Figure 3.
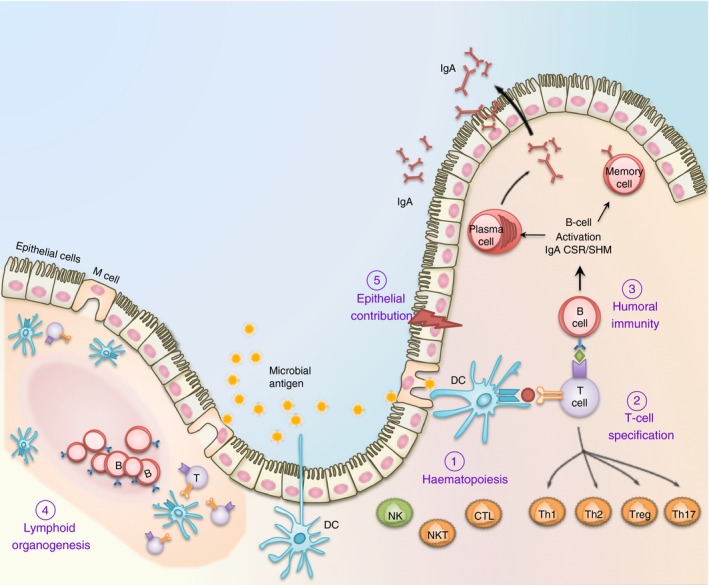
The multifaceted contribution of RUNX in mucosal immunity. (1) Runx proteins are essential for the differentiation and effector functions of diverse cell lineages that participate in the mucosal immune system. These include T cells, B cells and dendritic cells (DC) for adaptive immunity; as well as macrophages, natural killer (NK) cells and epithelial cells for innate immunity. (2) Runx1 and Runx3 are critically involved in the specification of CD8+ cytotoxic T (Tc) and naive CD4+ helper T (Th) lymphocytes before their entrance into the periphery. Following antigen engagement and T cell receptor (TCR) activation, Runx1/3 coordinate the terminal differentiation of CD4+ T cells into Th1, Th2, Th17 and Treg cells, and CD8+ T cells into cytotoxic T lymphocyte (CTL) effector T‐cell lineages. (3) In B lymphocytes, Runx proteins play a crucial role in transforming growth factor‐β (TGF‐β) ‐mediated IgA class switching following antigen engagement. Runx proteins are also necessary for the maximal surface expression of IgA on activated B lymphoblasts and the maintenance of specialized memory B (Bmem) cells distributed at the mucosa. (4) The Runx complex is required for the formation of anlagen that initiate Peyer's patches and peripheral lymph node biogenesis. These secondary lymphoid tissues are home to naive peripheral lymphocytes and dendritic cells and are necessary for maintaining surveillance and homeostasis. (5) The mucosal epithelium is in direct contact with the microbial load and functions as a physical as well as an immune barrier. RUNX proteins are functionally important in a diverse range of mucosal epithelial cells, including those in the lung and gastrointestinal tract. RUNX3 is necessary for the homeostasis of an intact mucosal epithelium by regulating the proliferation and apoptosis of epithelial cells.1 RUNX proteins may further contribute to innate immunity within the epithelium by regulating cytokine production (such as IL23A) during infection and inflammation.129
