models because the stochastic component of utility has different variances in these models (Hensher, Rose, and Greene 2005). To explore preference heterogeneity within disease groups, we defined subgroups based on their general orientation toward choices in health care (patients classified themselves into one of two categories: outcome‐focused or trust‐focused), the self‐assessed severity of disease, and their level of education. We compared the preference structures between these subgroups by plotting their estimated coefficients against each other. This has proven to be a very convenient method to detect differences in attribute strengths between subgroups (Hall et al. 2006).
Data Quality
We explored whether inclusion of respondents with missing responses and inclusion of respondents who continually preferred the same option introduced bias in the analysis. Hereto, the regression analysis of the DCE was done twice: first based on all obtained responses, and second based only on completed questionnaires by respondents who did not always choose the same option. As not all attributes have a logical preference ordering, it was not possible to do an internal consistency test based on a dominant choice.
Results
Questionnaires were returned by 616 patients with knee arthrosis (76 percent), 368 patients with chronic depression, and 421 representatives of patients with Alzheimer's disease (77 percent). As regards the patients with chronic depression, 1,626 of the 3,500 persons who were invited to participate began the questionnaire, 449 of them met the DSM‐IV‐TR‐criteria for Dysthymic Disorder, and 368 persons completed the questionnaire (82 percent of the target group). Table 4 presents the personal, disease‐related, and health care‐related characteristics of the three samples.
Table 4.
Sample Characteristics
| Characteristica | Knee Arthrosis | Chronic Depression | Alzheimer's Disease |
|---|---|---|---|
| Personal | |||
| Gender | |||
| Male | 229/609 (38) | 83/368 (23) | 78/421 (19) |
| Female | 380/609 (62) | 285/368 (77) | 343/421 (81) |
| Mean (SD) age (years) | 66 (10.5) | 41 (10.9) | 57 (10.1) |
| Education level | |||
| Low | 407/607 (67) | 72/368 (19.5) | 157/406 (39) |
| Middle | 106/607 (18) | 219/368 (60) | 136/406 (33) |
| High | 94/607 (15) | 77/368 (19.5) | 113/406 (28) |
| Urbanization | |||
| City | 189/606 (31) | 245/368 (67) | 262/418 (63) |
| Countryside | 417/606 (69) | 123/368 (33) | 156/418 (37) |
| Disease‐related | |||
| Mean (SD) disease severity (0–10) |
4.4 (2.9) pain 5.1 (2.8) limitation |
6.2 (2.0)b | 7.0 (2.0) |
| Mean (SD) perceived health (0–10) | 7.1 (1.3) | 4.5 (1.6) | 7.6 (1.4) |
| Mean (SD) period of complaints (years) | 12.5 (12.1) | 14.6 (9.5) | 7.1 (5.1) |
| Health care‐related | |||
| Choice profile | |||
| Outcome‐focused | 480/598 (80) | 58/368 (16) | 357/416 (86) |
| Trust‐focused | 118/598 (20) | 310/368 (84) | 59/416 (14) |
| Choice behavior | |||
| Passive | 428/609 (70) | 194/368 (53) | 132/421 (31) |
| Active | 181/609 (30) | 174/368 (47) | 289/421 (69) |
| Member of patient organization | |||
| Yes | 31/595 (5) | 54/368 (15) | 73/419 (17) |
| No | 564/595 (95) | 314/368 (85) | 346/419 (83) |
| Type of current/most recent care | 484/596 (81) GH | 186/368 (51) AmMC | 111/421 (26) Am |
| 86/596 (14) UH | 107/368 (29) Psych | 238/421 (57) In | |
| 13/596 (2.5) OC | 81/368 (22) GP | 71/421 (17) Cd | |
| 13/596 (2.5) Other | 25/368 (7) PH | ||
| 80/368 (22) Other | |||
| Treatment status | |||
| Treatment received | 403/616 (65) | – | – |
| Still on waiting list | 213/616 (35) | ||
The denominator in patient characteristics is not always the same as the number of respondents that completed their DCE because of missing data on some questions about the respondents' background characteristics (e.g., about choice behavior).
Scores on Beck Depression Inventory‐II‐scale: 0 (not depressed): 14/368 (3.8%); 1 (mildly depressed): 78/338 (21.2%); 2 (modestly depressed): 47/368 (12.8%); 3 (seriously depressed): 142/368 (38.6%); 4 (very seriously depressed): 87/368 (23.6%).
Am, ambulatory care facility; AmMC, ambulatory mental care facility; Cd, client has died; GH, general hospital; GP, general practitioner; In, institutional care facility; OC, orthopedic hospital; PH, psychiatric hospital; Psych, independent psychologist or psychiatrist; UH, university hospital.
The returned DCE questionnaires included missing values for 142 of the 616 knee patients and for 15 of the 421 representatives of patients with Alzheimer's. No missing values were present in chronic depression, because the marketing agency only returned questionnaires of those who had completed at least the DCE part. Few respondents always preferred the same option (two in knee patients, one in chronic depression, and two in Alzheimer's). It appeared to make little difference whether the data obtained from these respondents were excluded from the analysis. The effect on parameter estimates was trivial; in all three groups, the Pearson's correlation between the two sets of parameter estimates was >0.99. Therefore, we decided to base our analysis on all obtained responses.
Three Disease Group Models
In the “knee arthrosis model” (see Table 2), all statistically significant coefficients showed positive signs, indicating that, as presumed, levels indicating better care were preferred over levels indicating worse care. Relative to the reference level of each attribute, improvement of the expected outcome of the operation (represented by the indicator “average before‐after degree of bending of the knees that were operated on by a surgeon”) had the strongest impact on the search and selection process of patients with knee arthrosis. Respondents also were more likely to opt for a scenario that included better prior experience with the hospital or medical specialist during earlier contact, or one that included a 50 km reduction in travel distance. As may have been expected for this type of elective surgery, reduction in waiting time with a week was not considered of great importance compared to the aforementioned attributes. Some other attributes and levels, like the type of hospital and the provision of information before treatment, played no part in patients' decisions.
In the “chronic depression model” (see Table 3), relative to the attribute‐specific reference levels, the chance that a scenario was chosen was increased most by better continuity of care, a better personal match with the therapist during earlier contact, or introduction of the possibility to participate in decisions about the care process. The coefficients for the levels “medical doctor” and “no good relationship during earlier contact” showed negative signs, indicating that patients preferred the preceding levels “social‐psychiatric nurse” and “no relationship yet,” respectively, which is a plausible result. In this model, all attributes turned out to be relevant in the choice of health care provider, but not all attribute levels.
In the “Alzheimer's model” (see Table 4), better levels of caregiver expertise, reduced travel distance, and care delivery in accordance with agreements were the most important factors in the choice of care provider by representatives of patients with Alzheimer's disease. All attributes were relevant for the search and selection process, and all coefficients showed positive signs.
Differences and Similarities between Disease Group Models
The data seem to suggest that to the three patient groups, different types of quality indicators mattered. Knee patients above all considered clinical outcomes, while depressed patients focused on indicators about patient‐centeredness. Those who represent patients with Alzheimer's disease considered many indicators. The observed differences largely corresponded to the percentages of patients who qualified themselves as outcome‐focused or trust‐focused (see Table 1). Furthermore, the results seem intuitive in relation to the vulnerabilities one expects in the three patient groups. For example, knee patients can hardly be considered vulnerable, which gives them the opportunity to wait for optimal results and put different weight on attributes not directly related to outcomes. In contrast, patients with Alzheimer's may experience physical, social, and mental vulnerabilities so that no single indicator can dominate the others.
Differences and Similarities within Disease Groups
Preferences concerning attributes also differed within disease groups. Table 5 shows the coefficients for the most important subgroups based on “choice profile,” “severity of the disease,” and “education level.” Figure 2 shows scatter plots of the estimated coefficients for these subgroups. In these graphs, a strong linear relationship between the estimated coefficients of two subgroups indicates that the groups have comparable preferences. The nine plots show that the preference structure varied more with choice profile than with severity characteristics or education level, and that it varied more in representatives of patients with Alzheimer's disease than in patients suffering from knee arthrosis or chronic depression.
Table 5.
Modeling for Subgroups of Patients
| Attributes | Choice Profile | Severityb | Education Level | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Trust | Low | High | Low | High | ||||||||||
| Coef. | SE | Coef. | SE | Coef. | SE | Coef. | SE | Coef. | SE | Coef. | SE | ||||
| Knee | |||||||||||||||
| Travel distance 50 km | 0.365c | 0.058 | 0.407c | 0.115 | 0.321c | 0.068 | 11 | 0.461c | 0.079 | 0.418c | 0.062 | 0.454c | 0.166 | ||
| Travel distance 10 km | 0.775c | 0.067 | 0.873c | 0.121 | 0.794c | 0.081 | 10 | 0.811c | 0.085 | 0.852c | 0.068 | 16 | 0.984c | 0.244 | |
| 8 knee operations per month | 0.216c | 0.062 | 0.314c | 0.115 | 0.232c | 0.072 | 0.190c | 0.083 | 0.191c | 0.064 | 0.429c | 0.207 | |||
| 10 knee operations per month | 0.258c | 0.059 | 0.179 | 0.118 | 0.269c | 0.070 | 0.220c | 0.080 | 0.184c | 0.062 | 0.496c | 0.165 | |||
| University hospital | −0.018 | 0.062 | −0.023 | 0.118 | −0.116 | 0.072 | 0.026 | 0.083 | −0.128c | 0.064 | 0.328 | 0.205 | |||
| Orthopedic hospital | 0.090 | 0.060 | 0.091 | 0.116 | 0.012 | 0.071 | 0.127 | 0.080 | 0.010 | 0.063 | 0.348c | 0.170 | |||
| Recommended by patients | 0.096 | 0.059 | −0.237c | 0.116 | 0.061 | 0.069 | 0.005 | 0.079 | 0.052 | 0.061 | 0.128 | 0.161 | |||
| Recommended by GP | 0.228c | 0.059 | 0.173 | 0.116 | 0.208c | 0.070 | 0.163c | 0.080 | 0.230c | 0.062 | 0.110 | 0.168 | |||
| Waiting time 8 weeks | 0.121 | 0.064 | 0.065 | 0.117 | 0.090 | 0.075 | 0.111 | 0.084 | 0.048 | 0.064 | 0.251 | 0.214 | |||
| Waiting time 2 weeks | 0.144c | 0.058 | 0.132 | 0.117 | 0.179c | 0.068 | 0.073 | 0.079 | 0.126c | 0.061 | 0.066 | 0.163 | |||
| Written/oral info before | 0.132c | 0.060 | −0.003 | 0.116 | 0.072 | 0.071 | 0.145 | 0.080 | 0.143c | 0.062 | 0.151 | 0.169 | |||
| Written/oral info continuously | 0.130c | 0.058 | 0.156 | 0.116 | 0.142c | 0.069 | 0.144 | 0.079 | 0.116 | 0.062 | 0.159 | 0.149 | |||
| No prior exp. hospital | 0.187c | 0.061 | 0.268c | 0.115 | 0.266c | 0.072 | 0.111 | 0.082 | 0.225c | 0.063 | 0.257 | 0.190 | |||
| Good prior exp. hospital | 0.607c | 0.067 | 0.741c | 0.122 | 0.695c | 0.081 | 0.568c | 0.086 | 0.634c | 0.068 | 0.951c | 0.242 | |||
| No prior exp. specialist | 0.018 | 0.068 | 0.282c | 0.123 | −0.071 | 0.081 | 0.178c | 0.088 | 0.126 | 0.069 | −0.300 | 0.215 | |||
| Good prior exp. specialist | 0.325c | 0.057 | 2 | 0.663c | 0.112 | 0.300c | 0.067 | 12 | 0.459c | 0.076 | 0.406c | 0.059 | 0.268 | 0.167 | |
| Average knee bending 90° | 1.197c | 0.061 | 0.659c | 0.115 | 1.175c | 0.072 | 0.932c | 0.081 | 0.928c | 0.063 | 1.751c | 0.179 | |||
| Average knee bending 120° | 1.720c | 0.068 | 1 | 0.750c | 0.121 | 1.716c | 0.082 | 1.255c | 0.086 | 1.205c | 0.068 | 15 | 2.905c | 0.240 | |
| Risk wound infections 2.5% | 0.384c | 0.057 | 0.196c | 0.114 | 0.369c | 0.067 | 0.282c | 0.078 | 0.256c | 0.060 | 0.535c | 0.157 | |||
| Risk wound infections 1% | 0.585c | 0.058 | 0.322c | 0.112 | 0.610c | 0.068 | 0.475c | 0.079 | 0.487c | 0.061 | 0.835c | 0.152 | |||
| Chronic depression | |||||||||||||||
| Costs per consultation 15€ | 0.755c | 0.153 | 0.684c | 0.068 | 0.642c | 0.126 | 0.656c | 0.128 | 0.585c | 0.139 | 0.573c | 0.145 | |||
| Costs per consultation 0€ | 0.932c | 0.175 | 0.870c | 0.071 | 0.728c | 0.130 | 0.858c | 0.139 | 1.004c | 0.148 | 18 | 0.777c | 0.151 | ||
| Provider is a medical doctor | 0.152 | 0.180 | −0.279c | 0.071 | −0.218 | 0.135 | −0.273c | 0.138 | −0.020 | 0.150 | −0.394c | 0.156 | |||
| Provider is a psychiatrist | 0.352c | 0.164 | 0.087 | 0.068 | −0.082 | 0.127 | 0.227c | 0.131 | 0.209 | 0.147 | 0.304c | 0.143 | |||
| Vision on treatment: no match | 0.223 | 0.155 | −0.148c | 0.066 | −0.108 | 0.126 | −0.128 | 0.124 | −0.268c | 0.133 | −0.272 | 0.145 | |||
| Vision on treatment: match | 0.723c | 0.167 | 0.487c | 0.069 | 0.494c | 0.131 | 0.551c | 0.137 | 0.058 | 0.139 | 17 | 0.576c | 0.143 | ||
| Waiting time 12 weeks | 0.322 | 0.186 | 0.145c | 0.073 | 0.224 | 0.134 | 0.244 | 0.142 | 0.109 | 0.153 | 0.253 | 0.152 | |||
| Waiting time 2–3 weeks | 0.427c | 0.178 | 0.432c | 0.070 | 0.481c | 0.132 | 0.471c | 0.137 | 0.572c | 0.145 | 0.613c | 0.152 | |||
| Extensive intake, no clear care plan | 0.0238 | 0.173 | 0.095 | 0.071 | 0.181 | 0.130 | 0.049 | 0.136 | −0.001 | 0.151 | 0.148 | 0.156 | |||
| Extensive intake, clear care plan | 0.517c | 0.171 | 0.317c | 0.066 | 0.225c | 0.127 | 0.245 | 0.127 | 0.296c | 0.137 | 0.434c | 0.145 | |||
| Interpersonal treatm.: 50% satisfied | 0.032 | 0.176 | 0.283c | 0.069 | 0.062c | 0.132 | 0.210 | 0.136 | 0.463c | 0.147 | 20 | 0.213 | 0.147 | ||
| Interpersonal treatm.: 80% satisfied | 0.404c | 0.187 | 0.435c | 0.073 | 0.249 | 0.135 | 0.467c | 0.142 | 0.526c | 0.154 | 0.664c | 0.160 | |||
| Prior exp. therapist not very good | −0.722c | 0.177 | −0.644c | 0.070 | −0.477c | 0.130 | −0.639c | 0.133 | −0.520c | 0.153 | −0.607c | 0.150 | |||
| Prior exp. therapist good | 0.203 | 0.162 | 0.238c | 0.070 | 0.203 | 0.134 | 0.380c | 0.136 | 0.185 | 0.138 | 0.252 | 0.152 | |||
| Continuity: Fixed team | 0.415c | 0.181 | 0.697c | 0.070 | 0.622c | 0.133 | 0.602c | 0.134 | 0.594c | 0.139 | 0.635c | 0.151 | |||
| Continuity: Always the same person | 0.367 | 0.162 | 4 | 0.854c | 0.070 | 0.635c | 0.128 | 0.826c | 0.135 | 0.843c | 0.147 | 19 | 0.687c | 0.151 | |
| Participation: professional in control | 0.711c | 0.174 | 0.483c | 0.069 | 0.393c | 0.130 | 0.542c | 0.133 | 0.535c | 0.142 | 0.660c | 0.145 | |||
| Participation: client in control | 0.767c | 0.177 | 0.632c | 0.071 | 0.483c | 0.130 | 0.697 | 0.136 | 0.618 | 0.144 | 0.771c | 0.151 | |||
| 50% report good results | 0.465c | 0.159 | 0.301c | 0.067 | 0.420c | 0.131 | 0.207 | 0.129 | 0.176 | 0.143 | 0.463c | 0.140 | |||
| 80% report good results | 0.931c | 0.177 | 3 | 0.537c | 0.069 | 0.594c | 0.129 | 0.517 | 0.133 | 0.496 | 0.148 | 0.693c | 0.150 | ||
| Alzheimer's | |||||||||||||||
| Travel distance 20 km | 0.627c | 0.069 | 0.889c | 0.194 | 0.897c | 0.138 | 0.680c | 0.087 | 0.723c | 0.119 | 0.597c | 0.116 | |||
| Travel distance 5 km | 1.035c | 0.072 | 5 | 1.516c | 0.200 | 1.493c | 0.147 | 13 | 0.973c | 0.088 | 1.163c | 0.120 | 1.091c | 0.120 | |
| Institution with a specialized ward | 0.554c | 0.071 | 0.571c | 0.198 | 0.578c | 0.138 | 0.584c | 0.090 | 0.640c | 0.122 | 0.355c | 0.120 | |||
| Institution specialized in Alzh. Dis. | 0.666c | 0.065 | 0.524c | 0.190 | 0.654c | 0.129 | 0.687c | 0.083 | 0.754c | 0.116 | 0.418c | 0.110 | |||
| Recommended by family or friends | 0.025 | 0.071 | 0.279 | 0.201 | 0.096 | 0.139 | −0.028 | 0.091 | 0.109 | 0.122 | 0.099 | 0.119 | |||
| Recommended by GP/specialist | 0.149c | 0.069 | 0.274 | 0.194 | 0.254 | 0.139 | 0.117 | 0.087 | 0.243c | 0.120 | 0.160 | 0.118 | |||
| Waiting time 8 months | 0.131 | 0.070 | 0.201 | 0.189 | 0.267 | 0.138 | 0.098 | 0.086 | 0.101 | 0.107 | 0.155 | 0.120 | |||
| Waiting time 4 months | 0.301c | 0.066 | 8 | 0.699c | 0.178 | 0.352c | 0.126 | 0.430c | 0.083 | 0.414c | 0.097 | 0.289c | 0.117 | ||
| 10 hours of personal care/ week | 0.476c | 0.072 | 0.522c | 0.202 | 0.466c | 0.148 | 0.553c | 0.091 | 0.623c | 0.123 | 0.437c | 0.121 | |||
| 16 hours of personal care/ week | 0.779c | 0.064 | 7 | 0.496c | 0.158 | 0.533c | 0.114 | 14 | 0.829c | 0.078 | 0.703c | 0.092 | 0.805c | 0.111 | |
| Good interpersonal treatment: 50% | 0.392c | 0.069 | 0.289 | 0.197 | 0.482c | 0.142 | 0.389c | 0.087 | 0.438c | 0.120 | 0.295c | 0.118 | |||
| Good interpersonal treatment: 75% | 0.709c | 0.069 | 0.520c | 0.173 | 0.664c | 0.132 | 0.678c | 0.085 | 0.556c | 0.100 | 0.682c | 0.118 | |||
| Communication staff: 70% satisfied | 0.084 | 0.067 | 0.250 | 0.197 | 0.025 | 0.136 | 0.102 | 0.085 | 0.190 | 0.119 | 0.002 | 0.114 | |||
| Communication staff: 90% satisfied | 0.216c | 0.069 | 0.418 | 0.193 | 0.232 | 0.134 | 0.260c | 0.088 | 0.313c | 0.120 | 0.170 | 0.118 | |||
| Deliver care as agreed: sometimes | 0.131 | 0.069 | 0.130 | 0.183 | 0.309c | 0.129 | 0.050 | 0.087 | −0.007 | 0.104 | 0.042 | 0.118 | |||
| Deliver care as agreed: always | 0.555c | 0.068 | 0.338c | 0.167 | 0.701c | 0.132 | 0.456c | 0.082 | 0.411c | 0.097 | 0.408c | 0.117 | |||
| Feeling safe and comfortable: 70% | 0.560c | 0.070 | 0.382 | 0.199 | 0.735c | 0.144 | 0.531c | 0.088 | 0.564c | 0.120 | 0.399c | 0.116 | |||
| Feeling safe and comfortable: 90% | 0.923c | 0.068 | 6 | 0.619c | 0.192 | 1.088c | 0.136 | 0.842c | 0.085 | 0.843c | 0.118 | 0.782c | 0.116 | ||
| Risk of pressure ulcers: 10% | 0.137 | 0.072 | 9 | 0.489c | 0.200 | 0.300c | 0.144 | 0.169 | 0.090 | 0.310c | 0.122 | 0.198 | 0.122 | ||
| Risk of pressure ulcers: 2% | 0.497c | 0.061 | 0.606c | 0.162 | 0.594c | 0.110 | 0.463c | 0.076 | 0.522c | 0.090 | 0.598c | 0.108 | |||
| 2 a personnel per 15 residents | 0.320c | 0.070 | 0.501c | 0.199 | 0.381c | 0.138 | 0.376c | 0.089 | 0.464c | 0.122 | 0.376c | 0.119 | |||
| 3 a personnel per 15 residents | 0.617c | 0.067 | 0.769c | 0.193 | 0.650c | 0.133 | 0.717c | 0.087 | 0.736c | 0.118 | 0.632c | 0.113 | |||
Figure 2.
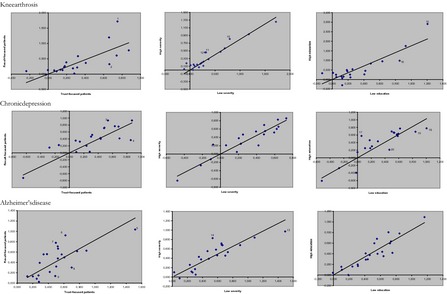
Comparison of the Model Outcomes for Different Subgroups (Numbers correspond to text and Table 5)
Primary treatment outcomes were in general more important to patients with a outcome‐focused choice profile, while those with a trust‐focused choice profile attached more important to patient‐centeredness. In the knee arthrosis group, this is illustrated by the differences in weights attributed to an improvement in knee bending from 60 to 120 degrees (dot 1) and to their different appreciation of moving from a situation where patient and physician are no good match into the situation where they are a good match (2). In the chronic depression group, it is illustrated by differences in the weights attached to improving the percentage of patients reporting good results from 20 percent to 80 percent (3) and to “always treated by the same person” instead of having to deal with “changes in treating professional” (4). Interpretation was less straightforward in representatives of patients with Alzheimer's disease. They all valued reducing the travel distance from 60 km to 5 km very highly (5). Outcome‐focused representatives attributed greater importance than trust‐focused representatives to improving the percentage of patients who feel “safe and comfortable” from 50 percent to 90 percent (6) and to increasing the hours of care per week from 4 to 16 (7). Those who were categorized as being trust‐oriented were more concerned with reducing the waiting time from 12 to 4 months (8) and reducing the risk of pressure ulcers from 20 percent to 10 percent (9).
Higher severity of disease was in knee patients associated with higher weight for having the travel distance reduced from 150 km to 50 km (11) or 10 km (10). In chronic depression, preference structure was quite similar over the severity groups. In representatives of patients with Alzheimer's disease, we found that severity of disease affected the weight for reducing the travel distance from 60 km to 5 km (13) and increasing the “hours of care per week” from 4 to 16 (14). Higher severity was associated with lower value to reduced travel distance and higher values for increased hours of care.
Finally, there were some differences noted between people with a high or low level of education. In knee, those with a high level of education attached a higher weight to improvement in knee bending from 60 to 120 degrees (15) and a lower weight to reducing the travel distance from 150 km to 10 km (16) than those with a low level of education. In chronic depression, we found the strongest effects of level of education. Those with a high level of education base their choice for a provider on a larger number of attributes. This generated differences between the educational groupings in their appreciation for level changes in the attributes “vision on treatment” (17), “cost per consultation” (18), “continuity” (19), and “interpersonal treatment” (20). Those with a high education valued it higher when vision on treatment changed from “no clear vision” to a “match.” They associated a lesser weight to reducing the cost per consultation from 80€ to 0€, to increasing the percentage of patients who are satisfied with interpersonal treatment from 25 percent to 50 percent, and to improving the continuity of care by “always treated by the same person” rather than having to deal with changes in who the provider of treatment changes in the treating professional. The preference structure of representatives of patients with Alzheimer's disease did not differ for the educational groupings.
Discussion
This study sought to investigate which actors and factors influence patients' health care decisions, how these preferences differ between and within patient groups, and what the implications are for providing information on the quality of health care providers to patients. We found that patients' preferences were conditional upon the type of disease, whether the individual had an outcome‐ or trust‐focused choice profile, the phase or severity of disease, and some background characteristics such as education level. Some subgroups of patients attached more importance to measures of outcome, others to measures of process or (infra)structure. This supports findings from earlier studies indicating that both interpersonal (Fasolo et al. 2010) and technical quality or even outcomes (Fung et al. 2005; Harris 2003; Viktoor, 2012a, b) play a part in patients' search and selection processes. Factors that other authors reported to be dominant—if patients were to choose at all—such as advice from family or friends (Lux et al. 2011), referral by a general practitioner (Dijs‐Elsinga et al. 2010; De Groot et al. 2011), waiting time (Dawson et al. 2007; Siciliani and Martin 2007), or information during treatment (Grumbach et al. 1999) appeared to be less important to patients in the current study. Finally, these findings suggest that a proportion of patients will benefit from comparative quality information about care providers. We think these results are relevant for policy makers and organizations in the health care sector interested in patient preferences for care providers, for example, because they are involved in developing patient information or quality report cards, or because they purchase or supply health services and want these to be demand‐oriented.
Given a priori expectations, the results were plausible and support the validity of the techniques applied. Nevertheless, there are some limitations to this study that need to be discussed. First, we used forced choice to elicit preferences. This means that respondents had no opt‐out option, that is, the possibility not to choose any provider at all, or the possibility to go to the same provider as usual. As such, the study might overestimate active choice behavior. We tried to accommodate preferences of passive choosers by including the attributes “advice/referral,” “travel distance,” and “earlier experience.” However, the mere fact that the choice sets presented information that is in real life more difficult to access, may have let respondents to consider information that they would not consider in real life, even in case the health care system gives consumers access to comparative (quality) information. If in real life comparative (quality) information would be made available, one might therefore expect a lesser impact than observed in the current study.
Second, we used a relatively large number of attributes (10 and 11). Some have claimed that people can only handle a limited amount of information at a time and therefore recommend a maximum of between five and nine attributes (Hochhauser 1998; AHRQ 2007). But one should also consider that it is important to capture all salient attributes to avoid unobserved variance related to the fact that respondents make inferences about omitted attributes (Lancsar and Louviere 2008). In that respect, the selection of attributes always involves a trade‐off between realism—which often demands more attributes and levels—and feasibility for respondents. Given the response rates in all three disease groups, we do not believe that the number of attributes posed serious problems to respondents. Moreover, the attributes were selected on the basis of extensive preliminary research involving consultation with the target population (Groenewoud, Kreuger, and Van Exel 2007), which we regard as a clear strength of this study and which may have contributed to greater realism and appeal of the choice sets to respondents.
Third, this study focused on main effects only and thus disregarded possible interaction effects between attributes. Addressing such interdependencies would have required a much larger set of scenarios (or a smaller number of attributes) to be evaluated by respondents, and would have left too little statistical power to identify differences between (sub)groups of patients, which was one of the primary purposes of the study.
Fourth, the sampling strategy may have implications for the ability to generalize results. For example, our sample includes more representatives of patients who receive institutional care (57 percent) than representatives of patients who receive ambulatory care (26 percent). This proportion is different for the national level of 35 percent and 65 percent, respectively (NIZW 2005). Second, women seem to be overrepresented in our sample. However, prevalence data show that far more women than men suffer from knee arthrosis or chronic depression (RIVM 2010), and it is a well‐known phenomenon that informal care for patients with Alzheimer's disease is mostly given by women (especially wives and daughters) (Max, Webber, and Fox 1995). Third, because we used the Beckscale to identify the severity of patients' depression, we can conclude that respondents in our sample are representatively spread over the severity groups (Bijl et al. 1997b). Furthermore, the use of an Internet panel to recruit patients with chronic depression might have biased the sample toward more assertive decision makers within this disease group. This means that in institutional care settings (which we were not able to include), the group of dependent, passive patients might be larger than in our sample, even though the latter contained a significant group of severely depressed patients. On the other hand, the number of people who regularly use the Internet is growing fast, and future consumer information will mainly be disseminated through this medium. We therefore believe that our conclusions can be maintained for the potential target group of consumer information in the field of depression care.
Fifth, one could claim that the heterogeneities in preferences could have been investigated into more depth. There are nowadays mixed logit studies that estimate all coefficients as random parameters. However, modeling random effects is a rather time consuming method and because we were more interested in the heterogeneity in the observed characteristics, which we do report in this study, we did not opt for the mixed logit model.
Finally, despite the assertion that DCE “is likely to be somewhat deficient when judged against its stated aim of eliciting consumer preferences in health care contexts” (McDonald et al. 2007) because “it does not embed patients' decisions in their social context, but focuses on rational trade‐offs, based on ‘product‐characteristics’ in a laboratory‐setting” (Light and Hughes 2001), DCEs have generally been shown to be reliable and valid (Ryan and Gerard 2003). Besides, the current situation in Dutch health care provides no opportunities to study the (potential) role of consumer information in patients' revealed choices, even if we had preferred to do so. Such a study would only be possible in the hypothetical situation where there are no shortages in the provision of health care and where patients have sufficient accessible, reliable, and understandable information at their disposal, which is not yet the case in the Netherlands. In addition, simulating patients' choices gives control over the experimental design, which not only ensures statistical robustness (Ubach et al. 2003) but also makes it possible to simulate a situation with understandable quality information about care providers across a broad spectrum of aspects.
Notwithstanding these limitations, our findings clearly suggest that publicly disclosed comparative quality information on health care providers will empower patients to fulfill their role of critical consumers in a competitive health care environment. Consumer information will, however, only contribute to patient empowerment if it is made disease‐specific and sensitive to patients' choice profile and the severity of their disease, and differentiates for important background characteristics such as education level. This is consistent with earlier findings indicating that, although there is no such thing as the patient in this context, there is a diversity of choice profiles which emerges when searching for and selecting a health care provider (Groenewoud, Kreuger, and Van Exel 2007; Zwijnenberg et al. 2012). We therefore argue that the development of effective consumer information requires a tailor‐made approach to provide groups of patients with convenient and relevant comparative quality information about care providers.
What do our research findings mean for the further dissemination of consumer information and for the future research agenda? Regarding the future development and dissemination of consumer information, we advise that, in the short term, more outcome indicators have to be developed, measured, and publicly disclosed at the level of health care products (e.g., DRGs). This information has to fit the needs of relevant segments of patients and must be disseminated via professionals who refer patients to health care providers (e.g., GPs) or via institutions that allocate care to patients.
Future research should map out both the stated and the revealed choice processes through which patients progress in the search for a care provider/doctor for a range of diseases or care needs. It should also study the role and desired content of decision‐supporting (quality) information in that process. This will create a clearer insight into the dilemmas confronting patients who are in search of care and would enable decision‐supporting information to be developed in a more targeted way. Only these conditions can help patients to fulfill their roles as a change agent in health care.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We are grateful to The Netherlands Organisation for Health Research and Development (ZonMw) for funding this study.
Disclosures: None.
Disclaimers: None.
References
- AHRQ . 2007. Talking to Consumers about Health Care Quality. Rockville, MD: Agency for Health Research and Quality. [Google Scholar]
- American Psychiatric Association . 2000. DSM‐IV‐TR Criteria for Dysthymic Disorder. Washington, DC: American Psychiatric Association. [Google Scholar]
- Appleby, J. , Harrison A., and Devlin N.. 2003. What Is the Real Cost of More Patient Choice? London: Kings Fund. [Google Scholar]
- Beck, A. T. , and Steer R. A.. 1984. “Internal consistencies of the original and revised Beck depression inventory.” Journal of Clinical Psychology 40 (6): 1365–7. [DOI] [PubMed] [Google Scholar]
- Bijl, R. V. , van Zessen G., Ravelli A., de Rijk C., and Langendoen Y.. 1997b. “Psychiatrische morbiditeit onder volwassenen in Nederland: Het NEMESIS‐onderzoek. I. Doelstellingen, opzet en methoden.” Nederlands Tijdschrift voor Geneeskunde 141: 2448–52. [PubMed] [Google Scholar]
- Brown, S. R. 1980. Political Subjectivity: Applications of Q Methodology in Political Science. New Haven, CT: Yale University Press. [Google Scholar]
- Burge, P. , Devlin N., Appleby J., Rohr C., and Grant J.. 2005. London Patient Choice Project Evaluation. A Model of Patients' Choices of Hospital from Stated and Revealed Preference Choice Data. Santa Monica, CA: RAND Corporation. [Google Scholar]
- Cheng, S. H. , and Song H. Y.. 2004. “Physician Performance Information and Consumer Choice: A Survey of Subjects with the Freedom to Choose between Doctors.” Quality and Safety in Health Care 13 (2): 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast, J. , and Horrocks S.. 2007. “Developing Attributes and Levels for Discrete Choice Experiments Using Qualitative Methods.” Journal of Health Services Research and Policy 12: 25–30. [DOI] [PubMed] [Google Scholar]
- Cross, R. M. 2005. “Exploring Attitudes: The Case for Q Methodology.” Health Education Research 20 (2): 206–13. [DOI] [PubMed] [Google Scholar]
- Dawson, D. , Gravelle H., Jacobs R., Martin S., and Smith P. C.. 2007. “The Effects of Expanding Patient Choice of Provider on Waiting Times: Evidence from a Policy Experiment.” Health Economics 16: 113–28. [DOI] [PubMed] [Google Scholar]
- De Groot, I. , Otten W., Smeets H., and Marang‐van de Mheen P.. 2011. “Is the Impact of Hospital Performance Data Greater in Patients Who Have Compared Hospitals?” BMC Health Serv Res 11: 214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health . 2004. Choosing Health. Making Healthy Choices Easier. London: Department of Health. [Google Scholar]
- Devellis, R. F. 2006. Scale Development: Theory and Applications. Thousand Oaks, London, New Delhi: Sage Publications. [Google Scholar]
- Dijs‐Elsinga, J. , Otten W., Versluijs M., Smeets H., Kievit J., Vree R., van der Made W. J., and Marang‐van de Mheen P. J.. 2010. “Choosing a Hospital for Surgery: The Importance of Information on Quality of Care.” Medical Decision Making 30: 544. [DOI] [PubMed] [Google Scholar]
- Dixon, A. , Robertson R., and Bal R.. 2010a. “The Experience of Implementing Choice at Point of Referral: A Comparison of the Netherlands and England.” Health Econ Policy Law 5: 295–317. [DOI] [PubMed] [Google Scholar]
- Dixon, A. , Robertson R., Appleby J., Burge P., Devlin N., and Magee H.. 2010b. “Patient Choice. How Patients Choose and How Providers Respond Kings Fund, June 2010” [accessed on October 20, 2014]. Available at http://www.kingsfund.org.uk/sites/files/kf/Patient-choice-final-report-Kings-Fund-Anna%20Dixon-Ruth-Robertson-John-Appleby-Peter-Purge-Nancy-Devlin-Helen-Magee-June-2010.pdf
- Exworthy, M. , and Peckham S.. 2010. “Access, Choice and Travel: Implications for Health Policy.” Social Policy & Administration 40: 267–87. [Google Scholar]
- Faber, M. , Bosch M., Wollersheim H., Leatherman S., and Grol R.. 2009. “Public Reporting in Health Care: How Do Consumers Use Quality‐of‐Care Information? A Systematic Review” Medical Care 47: 1–8. [DOI] [PubMed] [Google Scholar]
- Fasolo, B. , Reutskaja E., Dixon A., and Boyce T.. 2010. “Helping Patients Choose: How to Improve the Design of Comparative Scorecards of Hospital Quality.” Patient Education and Counseling 78: 344–9. [DOI] [PubMed] [Google Scholar]
- Fotaki, M. , Roland M., Boyd A., McDonald R., Scheaff R., and Smith L.. 2008. “What Benefits Will Choice Bring to Patients? Literature Review and Assessment of Implications.” Journal of Health Services Research and Policy 13: 178–84. [DOI] [PubMed] [Google Scholar]
- Fredriksson, M. 2013. “Is Patient Choice Democratising Swedish Primary Care?” Health Policy 111 (1): 95–8. [DOI] [PubMed] [Google Scholar]
- Fung, C. H. , Elliott M. N., Hays R. D., Kahn K. L., Kanouse D. E., McGlynn E. A., Spranca M. D., and Shekelle P. G.. 2005. “Patients' Preferences for Technical versus Interpersonal Quality When Selecting a Primary Care Physician.” Health Services Research 40 (4): 957–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewoud, S. , Kreuger L., and Van Exel N. J. A.. 2007. Decision Processes of Health Care Consumers with Knee Arthrosis, Alzheimer's Disease or Chronic Depression: Three Q‐Methodological Studies [in Dutch; original title: Keuzeprocessen van zorgconsumenten met knieartrose, de ziekte van Alzheimer of chronische depressie: drie Q‐methodologische studies. iMTA report 07.98] [accessed on October 20, 2014]. Available at http://www.bmg.eur.nl/fileadmin/ASSETS/bmg/english/iMTA/Publications/Reports/200798.pdf [Google Scholar]
- Grumbach, K. , Selby J. V., Damberg C., Bindman A. B., Quesenberry C. J., Truman A., and Uratsu C.. 1999. “Resolving the Gatekeeper Conundrum: What Patients Value in Primary Care and Referrals to Specialists.” Journal of the American Medical Association 282 (3): 261–6. [DOI] [PubMed] [Google Scholar]
- Gyrd‐Hansen, D. , and Sogaard J.. 2001. “Analysing Public Preferences for Cancer Screening Programmes.” Health Economics 10 (7): 617–34. [DOI] [PubMed] [Google Scholar]
- Hall, J. , Fiebig D. G., King M. T., Hossain I., and Louviere J. J.. 2006. “What Influences Participation in Genetic Carrier Testing? Results from a Discrete Choice Experiment.” Journal of Health Economics 25 (3): 520–37. [DOI] [PubMed] [Google Scholar]
- Harris, K. M. 2003. “How Do Patients Choose Physicians? Evidence from a National Survey of Enrollees in Employment‐Related Health Plans.” Health Services Research 38 (2): 711–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensher, D. A. , Rose J. M., and Greene W. H.. 2005. Applied Choice Analysis: A Primer. Cambridge: Cambridge University Press. [Google Scholar]
- Hibbard, J. , Greene J., and Daniel D.. 2010. “What Is Quality Anyway? Performance Reports That Clearly Communicate to Consumers the Meaning of Quality of Care.” Medical Care Research and Review: MCRR 67: 275–93. [DOI] [PubMed] [Google Scholar]
- Hibbard, J. H. , and Peters E.. 2003. “Supporting Informed Consumer Health Care Decisions: Data Presentation Approaches That Facilitate the Use of Information in Choice.” Annual Review of Public Health 24: 413–33. [DOI] [PubMed] [Google Scholar]
- Hibbard, J. H. , Slovic P., and Jewett J. J.. 1997. “Informing Consumer Decisions in Health Care: Implications from Decision Making Research.” Millbank Quarterly 75 (3): 395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhauser, M. 1998. “Why Patients Have Little Patience for Report Cards.” Managed Care 7 (3): 31–2. [PubMed] [Google Scholar]
- Ketelaar, N. A. , Faber M. J., Westert G. P., Elwyn G., and Braspenning J. C.. 2014. “Exploring Consumer Values of Comparative Performance Information for Hospital Choice.” Quality of Primary Care 22 (2): 81–9. [PubMed] [Google Scholar]
- Lancaster, K. 1966. “A New Approach to Consumer Theory.” Journal of Political Economy 74 (2): 132–57. [Google Scholar]
- Lancsar, E. , and Louviere J.. 2008. “Conducting Discrete Choice Experiments to Inform Healthcare Decision Making: A User's Guide.” Pharmaco Economics 26 (8): 661–77. [DOI] [PubMed] [Google Scholar]
- Light, D. W. , and Hughes D.. 2001. “Introduction: A Sociological Perspective on Rationing: Power, Rhetoric and Situated Practices.” Sociology of Health and Illness 23: 551–69. [Google Scholar]
- Linder‐Pelz, S. U. 1982. “Toward a Theory of Patient Satisfaction.” Social Science & Medicine 16 (5): 577–82. [DOI] [PubMed] [Google Scholar]
- Lux, M. P. , Fasching P. A., Schrauder M., Lohberg C., Thiel F., Bani M. R., Hildebrandt T., Grun A. H., Beckmann M. W., and Goecke T. W. 2011. “The Era of Centers: The Influence of Establishing Specialized Centers on Patients' Choice of Hospital.” Archives of Gynecology and Obstetrics 283: 559–68. [DOI] [PubMed] [Google Scholar]
- Marang‐Van‐De Mheen, P. J. , Dijs‐Elsinga J., Otten W., Versluijs M., Smeets H. J., Van der Made W. J., Vree R., and Kievit J.. 2010. “The Importance of Experienced Adverse Outcomes on Patients' Future Choice of a Hospital for Surgery.” Quality and Safety in Health Care 19: 1–6. [DOI] [PubMed] [Google Scholar]
- Marshall, M. N. , Hiscock J., and Sibbald B.. 2002. “Attitudes to the Public Release of Comparative Information on the Quality of General Practice Care: Qualitative Study.” British Medical Journal 325 (7375): 1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, M. N. , Shekelle P. G., Leatherman S., and Brook R. H.. 2000. “The Public Release of Performance Data: What Do We Expect to Gain? A Review of the Evidence.” Journal of the American Medical Association 283 (14): 1866–74. [DOI] [PubMed] [Google Scholar]
- Max, W. , Webber P., and Fox P.. 1995. “Alzheimer's Disease. The Unpaid Burden of Caring.” Journal of Aging and Health 7 (2): 179–99. [DOI] [PubMed] [Google Scholar]
- McDonald, R. , Mead N., Cheragi‐Sohi S., Bower P., Whalley D., and Roland M.. 2007. “Governing the Ethical Consumer: Identity, Choice, and the Primary Care Medical Encounter.” Sociology of Health and Illness 29 (3): 430–56. [DOI] [PubMed] [Google Scholar]
- McFadden, D. 1974. Conditional Logit Analysis of Qualitative Choice Behavior. New York: New York Academic Press. [Google Scholar]
- Ministry of Health Welfare and Sports . 2002. Choosing with Care. The Equipping of Patients and Consumers in a Demand Driven Care Sector. The Netherlands: The Hague. [Google Scholar]
- NIZW . 2005. [accessed on October 20, 2014]. Available at http://www.rivm.nl/vtv/object_binary/o3057_Factsheet%20Mantelzorg%20en%20dementie%20NIZW%202005.pdf
- Patton, M. Q. 2002. Qualitative Research & Evaluation Methods. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- RIVM . 2010. [accessed on October 20, 2014]. Available at http://www.rivm.nl/vtv/object_document/o1275n17537.html (Prevalence Depression) and http://www.rivm.nl/vtv/object_document/o1778n18371.html (Prevalence Knee Arthrosis)
- Ryan, M. 2004. “Discrete Choice Experiments in Health Care. NICE Should Consider Using Them for Patient Centred Evaluations of Technologies.” British Medical Journal 328: 360–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, M. , and Gerard K.. 2003. “Using Discrete Choice Experiments to Value Health Care Programmes: Current Practice and Future Research Reflections.” Applied Health Economics and Health Policy 2 (1): 55–64. [PubMed] [Google Scholar]
- Ryan, M. , Gerard K., and Amaya‐Amaya M.. 2008. Using Discrete Choice Experiments to Value Health and Health Care. The Economics of Non‐Market Goods and Resources. Dordrecht, The Netherlands: Springer. [Google Scholar]
- Salisbury, C. J. 1989. “How Do People Choose Their Doctor?” British Medical Journal 299 (6699): 608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, E. C. , and Lieberman T.. 2001. “Publicly Disclosed Information about the Quality of Health Care: Response of the US Public.” Quality in Health Care 10 (2): 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, L. , Woloshin S., and Birkmeyer J.. 2005. “How Do Elderly Patients Decide Where to Go for Major Surgery? Telephone Interview Survey” British Medical Journal 331: 821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliani, L. , and Martin S.. 2007. “An Empirical Analysis of the Impact of Choice on Waiting Times.” Health Economics 16: 763–79. [DOI] [PubMed] [Google Scholar]
- Sloane, N. J. A . 2010. “A Library of Orthogonal Arrays” [accessed on October 20, 2014]. Available at http://neilsloane.com/oadir/
- Steer, P. J. 2006. “So What's So New about Patient Choice?” British Medical Journal 332 (7547): 981. [Google Scholar]
- Street, D. , Burgess L., and Louviere J. J.. 2005. “Quick and Easy Choice Sets: Constructing Optimal and Nearly Optimal Stated Choice Experiments.” International Journal of Research in Marketing 22: 459–70. [Google Scholar]
- Thomson, S. , and Dixon A.. 2006. “Choices in Health Care: The European Exp.” Journal of Health Services Research & Policy 11 (3): 167–71. [DOI] [PubMed] [Google Scholar]
- Ubach, C. , Scott A., French F., Awramenko M., and Needham G.. 2003. “What Do Hospital Consultants Value about Their Jobs? A Discrete Choice Experiment.” British Medical Journal 326 (7404): 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkevisser, M. , van der Geest S., and Schut F.. 2009. “Quality Competition in Regulated Hospital Markets: Consumer Information and Patient Choice for Angioplasty” In Patient Choice, Competition and Antitrust Enforcement in Dutch Hospital Markets,, edited by Varkevisser M., pp. 117–49. Rotterdam: Erasmus Universiteit. [Google Scholar]
- Victoor, A. , Delnoij D. M., Friele R. D., and Rademakers J. J.. 2012a. “Determinants of Patient Choice of Healthcare Providers: A Scoping Review.” BMC Health Services Research 12: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoor, A. , Friele R. D., Delnoij D. M., and Rademakers J. J.. 2012b. “Free Choice of Healthcare Providers in The Netherlands is Both a Goal in Itself and a Precondition: Modelling the Policy Assumptions Underlying the Promotion of Patient Choice through Documentary Analysis and Interviews.” BMC Health Services Research 12 (1): 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, S. , and Stenner P.. 2005. “Doing Q Methodology: Theory, Method and Interpretation.” Qualitative Research in Psychology 2: 67–91. [Google Scholar]
- Wensing, M. 2000. “Evidence‐Based Patient Empowerment.” Quality in Health Care 9 (4): 200–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijnenberg, N. C. , Hendriks M., Damman O. C., Bloemendal E., Wendel S., de Jong J. D., and Rademakers J.. 2012. “Understanding and Using Comparative Healthcare Information; The Effect of the Amount of Information and Consumer Characteristics and Skills.” BMC Medical Informatics and Decision Making 12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


