Summary
Systemic lupus erythematosus (SLE) is a heterogeneous disease in which excessive inflammation, autoantibodies and complement activation lead to multisystem tissue damage. The contribution of the individual genetic composition has been extensively studied, and several susceptibility genes related to immune pathways that participate in SLE pathogenesis have been identified. It has been proposed that SLE takes place when susceptibility factors interact with environmental stimuli leading to a deregulated immune response. Experimental evidence suggests that such events are related to the failure of T‐cell and B‐cell suppression mediated by defects in cell signalling, immune tolerance and apoptotic mechanism promoting autoimmunity. In addition, it has been reported that dendritic cells (DCs) from SLE patients, which are crucial in the modulation of peripheral tolerance to self‐antigens, show an increased ratio of activating/inhibitory receptors on their surfaces. This phenotype and an augmented expression of co‐stimulatory molecules is thought to be critical for disease pathogenesis. Accordingly, tolerogenic DCs can be a potential strategy for developing antigen‐specific therapies to reduce detrimental inflammation without causing systemic immunosuppression. In this review article we discuss the most relevant data relative to the contribution of DCs to the triggering of SLE.
Keywords: dendritic cells, immune tolerance, immunotherapy, lupus, systemic autoimmunity
Abbreviations
- ANA
anti‐nuclear antibodies
- APCs
antigen‐presenting cells
- cDCs
conventional dendritic cells
- DCs
dendritic cells
- EAE
experimental autoimmune encephalomyelitis
- IC
immune complex
- IFN
interferon
- IL
interleukin
- IRF
interferon regulatory factor
- PD‐1
programmed death 1
- pDCs
plasmacytoid dendritic cells
- SLAM
signalling lymphocyte activation molecule
- SLE
systemic lupus erythematosus
- Th
T helper
- TLRs
Toll‐like receptors
- tolDC
tolerogenic dendritic cells
- Treg
regulatory T
Introduction
Immunological tolerance is crucial for the development and maintenance of functional and non‐harmful T and B cells.1 Although the aetiology of autoimmune diseases is unknown and may involve several factors, it can be initiated when specific gene products interact with environmental stimuli, resulting in a deregulated immune response.2, 3 Alteration in T‐cell and B‐cell signalling, immune tolerance and clearance of apoptotic cells may result in immune failure, leading to autoimmunity in susceptible individuals.2 Although physiological expression of inhibitory as well as activating receptors is fundamental for antigen‐presenting cells (APCs) to trigger a protective immune response or to promote immune tolerance, the precise molecular mechanisms responsible for peripheral T‐cell tolerance remain to be elucidated.4
Systemic lupus erythematosus (SLE) is a chronic and heterogeneous disease that majorly affects joints, kidneys, nervous systems, skin and mucosa, in which innate and adaptive immune cells are involved.5, 6, 7 It has been shown that dendritic cells (DCs) from patients with SLE exhibit an altered expression of CD40, CD86 and Fcγ receptors (FcγRs) compared with healthy controls.6 Similarly, it has been found that the anti‐inflammatory enzyme haemeoxygenase‐1 is less expressed in monocytes from SLE compared with healthy controls, suggesting that phenotypic alterations in APCs may impact T‐cell homeostasis, resulting in immune‐mediated diseases.6, 8 Recent advances in the modulation of DCs in vitro has increased the potential of cell‐based approaches for autoimmune disease treatment, in which immunotherapy is restricted to autoantigens involved in tissue damage.9, 10 Several autoimmune diseases show an imbalance in the homeostasis of DC subpopulations. Indeed, there is increasing evidence supporting the notion that DCs may play a key role in SLE pathogenesis.11 Herein, we discuss recent data concerning how phenotypic alterations in DCs may drive SLE.
DC phenotype in SLE patients
The diversity of DC function is the result of their complex differentiation and maturation mechanisms, which lead to the generation of specialized DCs that conform to wide subsets of different subpopulations.12 In addition, several findings related to a more immunogenic phenotype have been reported in DCs from SLE patients.6, 13 It was first reported the 1980s that patients with SLE had increased levels of serum interferon‐α (IFN‐α), which correlates with disease activity and anti‐dsDNA antibody titres.14, 15 A consistent finding is that the majority of patients with SLE show an increased expression of IFN‐α‐stimulated genes, which is known as IFN‐α signature.16
It has been reported that plasmacytoid DCs (pDCs) from patients with SLE show an enhanced expression of interferon regulatory factor 3 (IRF3) and IRF5 compared with healthy controls, which is associated with higher circulating levels of IFN‐α 13 (Fig. 1). Signalling lymphocyte activation molecule (SLAM) forms a family of receptors expressed by several immune cells and some polymorphisms in their locus have been associated with SLE diseases.17, 18 Interestingly, the expression of CD319 and CD48 (SLAM family receptors) is regulated by RNA containing immune complexes (ICs) and is diminished in pDCs from patients with SLE compared with healthy controls19 (Fig. 1). In addition, DCs from patients with active SLE show an altered homeostasis of the inducible programmed death ligand‐1 (PD‐L1), which plays a crucial role in immune tolerance and T‐cell suppression when binding to its ligand programmed death‐1 (PD‐1).20 Dendritic cells from patients with active SLE failed to induced PD‐L1 expression in vitro.20 Strikingly, DCs from SLE patients show a remarkable decrease of PD‐L1 expression during disease flares, whereas the expression of CD80/CD86 is increased. In contrast, DCs from SLE patients during remission show greater expression of PD‐L120 (Fig. 1).
Figure 1.
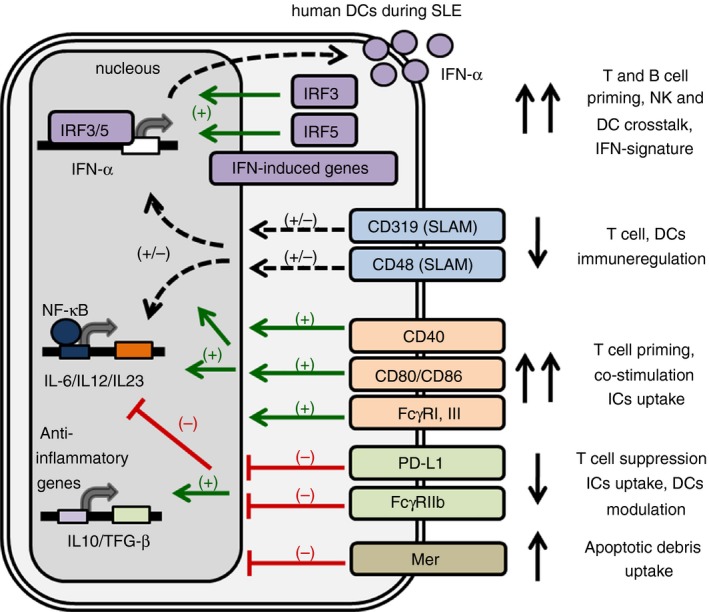
Human dendritic cell (DC) phenotype during systemic lupus erythematosus (SLE). DC modulation is mediated by different signals that may lead to DC activation and initiation of the immune response, or may lead to immunosuppression. DCs from SLE patients showed an increased expression of interferon‐α (IFN‐α), IFN regulatory factor 3 (IRF3), IRF5 and IFN‐induced genes, which correspond to the IFN signature. Similarly, SLE DCs show an increased expression of co‐stimulatory molecules, such as CD40, CD86 and activating FcγRs, showing a mature phenotype. In contrast, the expression of immunoregulatory molecules such as SLAM (CD319, CD48), the co‐inhibitor molecule PD‐L1 and the inhibitory FcγR are decreased in SLE. This mature‐type phenotype of DCs with higher expression of activating (and lower expression of inhibitory) signals may promote naive T‐cell priming and B‐cell activation, favouring a deregulated T helper type differentiation and antibody production.
Interestingly Mer, a surface immunoregulatory receptor involved in apoptotic cell recognition and removal, is increased in DCs from SLE patients.21 This phenotype correlates with serum levels of IFN‐α.22 Furthermore, prednisone‐treated SLE patients show higher expression of Mer in DCs than do patients that did not receive steroids, suggesting a role for Mer in inducing a tolerogenic response by DCs in the presence of apoptotic antigens22 (Fig. 1).
It has also been reported that patients with SLE show reduced numbers of conventional DCs (cDCs) in their bloodstream compared with healthy controls whereas pDCs were increased.23, 24 Both, cDCs and pDCs can sense pathogen‐associated molecular patterns by pathogen recognition receptors, such as Toll‐like receptor 7 (TLR7) and TLR9, contributing to protective immunity against viral and bacterial infections.25, 26, 27 TLR7 and TLR9 ligands (such as Imiquimod and CpG) induce the production of large amounts of type I IFN by pDCs via the IRF signalling pathway.28, 29 The fact that TLR7 ligand stimulation of pDCs induces high expression of interleukin‐1β (IL‐1β) and IL‐23, leading to a T helper 17 (Th17) differentiation, highlights the potential role of pDCs at modulating immunity and tolerance.27 In contrast, some studies suggest that DCs from patients with SLE produce fewer proinflammatory cytokines in response to CpG compared with DCs from healthy controls.30
Interestingly, it has been reported that serum from SLE patients induces monocytes from healthy controls to differentiate into DCs in a type I IFN‐dependent manner. Furthermore, authors demonstrated that IFN‐α and serum from patients with SLE induce the expression of CCR7 in monocytes from healthy subjects, suggesting that SLE serum may prime monocytes to migrate to lymphatic nodes such as DCs.31
We have shown that DCs from SLE patients show a higher expression of co‐stimulatory molecules such as CD40 and CD86, as well as an altered ratio of activating/inhibitory FcγRs compared with healthy controls, which may impact T‐cell priming leading to SLE6 (Fig. 1). However, some studies demonstrate that CD40 expression on SLE patient DCs can be decreased.30 All these data from human studies underscore the role of DCs in SLE pathogenesis and support the strategy of designing new therapies based on DC modulation and depletion.
Murine models of SLE to identify the role of DCs and IFN‐α in disease onset
Several murine strains have been reported to study the immunopathogenesis of SLE. These murine models of SLE include the F1 hybrid between the New Zealand Black (NZB) and New Zealand White (NZW) strains (NZB/W F1), the MRL.Faslpr, FcγRIIb knockout and BXSB/Yaa (TLR7 gene duplication) and the strains in which autoimmunity develops from polygenic factors, apoptosis failure, inhibitory receptor deficiency and gene duplication.32, 33, 34, 35
For instance, murine models have been key to determine the role of Blimp‐1 in SLE pathogenesis. In both murine models and humans, genome‐wide studies have determined that a polymorphism of Blimp‐1 can be associated with SLE susceptibility.36, 37 Plasmacytoid DCs stimulated with IFN‐α induced the expression of miRNAs that regulate Blimp‐1, suggesting that this mediator may be involved in SLE pathogenesis.38 Female mice lacking Blimp‐1 on DCs show an expansion of follicular helper T cells with an enhanced germinal center response and the development of anti‐nuclear antibodies (ANAs) and an SLE‐like syndrome, which is dependent on the production of IL‐6.36
Also, the relevant role of DCs in driving SLE pathogenesis is highlighted by the fact that DCs loaded with apoptotic cells could initiate an autoreactive immune response with the development of SLE‐like symptoms, such as ANAs and glomerulonephritis.39, 40, 41 Interestingly, IFN‐α can reduce the suppressive effect of apoptotic cells on DCs, promoting SLE pathogenesis through DC activation.42 In addition, the administration of adenovirus expressing IFN‐α to SLE NZB/NZW mice accelerated disease onset, increased serum levels of anti‐dsDNA antibodies being associated with increased B‐cell activating factor, IL‐6 and tumour necrosis factor‐α serum levels.43
In contrast, deleting DCs in SLE‐prone MRL.Faslpr mice ameliorates disease progression, and decreases inflammation and glomerulonephritis, highlighting the essential role of DCs in SLE pathogenesis and autoantibody development.11 Similarly, a transient ablation of pDCs in the BXSB/Yaa SLE mice ameliorates disease, and reduces lymphoproliferation and ANA development, which correlates with lower IFN‐α/β‐induced gene expression, suggesting a necessary role of DCs in SLE onset and progression.44 In addition, in NZB/W F1 mice the subpopulation of DCs that produce IFN‐α shows an altered phenotype, consisting of a higher production of IL‐12 and expression of TLR9 mRNA.45
Dendritic cells from B6.NZMSle1/Sle2/Sle3 (a lupus murine model derived from NZB/W F1) SLE‐prone mice show an overexpression of IFN‐responsive genes (IFN‐β and CXCL10) and members of the IFN signalling pathway (signal transducer and activator of transcription 1 and 2, and IRF7) that hyper‐respond to IFN‐α and TLR7‐TLR9 ligands.46 Similarly, DCs also show an IFN signature in vivo before SLE onset, suggesting that DCs may have a crucial role in SLE pathogenesis.46 In contrast, chronic administration of anti‐IFN‐α/β receptor antibodies to male BXSB/Yaa mice reduces autoimmunity, suggesting that IFN signalling is crucial for SLE pathology.47
Role of pathogen recognition receptors and immune complexes in SLE pathogenesis
Activation of TLRs could be triggered by either endogenous or foreign molecules, such as danger‐associated molecular patterns and pathogen‐associated molecular patterns, respectively. Some of most studied danger‐associated molecular patterns are mRNA, ssRNA, high mobility group box protein 1 (HMGB1), heat‐shock protein 60 (HSP 60), fibronectin, fibrinogen, hyaluronic acid fragments and chromatin.48, 49, 50 Plasmacytoid DCs activated by CpG suppress the function of regulatory T (Treg) cells, induce inflammatory cytokines (IL‐6, transforming growth factor‐β and IFN‐α) and promote Th17 polarization.51, 52, 53, 54, 55, 56 Danger‐associated molecular patterns binding to TLR initiate an innate immune response that contributes to tissue damage, as observed when circulating ICs containing self nucleic acids stimulate DCs and promote cell activation and tissue injury in SLE.57 Strikingly, HMGB1 bound to circulating nucleosome‐containing ICs triggers TLR2 on APCs and induces pathogenic anti‐dsDNA in SLE49 (Fig. 2). It has been shown that ICs containing nucleosomes derived from the plasma of patients with SLE carry HMGB1 tightly attached to the chromatin of apoptotic cells.58, 59 These HMGB1‐loaded ICs induce the secretion of IL‐1β, IL‐6, IL‐10 and tumour necrosis factor‐α and the expression of co‐stimulatory molecules in DCs via TLR258 (Fig. 2).
Figure 2.
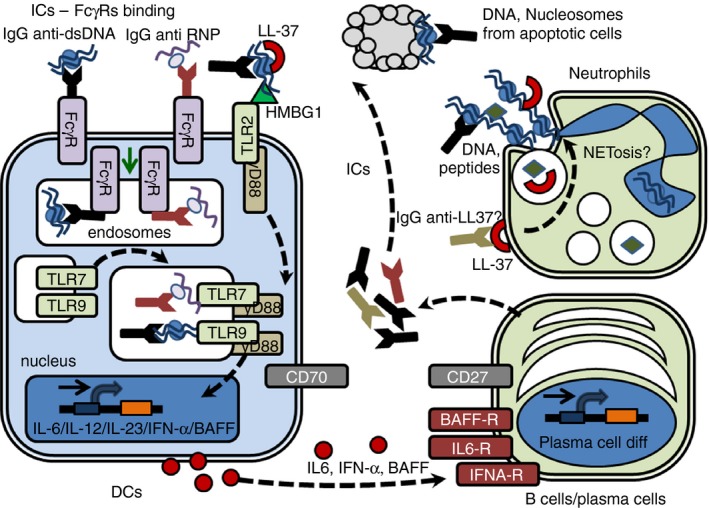
Hypothetical circuits and interactions between dendritic cells (DCs), neutrophils and B cells in systemic lupus erythematosus (SLE). Hypothetical circuits, interactions and mechanism between DCs, neutrophils, B cells and the stimulatory effects of nucleic acid containing immune complexes (ICs). Apoptotic antigens are recognized by autoantibodies present in SLE‐susceptible hosts. ICs containing self‐DNA or ‐RNA are internalized by FcγRs targeting Toll‐like receptor 9/7 (TLR9/7) expressing endosomes, a process enhanced by the presence of LL‐37. Extracellular ICs containing self‐DNA or ‐RNA need to be transported into TLR7/9‐containing endosomes to fully activate DCs. TLR9/7 ligation promotes DC activation with production of pro‐inflammatory cytokines, such as interferon‐α (IFN‐α), interleukin‐6 (IL‐6), IL‐12 and IL‐23, which may interact with B cells inducing cellular activation, plasma cell differentiation and antibody production. Also, ICs containing HMGB1 activate DCs by a TLR2‐dependent mechanism. The recognition of autoantibodies against neutrophil peptides (LL‐37) exposed on the cell surface may trigger neutrophil extracellular trap (NET)‐osis with the subsequent releasing of nuclear antigens and new ICs formation when interacting with anti‐nuclear antibodies produced by stimulated plasma cells.
It has also been reported that nucleosomes induce neutrophil activation, IL‐8 secretion and increased phagocytic activity independent of IC formation.60 Neutrophils from patients with SLE are sensitive to nucleosome‐induced activation, suggesting that nucleosomes may link innate immunity with loss of peripheral tolerance during SLE pathogenesis.60
Similarly, the activation of autoreactive B cells is mediated by DNA‐containing ICs upon ligation of both B‐cell receptor and TLR9, enhancing the immune response and promoting the development of SLE.50 To be recognized by TLR9, DNA‐ICs need to be taken up by cells through the endosome pathway and translocated to TLR9‐expressing vesicles, a process that might be mediated by FcγRIIa. Subsequently, DNA‐containing ICs are able to activate TLR9 in pDCs, leading to the production of IFN‐α 61 (Fig. 2). Although TLR9 promotes pDC activation and anti‐DNA autoantibody development in lupus mouse models, major features of lupus disease such as hypergammaglobulinaemia and glomerulonephritis are also observed in TLR9‐deficient mice.62, 63 Remarkably, the critical role of TLR9 in the development of anti‐DNA antibodies is supported by the fact that targeting TLR9 in different SLE‐prone mice decreases autoreactivity.64, 65 Similarly, TLR7 expression is necessary for the development of anti‐Smith (Sm) autoantibodies in the MRL. Faslpr mouse model, which is consistent with the presence of anti‐Sm antibodies in SLE patients63, 66 (Fig. 2). These mechanisms were corroborated in the MyD88‐deficient mice, in which anti‐DNA and anti‐RNA antibodies were absent, suggesting that TLRs could be involved in this process.67, 68
Recently, a new mechanism for IC pathogenesis has been described. The antimicrobial peptide LL‐37, a cathelicidin polypeptide, binds self‐DNA and directs this molecule to TLR9‐containing endosomes in DCs.69 TLR9 ligation leads to DC activation, IFN‐α production and possibly IC‐dependent pathogenesis during SLE and systemic autoimmune disease.69, 70, 71, 72
Hydroxychloroquine (HCQ), which has been demonstrated to decrease SLE flares and mortality, is able to prevent pDC activation and IFN‐α production by limiting acidification and maturation of endosomes after stimulation with ligands for TLR7 and TLR9.73, 74 HCQ may inhibit TLR ligation of internalized self‐DNA/RNA ICs.61 New compounds are being studied to antagonize TLR7 and TLR9 signalling by IC containing DNA/RNA, which accumulate in TLR‐expressing endosomes and decrease the affinity of nucleic acid to TLR7 and TLR9.75, 76 Interestingly, when female MRL.Faslpr mice were treated with the drug E6446, a TLR9 antagonist, development of ANAs was suppressed in a dose‐dependent manner.75
Immune cells, including DCs, differentially express several FcγRs, such as FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa and FcγRIIIb in humans, and FcγRI, FcγRIIb, FcγRIII and FcγRIV in mice.77, 78, 79 In both SLE patients and murine models of this disease, ICs binding to FcγRs on DCs induce migration to lymph nodes, up‐regulating CCR7 expression, which is essential in this process to expand the immune response to self‐antigens captured by DCs.80 Furthermore, absence of the inhibitory receptor FcγRIIb enhanced this process, suggesting that fine tuning between activating/inhibitory signalling from different FcγRs and co‐stimulatory molecules is crucial for keeping the homeostasis of the immune system.80, 81
As previously mentioned, DNA/RNA containing ICs lead to DC/B‐cell activation and might initiate SLE.61, 82, 83 The pDCs are activated through TLR signalling by ICs from SLE patients containing IgG and DNA/RNA after FcγRIIa‐mediated internalization. In contrast, IgGs from healthy controls do not induce TLR‐mediated activation of DCs.61, 83, 84
Similarly, ICs binding to FcγRI and FcγRIII (activating receptors) on DCs leads to maturation. In contrast, engagement of FcγRIIb, an inhibitory receptor, keeps DCs in an immature state.85, 86, 87 The inhibitory FcγRIIb is expressed by innate and adaptive immune cells including monocytes, DCs and B cells.88 FcγRIIb deficiency in DCs improves T‐cell priming and leads to protective tumour immunity.87 Interestingly, FγRIIb knockout mice develop spontaneous SLE‐like disease with production of anti‐DNA antibodies, IC deposition in kidneys and glomerulonephritis, as well as increased susceptibility to other autoimmune diseases, such as collagen‐induced arthritis and experimental autoimmune encephalomyelitis (EAE).89 In contrast, FcγRIII knockout mice are more resistant to EAE induction than WT mice.81
As discussed above, cDCs from SLE patients show a higher ratio of activating/inhibitory FcγRs expression, suggesting that these cells are more susceptible to being activated by ICs.6 Furthermore, the increased ratio of activating/inhibitory FcγRs in DCs correlates with the activity of SLE6 (Fig. 1).
The pentraxin C‐reactive protein is a serum molecule that binds phosphorilcolin on apoptotic cells.90 Interestingly, C‐reactive protein can prevent DC activation and IFN‐α production after stimulation with ICs containing anti‐U1 RNP‐snRNP and anti‐DNA‐DNA.91 In addition, C‐reactive protein may modify intracellular trafficking of endocytosed self‐antigens to prevent TLR engagement in DCs.91
In addition, it has been reported that sera from SLE patients show ICs containing neutrophil extracellular trap components, such as antimicrobial peptides and self‐DNA that could promote pDC activation.92 Interestingly, the production of neutrophil extracellular traps by SLE neutrophils is higher than in healthy donors, suggesting that this mechanism enhances DC immunogenicity.93 The underlying mechanism could be mediated in the production of anti‐LL37 autoantibodies that bind surface‐exposed specific ligands and trigger the release of neutrophil extracellular traps93 (Fig. 2).
The selective engagement of inhibitory FcγRs with ICs containing immunodominant self‐antigens to modulate DC function may restore antigen‐specific tolerance in immune‐mediated and autoimmune diseases.94
Control of DC immunogenicity by co‐stimulatory molecules
In autoimmune susceptible individuals, central and/or peripheral tolerance could be experimentally overcome by an altered APC function that primes self‐reactive T cells.1 The expression of a wide spectrum of co‐stimulatory molecules by DCs constitutes a major aspect in T‐cell activation.95, 96 CD28 (T cells)/CD80/CD86 (DCs) interaction is crucial for clonal expansion and survival of antigen‐specific T cells by promoting optimal mRNA stabilization and IL‐2 production.97, 98 As previously mentioned, our group and others have shown that cDCs obtained from SLE patients show greater expression of co‐stimulatory molecules (such as CD86 and CD40) than healthy controls.99, 100 These data suggest a more immunogenic state for these APCs and an increased capacity to prime T cells.6
Similarly, activating signals from the interaction between CD40L and CD40 are important for T‐cell/B‐cell cooperation and T‐cell/DC crosstalk with subsequent cellular activation.101 The relevance of this interaction is highlighted by the fact that pharmacological blockade with anti‐CD40L monoclonal antibodies ameliorates autoimmune diseases.102, 103 OX40 ligand (OX40L) is an inducible co‐stimulatory molecule expressed on APCs and non‐immune cells that binds to OX40 on T cells and promotes cellular survival as well as Th2 polarization.104, 105 Similarly as observed with CD40/CD40L interaction, a deficiency (or blockade) of OX40/OX40L delays the onset of autoimmune symptoms by inhibiting the expression of the effector cytokines IFN‐γ and IL‐4.106 Dendritic cells also express ICOS‐L (B7‐H2), which binds to ICOS on T cells, partially modulating T‐cell fate.107, 108 The blockade of ICOS/ICOS‐L interaction limits the production of IL‐10 release without affecting IL‐2 secretion, suggesting an active role in immune regulation and peripheral tolerance.109 Interestingly a Treg cell subpopulation expresses ICOS, suggesting that this molecule is also involved in Treg–DC crosstalk and immune suppression mediated by IL‐10.110, 111 ICOS‐L deficiency can impair Th2 bias, mainly limiting IL‐4 and IL‐10 production by T cells.112
Flt3L, a growth factor involved in development and differentiation of DCs, has also been associated with autoimmune pathogenesis, such as rheumatoid arthritis. In this disease, Flt3L is increased in synovial fluid and correlated with active lesions.113, 114, 115, 116
DC modulation by co‐inhibitory molecules
The most studied interaction between co‐inhibitors on T cells and DCs is the PD‐1/PD‐L1 (and PD‐L2) axis.117, 118, 119 PD‐L1 expressed on DCs drives T‐cell inhibition.120, 121 In addition, Treg cell development is also enhanced by PD‐L1 expression on DCs, which may prevent autoimmunity as observed with PD‐1+ Treg cells during EAE.117, 122 Similarly, PD‐1/PD‐L1 interaction can suppress Th1 differentiation during EAE.123 Interestingly, PD‐1/PD‐L1 deficiency in mice promotes IFN‐γ overproduction by T cells and the activation of CD8+ T‐cell responses, making these animals more susceptible to autoimmunity and SLE‐like disease.124, 125 TIM‐3, an exhausted cell marker, has been reported to display an inhibitory function on DCs that are resistant to maturation.126, 127 TIM‐3 deficiency in DCs leads to overproduction of pro‐inflammatory cytokines after stimulation with TLR ligands.126
Because no single DC/T‐cell interaction is fully immunogenic or tolerogenic, further research is needed to understand the activation/inhibitory signalling network controlling DC immunogenicity. Strategies based on interfering DC/T‐cell interactions are of clinical interest mainly in autoimmune diseases, where the immune response is deregulated.
DC interaction with B cells and Treg cells during SLE pathogenesis
Dendritic cells could also interact with the main factor responsible for tissue damage during SLE pathogenesis: B cells.5 The DCs and macrophages efficiently transfer conformational antigens such as particulates and ICs and present them to naive B cells in lymphoid organs.128 In addition, it has been shown that DCs induced surface IgA expression on B cells, which is enhanced by IL‐10 and transforming growth factor‐β, suggesting that DCs directly modulate B‐cell activation and differentiation.129 Similarly, activated DCs in SLE‐prone mice enhanced B‐cell proliferation, IL‐6 and IFN‐γ production and ANA production.130, 131 Moreover, SLE DCs induce chemokine receptor expression on B cells that target them to initiate germinal centre responses with subsequent IgG production.130 The pDCs can induce plasma cell differentiation and promote antibody secretion through type 1 IFN and CD70.132 Also, activated DCs produce BLyS and APRIL, which are key mediators in B‐cell homeostasis and may induce immunoglobulin class‐switch DNA recombination.133
Recently, it has been reported that a subset of splenic regulatory CD11bhi Ialow DCs induce B‐cell differentiation into an IL‐10 producing regulatory CD19hi FcγIIbhi B cells, which could prevent T‐cell response via IL‐10.134 These data highlight the potential of tolerogenic DC (tolDC) ‐based therapy to promote immunesuppression in immunoglobulin‐mediated diseases such as SLE.
It has been reported that activated DCs impair Treg function.135 TLR ligand stimulation of DCs prevents Treg cell suppression, leading to T‐cell priming by an IL‐6‐dependent mechanism.135, 136 During SLE, elevated cytokines such as IFN‐α, IL‐6 and IL‐18 might affect the capacity of Treg cells to modulate DC immunogenicity, promoting T‐cell proliferation.137, 138, 139 Accordingly, pharmacological blockade of IL‐6 by monoclonal antibodies in experimental SLE mouse models improves clinical symptoms, highlighting the role of this pro‐inflammatory cytokine in DC interaction with B cells and Treg cells during systemic autoimmunity.140
Targeting DC function as a therapeutic approach for autoimmune diseases
Pharmacological targeting of DCs to restore tolerance by either Treg cell promotion or phenotype skewing of autoantigen‐specific T‐cell responses has been studied.141 For T‐cell tolerance induction, DCs might exhibit low expression of different surface molecules involved in T‐cell priming, such as MHC‐II, CD40, CD80, CD86, and a reduced production of pro‐inflammatory cytokines while promoting the secretion of the anti‐inflammatory cytokine IL‐10.142, 143 The generation of tolDCs could be assessed by DC modulation with chemicals, biological agents and gene therapy, mainly targeting the maturation capacity.143, 144
Dexamethasone is one of the most studied inhibitors of DC maturation. Dexamethasone‐treated DCs show a semi‐mature phenotype with low expression of co‐stimulatory molecules, such as MHC‐II and CD86; are resistant to maturation (keeping IL‐10 production unaffected); induce Treg cell differentiation; suppress T‐cell priming; and modulate nuclear factor‐κB signalling.10, 143, 145 Similarly, 1α,25‐dihydroxyvitamin D3 and acetylsalicylic acid (aspirin) induce tolDCs with low co‐stimulatory molecule expression and resistant to maturation.10, 146 However, due to the capacity of dexamethasone and 1α,25‐dihydroxyvitamin D3 to induce cell death that could cause immune suppression, further research is required to confirm a direct role of these drugs in DC tolerance induction.147, 148, 149
Rapamycin‐ or rosiglitazone‐treated DCs show a tolerogenic phenotype that induces Treg cell differentiation and prevents pro‐inflammatory cytokine production.150 Dendritic cells treated with BAY‐117085 and andrographolide, two nuclear factor‐κB blockers, show a tolerogenic phenotype inducing Treg cell expansion and ameliorating autoimmune diseases.151, 152 Our group has reported that the chronic administration of andrographolide and rosiglitazone to FcγRIIb knockout mice prevents SLE onset. Splenic DCs from treated mice show lower CD40/CD86 expression, ANA levels and IC deposition in kidneys than untreated mice.153 Cobalt protoporphyrin promotes a tolerogenic phenotype on DCs by inducing the expression of hemeoxygenase‐1.154
Some biological compounds such as anti‐inflammatory cytokines are potent tolerogenic inducers. DCs treated with IL‐10 show a potent tolerogenic phenotype preventing T‐cell proliferation, decreasing pro‐inflammatory cytokine production, while increasing the expression of immunoglobulin‐like transcript‐2, which is an inhibitory receptor.144, 155, 156, 157 Similarly, transforming growth factor‐β induces tolDCs and prevents the expression of CD80/CD86, IL‐12 production and improved survival of grafted β‐cell islets in a diabetes model.158
Interference RNA and gene therapy technology provide a wide and new spectrum of therapeutic strategies for autoimmune diseases.159 Gene silencing of different pro‐inflammatory molecules, such as CD40, CD80, CD86 and IL‐12 in DCs promotes a tolerogenic phenotype that may improve autoimmune diseases, mainly by suppressing the activation of T and B cells and expanding Treg cell subsets.159, 160, 161 These data underscore the potential use of DC manipulation with lentivirus transduction expressing interference RNA for co‐stimulatory molecules, to induce a tolerogenic phenotype.162, 163
Although there are several studies reporting the generation of tolDCs from patients with multiple sclerosis, which induce hyporesponsiveness in myelin‐specific autologous T cells, to date there are no clinical trials with tolDC therapy in multiple sclerosis.164, 165, 166 Nevertheless, this strategy is a promising cell therapy for the treatment of immune‐mediated diseases.
Targeting DCs in clinical application
Advances in immune intervention with tolDCs, as well as the identification of the immunodominant self‐antigen in SLE, are crucial for designing an efficient and specific therapy based on autologous tolDCs.167 A Phase I randomized placebo‐controlled trial using tolDCs as a therapy for type 1 diabetes, an organ‐specific autoimmune disease, has already been published (ClinicalTrials.gov identifier NCT00445913).168 This approach was based on the administration of tolDCs induced ex vivo by the administration of anti‐sense oligonucleotides for the co‐stimulatory molecules CD40, CD80, and CD86 in patients with type 1 diabetes.168 These researchers reported that the tolDC transfer was well‐tolerated, with no adverse events after 1 year of follow up.168
A Phase I clinical trial in patients with rheumatoid arthritis evaluating the feasibility and safety of autologous tolDC therapy and describing its clinical and immune effects is being conducted. Autologous tolDCs were generated by stimulating DCs with BAY11‐7082, a nuclear factor‐κB inhibitor, and loading DCs with different citrullinated peptides. Only mild adverse effects were reported and, strikingly, disease activity improved in the treated group.169, 170 Another randomized, placebo‐controlled Phase I clinical trial based on tolDC therapy in patients with rheumatoid arthritis is currently recruiting participants [Autologous Tolerogenic Dendritic Cells for RheumatoidArthritis(AutoDECRA); ClinicalTrials.gov Identifier: NCT01352858]. In this study TolDCs from blood monocytes will be generated with dexamethasone and 1α,25‐dihydroxyvitamin D3 and will be administered to affected joints, using arthroscopy.
It has been reported that IL‐10 treatment of DCs from SLE patients may promote a tolerogenic phenotype, supporting the feasibility of using tolDCs in therapeutic clinical trials for patients with SLE.99
Concluding remarks
Development of more efficient therapies for SLE, avoiding systemic immunosuppression, is one of the major goals of rheumatologists. The inhibitory effects of tolDCs in T‐cell priming and B‐cell differentiation underlie the role of DCs in maintaining peripheral tolerance, promoting its potential use in clinical studies. The therapeutic efficacy of a tolDC‐based approach during immune pathogenesis in experimental autoimmune models such as EAE, type 1 diabetes and rheumatoid arthritis highlights the value of testing this strategy in human SLE. The achievement of tolDC intervention in SLE as a therapeutic approach includes specificity avoiding adverse effects from systemic immunosuppression. Identification of the immunodominant self‐antigens involved in SLE pathogenesis, as well as the understanding of DC function are crucial for designing more efficient therapies targeting DCs, which may have a major clinical impact in chronic autoimmune diseases such as SLE.
Disclosures
The authors declare no financial or commercial conflict of interest.
Acknowledgements
The authors are supported by grants FONDECYT no 1110518, FONDECYT no 1070352, FONDECYT no 1085281, FONDECYT no 1100926, FONDECYT no 3070018, FONDECYT no 3100090, FONDECYT no 11075060, FONDECYT no 1100926, FONDECYT no 1110397. CONICYT Capital Humano Avanzado en la Academia no 791100015, Vicerrectoría de Investigación de la Pontificia Universidad Católica de Chile No 04/2010 and Millennium Institute on Immunology and Immunotherapy (No P09/016‐F). AMK is a Chaire De La Région Pays De La Loire De Chercheur Étranger D'excellence and a CDD‐DR INSERM.
References
- 1. Gratz IK, Rosenblum MD, Maurano MM, Paw JS, Truong HA, Marshak‐Rothstein A, Abbas AK. Cutting edge: self‐antigen controls the balance between effector and regulatory T cells in peripheral tissues. J Immunol 2014; 192:1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo X, Yang W, Ye D‐Q et al A functional variant in microRNA‐146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet 2011; 7:e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kariuki SN, Ghodke‐Puranik Y, Dorschner JM et al Genetic analysis of the pathogenic molecular sub‐phenotype interferon‐α identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun 2015; 16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA‐4 engagement. Immunity 1997; 6:411–17. [DOI] [PubMed] [Google Scholar]
- 5. Bernatsky S, Boivin JF, Joseph L et al Mortality in systemic lupus erythematosus. Arthritis Rheum 2006; 54:2550–7. [DOI] [PubMed] [Google Scholar]
- 6. Carreño LJ, Pacheco R, Gutierrez MA, Jacobelli S, Kalergis AM. Disease activity in systemic lupus erythematosus is associated with an altered expression of low‐affinity Fcγ receptors and costimulatory molecules on dendritic cells. Immunology 2009; 128:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaipl US, Munoz LE, Grossmayer G et al Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 2007; 28:114–21. [DOI] [PubMed] [Google Scholar]
- 8. Herrada AA, Llanos C, Mackern‐Oberti JP et al Haem oxygenase 1 expression is altered in monocytes from patients with systemic lupus erythematosus. Immunology 2012; 136:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan A, Fu H, Tan LA, Harper JE, Beutelspacher SC, Larkin DFP, Lombardi G, McClure MO, George AJT. Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition. Eur J Immunol 2013; 43:734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unger WWJ, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte‐derived DC modulated by vitamin D3 or dexamethasone: differential role for PD‐L1. Eur J Immunol 2009; 39:3147–59. [DOI] [PubMed] [Google Scholar]
- 11. Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity 2010; 33:967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shortman K, Liu Y‐J. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002; 2:151–61. [DOI] [PubMed] [Google Scholar]
- 13. Santana‐de Anda K, Gómez‐Martín D, Monsivais‐Urenda AE, Salgado‐Bustamante M, González‐Amaro R, Alcocer‐Varela J. Interferon regulatory factor 3 as key element of the interferon signature in plasmacytoid dendritic cells from systemic lupus erythematosus patients: novel genetic associations in the Mexican mestizo population. Clin Exp Immunol 2014; 178:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 1979; 301:5–8. [DOI] [PubMed] [Google Scholar]
- 15. Dall'Era MC, Cardarelli PM, Preston BT, Witte A, Davis JC. Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis 2005; 64:1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol 2004; 16:801–7. [DOI] [PubMed] [Google Scholar]
- 17. Tsao BP, Cantor RM, Kalunian KC et al Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J Clin Invest 1997; 99:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Réthi B, Gogolák P, Szatmari I, Veres Á, Erdôs E, Nagy L, Rajnavölgyi É, Terhorst C, Lányi Á. SLAM/SLAM interactions inhibit CD40‐induced production of inflammatory cytokines in monocyte‐derived dendritic cells. Blood 2006; 107:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hagberg N, Theorell J, Schlums H, Eloranta M‐L, Bryceson YT, Rönnblom L. Systemic lupus erythematosus immune complexes increase the expression of SLAM family members CD319 (CRACC) and CD229 (LY‐9) on plasmacytoid dendritic cells and CD319 on CD56dim NK cells. J Immunol 2013; 191:2989–98. [DOI] [PubMed] [Google Scholar]
- 20. Mozaffarian N, Wiedeman AE, Stevens AM. Active systemic lupus erythematosus is associated with failure of antigen‐presenting cells to express programmed death ligand‐1. Rheumatology 2008; 47:1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol 2007; 178:5635–42. [DOI] [PubMed] [Google Scholar]
- 22. Hilliard B, Zizzo G, Ulas M, Linan M, Schreiter J, Cohen P. Increased expression of Mer tyrosine kinase in circulating dendritic cells and monocytes of lupus patients: correlations with plasma interferon activity and steroid therapy. Arthritis Res Ther 2014; 16:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gill MA, Blanco P, Arce E, Pascual V, Banchereau J, Palucka AK. Blood dendritic cells and DC‐poietins in systemic lupus erythematosus. Hum Immunol 2002; 63:1172–80. [DOI] [PubMed] [Google Scholar]
- 24. Jin O, Kavikondala S, Sun L, Fu R, Mok M‐Y, Chan A et al Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression. Lupus 2008; 17:654–62. [DOI] [PubMed] [Google Scholar]
- 25. Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001; 31:3388–93. [DOI] [PubMed] [Google Scholar]
- 26. Krug A, Towarowski A, Britsch S et al Toll‐like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL‐12. Eur J Immunol 2001; 31:3026–37. [DOI] [PubMed] [Google Scholar]
- 27. Yu CF, Peng WM, Oldenburg J et al Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol 2010; 184:1159–67. [DOI] [PubMed] [Google Scholar]
- 28. Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood 2006; 107:2423–31. [DOI] [PubMed] [Google Scholar]
- 29. Guiducci C, Ghirelli C, Marloie‐Provost M‐A, Matray T, Coffman RL, Liu Y‐J, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF‐7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med 2008; 205:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeuner RA, Klinman DM, Illei G, Yarboro C, Ishii KJ, Gursel M, Verthelyi D. Response of peripheral blood mononuclear cells from lupus patients to stimulation by CpG oligodeoxynucleotides. Rheumatology 2003; 42:563–9. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez‐Pla A, Patel P, Maecker HT et al IFN priming is necessary but not sufficient to turn on a migratory dendritic cell program in lupus monocytes. J Immunol 2014; 192:5586–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol 1997; 158:6019–28. [PubMed] [Google Scholar]
- 33. Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr‐induced autoimmunity. J Exp Med 1994; 180:1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA‐related antigens due to TLR7 gene duplication. Science 2006; 312:1669–72. [DOI] [PubMed] [Google Scholar]
- 35. Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(γ)RIIB‐deficient mice results from strain‐specific epistasis. Immunity 2000; 13:277–85. [DOI] [PubMed] [Google Scholar]
- 36. Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp‐1 in dendritic cells. J Exp Med 2011; 208:2193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han J‐W, Zheng H‐F, Cui Y et al Genome‐wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 2009; 41:1234–7. [DOI] [PubMed] [Google Scholar]
- 38. Parlato S, Bruni R, Fragapane P et al IFN‐α regulates blimp‐1 expression via miR‐23a and miR‐125b in both monocytes‐derived DC and pDC. PLoS ONE 2013; 8:e72833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bondanza A, Zimmermann VS, Dell'Antonio G, Dal Cin E, Capobianco A, Sabbadini MG, Manfredi AA, Rovere‐Querini P. Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol 2003; 170:24–7. [DOI] [PubMed] [Google Scholar]
- 40. Ma L, Chan KW, Trendell‐Smith NJ et al Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol 2005; 35:3364–75. [DOI] [PubMed] [Google Scholar]
- 41. Georgiev M, Agle LMA, Chu JL, Elkon KB, Ashany D. Mature dendritic cells readily break tolerance in normal mice but do not lead to disease expression. Arthritis Rheum 2005; 52:225–38. [DOI] [PubMed] [Google Scholar]
- 42. Abeler‐Dörner L, Rieger CC, Berger B et al Interferon‐α abrogates the suppressive effect of apoptotic cells on dendritic cells in an in vitro model of systemic lupus erythematosus pathogenesis. J Rheumatol 2013; 40:1683–96. [DOI] [PubMed] [Google Scholar]
- 43. Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, Davidson A. Interferon‐α accelerates murine systemic lupus erythematosus in a T cell–dependent manner. Arthritis Rheum 2011; 63:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med 2014; 211:1977–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lian Z‐X, Kikuchi K, Yang G‐X, Ansari AA, Ikehara S, Gershwin ME. Expansion of bone marrow IFN‐α‐producing dendritic cells in New Zealand black (NZB) mice: high level expression of TLR9 and secretion of IFN‐α in NZB bone marrow. J Immunol 2004; 173:5283–9. [DOI] [PubMed] [Google Scholar]
- 46. Sriram U, Varghese L, Bennett HL, Jog NR, Shivers DK, Ning Y, Behrens EM, Caricchio R, Gallucci S. Myeloid dendritic cells from B6.NZM Sle1/Sle2/Sle3 lupus‐prone mice express an IFN signature that precedes disease onset. J Immunol 2012; 189:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baccala R, Gonzalez‐Quintial R, Schreiber RD, Lawson BR, Kono DH, Theofilopoulos AN. Anti–IFN‐α/β receptor antibody treatment ameliorates disease in lupus‐predisposed mice. J Immunol 2012; 189:5976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang C, Wang H, Chang D‐Y, Hao J, Zhao M‐H, Chen M. High mobility group box 1 contributes to anti‐neutrophil cytoplasmic antibody‐induced neutrophils activation through receptor for advanced glycation end products (RAGE) and Toll‐like receptor 4. Arthritis Res Ther 2015; 17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wen Z, Xu L, Chen X, Xu W, Yin Z, Gao X, Xiong S. Autoantibody induction by DNA‐containing immune complexes requires HMGB1 with the TLR2/microRNA‐155 pathway. J Immunol 2013; 190:5411–22. [DOI] [PubMed] [Google Scholar]
- 50. Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak‐Rothstein A. Chromatin‐IgG complexes activate B cells by dual engagement of IgM and Toll‐like receptors. Nature 2002; 416:603–7. [DOI] [PubMed] [Google Scholar]
- 51. Chen DY, Chen YM, Wen MC, Hsieh TY, Hung WT, Lan JL. The potential role of Th17 cells and Th17‐related cytokines in the pathogenesis of lupus nephritis. Lupus 2012; 21:1385–96. [DOI] [PubMed] [Google Scholar]
- 52. Rana A, Minz RW, Aggarwal R, Anand S, Pasricha N, Singh S. Gene expression of cytokines (TNF‐α, IFN‐γ), serum profiles of IL‐17 and IL‐23 in paediatric systemic lupus erythematosus. Lupus 2012; 21:1105–12. [DOI] [PubMed] [Google Scholar]
- 53. Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL‐23 and IL‐17 in patients with systemic lupus erythematosus: implications for Th17‐mediated inflammation in auto‐immunity. Clin Immunol 2008; 127:385–93. [DOI] [PubMed] [Google Scholar]
- 54. Pan WC, Chen RM, Shen YC, Chen CC, Ueng YF. Suppressive effect of tobacco smoke extracts on oral P‐glycoprotein function and its impact in smoke‐induced insult to oral epidermal cells. Toxicol Lett 2009; 185:116–23. [DOI] [PubMed] [Google Scholar]
- 55. Xu L, Wang C, Zhou Y, Ren T, Wen Z. CpG oligonucleotides induce the differentiation of CD4+ Th17 cells by triggering plasmacytoid dendritic cells in adoptively cell transfer immunotherapy. Immunol Lett 2012; 142:55–63. [DOI] [PubMed] [Google Scholar]
- 56. Ouabed A, Hubert FX, Chabannes D, Gautreau L, Heslan M, Josien R. Differential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid versus conventional spleen dendritic cells. J Immunol 2008; 180:5862–70. [DOI] [PubMed] [Google Scholar]
- 57. Lu M, Yu S, Xu W, Gao B, Xiong S. HMGB1 promotes systemic lupus erythematosus by enhancing macrophage inflammatory response. J Immunol Res 2015; 2015:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Urbonaviciute V, Fürnrohr BG, Meister S et al Induction of inflammatory and immune responses by HMGB1–nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 2008; 205:3007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418:191–5. [DOI] [PubMed] [Google Scholar]
- 60. Rönnefarth VM, Erbacher AIM, Lamkemeyer T, Madlung J, Nordheim A, Rammensee H‐G, Decker P. TLR2/TLR4‐independent neutrophil activation and recruitment upon endocytosis of nucleosomes reveals a new pathway of innate immunity in systemic lupus erythematosus. J Immunol 2006; 177:7740–9. [DOI] [PubMed] [Google Scholar]
- 61. Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 2005; 115:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll‐like receptor 9 controls anti‐DNA autoantibody production in murine lupus. J Exp Med 2005; 202:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell Richard A, Shlomchik MJ. Toll‐like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006; 25:417–28. [DOI] [PubMed] [Google Scholar]
- 64. Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med 2006; 203:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lartigue A, Courville P, Auquit I, François A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti‐nucleosome and anti‐DNA antibody production in lpr mutation‐induced murine lupus. J Immunol 2006; 177:1349–54. [DOI] [PubMed] [Google Scholar]
- 66. Alba P, Bento L, Cuadrado MJ, Karim Y, Tungekar MF, Abbs I, Khamashta MA, D'Cruz D, Hughes GRV. Anti‐dsDNA, anti‐Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis 2003; 62:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lau CM, Broughton C, Tabor AS et al RNA‐associated autoantigens activate B cells by combined B cell antigen receptor/Toll‐like receptor 7 engagement. J Exp Med 2005; 202:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88‐deficient MRL/lpr mice. Arthritis Rheum 2007; 56:1618–28. [DOI] [PubMed] [Google Scholar]
- 69. Lande R, Gregorio J, Facchinetti V et al Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449:564–9. [DOI] [PubMed] [Google Scholar]
- 70. Doring Y, Manthey HD, Drechsler M et al Auto‐antigenic protein‐DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012; 125:1673–83. [DOI] [PubMed] [Google Scholar]
- 71. Sandgren S, Wittrup A, Cheng F, Jonsson M, Eklund E, Busch S, Belting M. The human antimicrobial peptide LL‐37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan‐dependent endocytosis. J Biol Chem 2004; 279:17951–6. [DOI] [PubMed] [Google Scholar]
- 72. Hwang YJ, Jung HJ, Kim MJ, Roh NK, Jung JW, Lee YW, Choe YB, Ahn KJ. Serum levels of LL‐37 and inflammatory cytokines in plaque and guttate psoriasis. Mediators Inflamm 2014; 2014:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon‐α and tumor necrosis factor‐α production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 2012; 14:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kužnik A, Benčina M, Švajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011; 186:4794–804. [DOI] [PubMed] [Google Scholar]
- 75. Lamphier M, Zheng W, Latz E et al Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo . Mol Pharmacol 2014; 85:429–40. [DOI] [PubMed] [Google Scholar]
- 76. Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll‐like receptor 9 inhibition confers protection from liver ischemia–reperfusion injury. Hepatology 2010; 51:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nimmerjahn F, Ravetch JV. Fc‐receptors as regulators of immunity. Adv Immunol 2007; 96:179–204. [DOI] [PubMed] [Google Scholar]
- 78. Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 2005; 23:41–51. [DOI] [PubMed] [Google Scholar]
- 79. Smith KG, Clatworthy MR. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 2010; 10:328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clatworthy MR, Aronin CEP, Mathews RJ, Morgan NY, Smith KGC, Germain RN. Immune complexes stimulate CCR7‐dependent dendritic cell migration to lymph nodes. Nat Med 2014; 20:1458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iruretagoyena MI, Riedel CA, Leiva ED, Gutierrez MA, Jacobelli SH, Kalergis AM. Activating and inhibitory Fcγ receptors can differentially modulate T cell‐mediated autoimmunity. Eur J Immunol 2008; 38:2241–50. [DOI] [PubMed] [Google Scholar]
- 82. Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 1998; 279:1052–4. [DOI] [PubMed] [Google Scholar]
- 83. Lovgren T, Eloranta ML, Kastner B, Wahren‐Herlenius M, Alm GV, Ronnblom L. Induction of interferon‐α by immune complexes or liposomes containing systemic lupus erythematosus autoantigen‐ and Sjogren's syndrome autoantigen‐associated RNA. Arthritis Rheum 2006; 54:1917–27. [DOI] [PubMed] [Google Scholar]
- 84. Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. FcγRIIa is expressed on natural IFN‐α‐producing cells (plasmacytoid dendritic cells) and is required for the IFN‐α production induced by apoptotic cells combined with lupus IgG. J Immunol 2003; 171:3296–302. [DOI] [PubMed] [Google Scholar]
- 85. Regnault A, Lankar D, Lacabanne V et al Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J Exp Med 1999; 189:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boruchov AM, Heller G, Veri M‐C, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 2005; 115:2914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J Exp Med 2002; 195:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Clatworthy MR, Harford SK, Mathews RJ, Smith KGC. FcγRIIb inhibits immune complex‐induced VEGF‐A production and intranodal lymphangiogenesis. Proc Natl Acad Sci USA 2014; 111:17971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. Deletion of fcγ receptor IIB renders H‐2(b) mice susceptible to collagen‐induced arthritis. J Exp Med 1999; 189:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chang M‐K, Binder CJ, Torzewski M, Witztum JL. C‐reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci USA 2002; 99:13043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mold C, Clos TWD. C‐reactive protein inhibits plasmacytoid dendritic cell interferon responses to autoantibody immune complexes. Arthritis Rheum 2013; 65:1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garcia‐Romo GS, Caielli S, Vega B et al Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lande R, Ganguly D, Facchinetti V et al Neutrophils activate plasmacytoid dendritic cells by releasing self‐DNA–peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra19–73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brownlie RJ, Lawlor KE, Niederer HA et al Distinct cell‐specific control of autoimmunity and infection by FcγRIIb. J Exp Med 2008; 205:883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bhardwaj N, Young JW, Nisanian AJ, Baggers J, Steinman RM. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp Med 1993; 178:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86‐induced phosphatidylinositol 3‐kinase signaling by NOTCH1 protein in interleukin‐6 and indoleamine 2,3‐dioxygenase production by dendritic cells. J Biol Chem 2014; 289:7747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med 1991; 173:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tseng S‐Y, Waite JC, Liu M, Vardhana S, Dustin ML. T cell‐dendritic cell immunological synapses contain TCR‐dependent CD28‐CD80 clusters that recruit protein kinase Cθ . J Immunol 2008; 181:4852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Crispín JC, Vargas‐Rojas MI, Monsiváis‐Urenda A, Alcocer‐Varela J. Phenotype and function of dendritic cells of patients with systemic lupus erythematosus. Clin Immunol 2012; 143:45–50. [DOI] [PubMed] [Google Scholar]
- 100. Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol 2006; 177:5878–89. [DOI] [PubMed] [Google Scholar]
- 101. Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, Janeway CA Jr, Flavell RA. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 1996; 273:1864–7. [DOI] [PubMed] [Google Scholar]
- 102. Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. CD40‐CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 1996; 93:2499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bagenstose LM, Agarwal RK, Silver PB, Harlan DM, Hoffmann SC, Kampen RL, Chan C‐C, Caspi RR. Disruption of CD40/CD40‐ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol 2005; 175:124–30. [DOI] [PubMed] [Google Scholar]
- 104. Burgess JK, Carlin S, Pack RA, Arndt GM, Au WW, Johnson PRA, Black JL, Hunt NH. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol 2004; 113:683–9. [DOI] [PubMed] [Google Scholar]
- 105. Ito T, Wang Y‐H, Duramad O et al TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gaspal F, Withers D, Saini M et al Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3‐deficient mice. J Exp Med 2011; 208:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yoshinaga SK, Whoriskey JS, Khare SD et al T‐cell co‐stimulation through B7RP‐1 and ICOS. Nature 1999; 402:827–32. [DOI] [PubMed] [Google Scholar]
- 108. Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez‐Ramos J‐C, Bachmann MF. Inducible costimulator protein (Icos) controls T helper cell subset polarization after virus and parasite infection. J Exp Med 2000; 192:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Witsch EJ, Peiser M, Hutloff A, Büchner K, Dorner BG, Jonuleit H, Mages HW, Kroczek RA. ICOS and CD28 reversely regulate IL‐10 on re‐activation of human effector T cells with mature dendritic cells. Eur J Immunol 2002; 32:2680–6. [DOI] [PubMed] [Google Scholar]
- 110. Sim GC, Martin‐Orozco N, Jin L et al IL‐2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest 2014; 124:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gao X, Zhao L, Wang S, Yang J, Yang X. Enhanced inducible costimulator ligand (ICOS‐L) expression on dendritic cells in interleukin‐10 deficiency and its impact on T‐cell subsets in respiratory tract infection. Mol Med 2013; 19:346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mak TW, Shahinian A, Yoshinaga SK et al Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell‐dependent B cell responses. Nat Immunol 2003; 4:765–72. [DOI] [PubMed] [Google Scholar]
- 113. Ramos MI, Tak PP, Lebre MC. Fms‐like tyrosine kinase 3 ligand‐dependent dendritic cells in autoimmune inflammation. Autoimmun Rev 2014; 13:117–24. [DOI] [PubMed] [Google Scholar]
- 114. McKenna HJ, Stocking KL, Miller RE et al Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95:3489–97. [PubMed] [Google Scholar]
- 115. Dehlin M, Bokarewa M, Rottapel R, Foster SJ, Magnusson M, Dahlberg LE, Tarkowski A. Intra‐articular Fms‐like tyrosine kinase 3 ligand expression is a driving force in induction and progression of arthritis. PLoS ONE 2008; 3:e3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Erlandsson MC, Forslind K, Andersson SEM, Lund A, Bokarewa MI. Metastasin S100A4 is increased in proportion to radiographic damage in patients with RA. Rheumatology 2012; 51:932–40. [DOI] [PubMed] [Google Scholar]
- 117. Yogev N, Frommer F, Lukas D et al Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD‐1 receptor+ regulatory T cells. Immunity 2012; 37:264–75. [DOI] [PubMed] [Google Scholar]
- 118. Yamazaki T, Akiba H, Iwai H et al Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002; 169:5538–45. [DOI] [PubMed] [Google Scholar]
- 119. McPherson RC, Konkel JE, Prendergast CT et al Epigenetic modification of the PD‐1 (Pdcd1) promoter in effector CD4+ T cells tolerized by peptide immunotherapy. eLife 2014; 3:e03416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Keir ME, Francisco LM, Sharpe AH. PD‐1 and its ligands in T‐cell immunity. Curr Opin Immunol 2007; 19:309–14. [DOI] [PubMed] [Google Scholar]
- 121. Dilek N, Poirier N, Hulin P et al Targeting CD28, CTLA‐4 and PD‐L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T‐cells. PLoS ONE 2013; 8:e83139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD‐L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD‐1 ligands expressed on myeloid‐derived APC in the CNS regulate T‐cell responses in EAE. Eur J Immunol 2008; 38:2706–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD‐L1‐deficient mice show that PD‐L1 on T cells, antigen‐presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA 2004; 101:10691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity 1999; 11:141–51. [DOI] [PubMed] [Google Scholar]
- 126. Chiba S, Baghdadi M, Akiba H et al Tumor‐infiltrating DCs suppress nucleic acid‐mediated innate immune responses through interactions between the receptor TIM‐3 and the alarmin HMGB1. Nat Immunol 2012; 13:832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein‐3 via Bruton's tyrosine kinase and c‐Src. J Immunol 2014; 193:3417–25. [DOI] [PubMed] [Google Scholar]
- 128. Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement‐dependent transport of immune complexes by lymph node B cells. Nat Immunol 2007; 8:992–1000. [DOI] [PubMed] [Google Scholar]
- 129. Fayette J, Dubois B, Vandenabeele S, Bridon J‐M, Vanbervliet B, Durand I, Banchereau J, Caux C, Brière F. Human dendritic cells skew isotype switching of CD40‐activated Naive B cells towards IgA1 and IgA2. J Exp Med 1997; 185:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wan S, Zhou Z, Duan B, Morel L. Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum 2008; 58:1741–50. [DOI] [PubMed] [Google Scholar]
- 131. Sang A, Zheng Y‐Y, Yin Y, Dozmorov I, Li H, Hsu H‐C, Mountz JD, Morel L. Dysregulated cytokine production by dendritic cells modulates B cell responses in the NZM2410 mouse model of lupus. PLoS ONE 2014; 9:e102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B‐cell growth and differentiation via CD70. Blood 2010; 115:3051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40‐independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 2002; 3:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L, Cao X. Regulatory dendritic cells program B cells to differentiate into CD19hiFcγIIbhi regulatory B cells through IFN‐β and CD40L. Blood 2012; 120:581–91. [DOI] [PubMed] [Google Scholar]
- 135. Pasare C, Medzhitov R. Toll pathway‐dependent blockade of CD4+ CD25+ T cell‐mediated suppression by dendritic cells. Science 2003; 299:1033–6. [DOI] [PubMed] [Google Scholar]
- 136. Wan S, Xia C, Morel L. IL‐6 produced by dendritic cells from lupus‐prone mice inhibits CD4+ CD25+ T cell regulatory functions. J Immunol 2007; 178:271–9. [DOI] [PubMed] [Google Scholar]
- 137. Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 2000; 18:565–70. [PubMed] [Google Scholar]
- 138. Amerio P, Frezzolini A, Abeni D, Teofoli P, Girardelli CR, De Pita O, Puddu P. Increased IL‐18 in patients with systemic lupus erythematosus: relations with Th‐1, Th‐2, pro‐inflammatory cytokines and disease activity. IL‐18 is a marker of disease activity but does not correlate with pro‐inflammatory cytokines. Clin Exp Rheumatol 2002; 20:535–8. [PubMed] [Google Scholar]
- 139. Abdel Galil SM, Ezzeldin N, El‐Boshy ME. The role of serum IL‐17 and IL‐6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine 2015; in press. pii: S1043‐4666(15)00187‐8 [DOI] [PubMed] [Google Scholar]
- 140. Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XYR. Anti‐interleukin‐6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology 2006; 119:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Terness P, Oelert T, Ehser S et al Mitomycin C‐treated dendritic cells inactivate autoreactive T cells: toward the development of a tolerogenic vaccine in autoimmune diseases. Proc Natl Acad Sci USA 2008; 105:18442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Raïch‐Regué D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett 2014; 161:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Xing N, ML LM, Bachman LA, McKean DJ, Kumar R, Griffin MD. Distinctive dendritic cell modulation by vitamin D(3) and glucocorticoid pathways. Biochem Biophys Res Commun 2002; 297:645–52. [DOI] [PubMed] [Google Scholar]
- 144. Kubsch S, Graulich E, Knop J, Steinbrink K. Suppressor activity of anergic T‐cells induced by IL‐10‐treated human dendritic cells: association with IL‐2‐ and CTLA‐4‐dependent G1 arrest of the cell cycle regulated by p27Kip1. Eur J Immunol 2003; 33:1988–97. [DOI] [PubMed] [Google Scholar]
- 145. Volchenkov R, Brun J, Jonsson R, Appel S. In vitro suppression of immune responses using monocyte‐derived tolerogenic dendritic cells from patients with primary Sjogren's syndrome. Arthritis Res Ther 2013; 15:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol 2001; 166:7053–62. [DOI] [PubMed] [Google Scholar]
- 147. Huang S‐TJ, Cidlowski JA. Phosphorylation status modulates Bcl‐2 function during glucocorticoid‐induced apoptosis in T lymphocytes. FASEB J 2002; 16:825–32. [DOI] [PubMed] [Google Scholar]
- 148. Sergeev IN. 125‐Dihydroxyvitamin D3 induces Ca2+‐mediated apoptosis in adipocytes via activation of calpain and caspase‐12. Biochem Biophys Res Commun 2009; 384:18–21. [DOI] [PubMed] [Google Scholar]
- 149. Dixon KO, O'Flynn J, van der Kooij SW, van Kooten C. Phagocytosis of apoptotic or necrotic cells differentially regulates the transcriptional expression of IL‐12 family members in dendritic cells. J Leukoc Biol 2014; 96:313–24. [DOI] [PubMed] [Google Scholar]
- 150. Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson A. Rapamycin inhibits IL‐4–induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo . Blood 2003; 101:4457–63. [DOI] [PubMed] [Google Scholar]
- 151. Volchenkov R, Karlsen M, Jonsson R, Appel S. Type 1 regulatory T cells and regulatory B cells induced by tolerogenic dendritic cells. Scand J Immunol 2013; 77:246–54. [DOI] [PubMed] [Google Scholar]
- 152. Martin E, Capini C, Duggan E, Lutzky VP, Stumbles P, Pettit AR, O'Sullivan B, Thomas R. Antigen‐specific suppression of established arthritis in mice by dendritic cells deficient in NF‐κB. Arthritis Rheum 2007; 56:2255–66. [DOI] [PubMed] [Google Scholar]
- 153. Kalergis AM, Iruretagoyena MI, Barrientos MJ et al Modulation of nuclear factor‐kappaB activity can influence the susceptibility to systemic lupus erythematosus. Immunology 2009; 128:e306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chauveau C, Remy S, Royer PJ et al Heme oxygenase‐1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL‐10 expression. Blood 2005; 106:1694–702. [DOI] [PubMed] [Google Scholar]
- 155. Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin‐10–treated human dendritic cells display antigen‐specific suppressor activity. Blood 2002; 99:2468–76. [DOI] [PubMed] [Google Scholar]
- 156. Li X, Yang A, Huang H, Zhang X, Town J, Davis B, Cockcroft DW, Gordon JR. Induction of Type 2 T helper cell allergen tolerance by IL‐10–differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol 2010; 42:190–9. [DOI] [PubMed] [Google Scholar]
- 157. Velten FW, Duperrier K, Bohlender J, Metharom P, Goerdt S. A gene signature of inhibitory MHC receptors identifies a BDCA3+ subset of IL‐10‐induced dendritic cells with reduced allostimulatory capacity in vitro . Eur J Immunol 2004; 34:2800–11. [DOI] [PubMed] [Google Scholar]
- 158. Thomas DC, Wong FS, Zaccone P, Green EA, Wållberg M. Protection of islet grafts through transforming growth factor‐β–induced tolerogenic dendritic cells. Diabetes 2013; 62:3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li R, Zheng X, Popov I et al Gene silencing of IL‐12 in dendritic cells inhibits autoimmune arthritis. J Transl Med 2012; 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zheng X, Suzuki M, Ichim TE et al Treatment of autoimmune arthritis using RNA interference‐modulated dendritic cells. J Immunol 2010; 184:6457–64. [DOI] [PubMed] [Google Scholar]
- 161. Zheng X, Suzuki M, Zhang X et al RNAi‐mediated CD40‐CD154 interruption promotes tolerance in autoimmune arthritis. Arthritis Res Ther 2010; 12:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ferenbach DA, Ramdas V, Spencer N, Marson L, Anegon I, Hughes J, Kluth DC. Macrophages expressing heme oxygenase‐1 improve renal function in ischemia/reperfusion injury. Mol Ther 2010; 18:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Dufait I, Liechtenstein T, Lanna A, Bricogne C, Laranga R, Padella A, Breckpot K, Escors D. Retroviral and lentiviral vectors for the induction of immunological tolerance. Scientifica 2012; 2012:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Raϊch‐Regué D, Grau‐López L, Naranjo‐Gómez M, Ramo‐Tello C, Pujol‐Borrell R, Martínez‐Cáceres E, Borràs FE. Stable antigen‐specific T‐cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol 2012; 42:771–82. [DOI] [PubMed] [Google Scholar]
- 165. Chiurchiù V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, Maccarrone M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 2013; 73:626–36. [DOI] [PubMed] [Google Scholar]
- 166. Mansilla MJ, Sellès‐Moreno C, Fàbregas‐Puig S, Amoedo J, Navarro‐Barriuso J, Teniente‐Serra A, Grau‐López L, Ramo‐Tello C, Martínez‐Cáceres EM. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther 2015; 21:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ginzler EM, Dooley MA, Aranow C et al Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. New Engl J Med 2005; 353:2219–28. [DOI] [PubMed] [Google Scholar]
- 168. Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011; 34:2026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Benham H, Nel HJ, Law SC et al Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype–positive rheumatoid arthritis patients. Sci Transl Med 2015; 7:290ra87. [DOI] [PubMed] [Google Scholar]
- 170. Thomas RSS, Ramnoruth N, Pahau H et al Feasibility, safety and clinical effects of Single intradermal administration of autologous tolerising dendritic cells exposed to citrullinated peptides in patients with rheumatoid arthritis. Arthritis Rheum 2011; 63:2430. [Google Scholar]


