Abstract
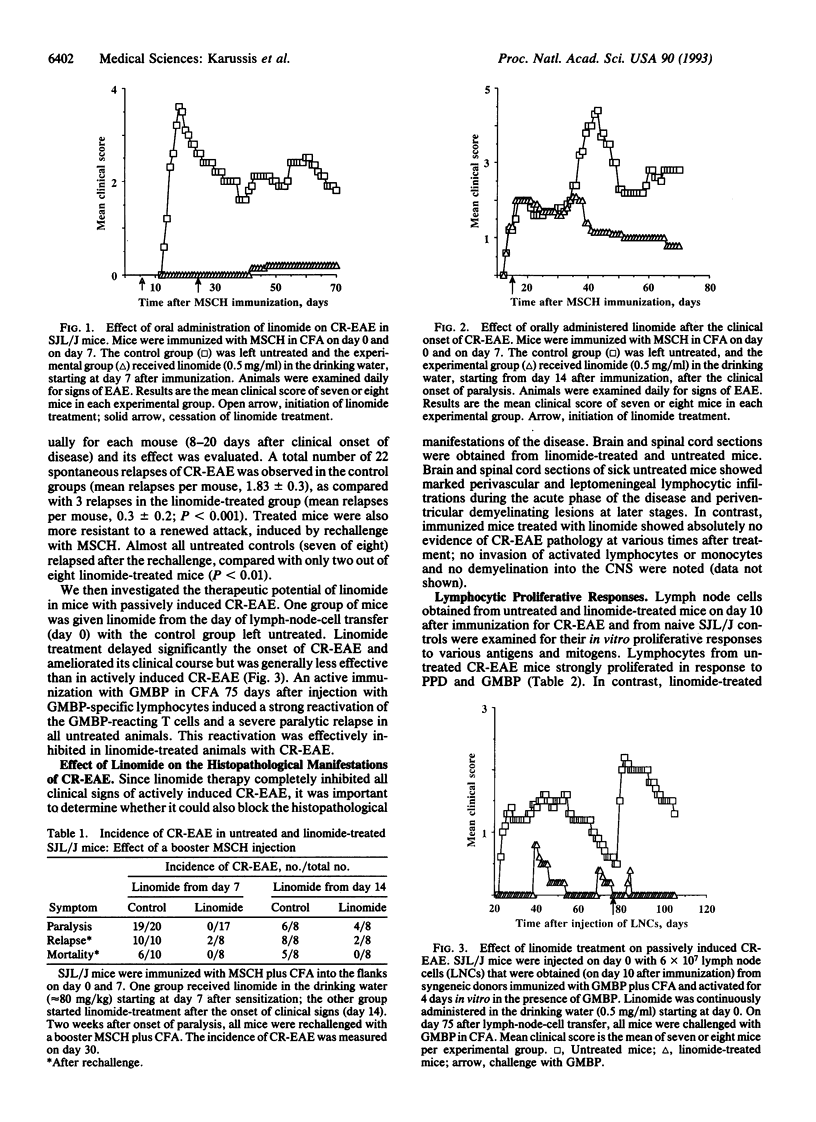
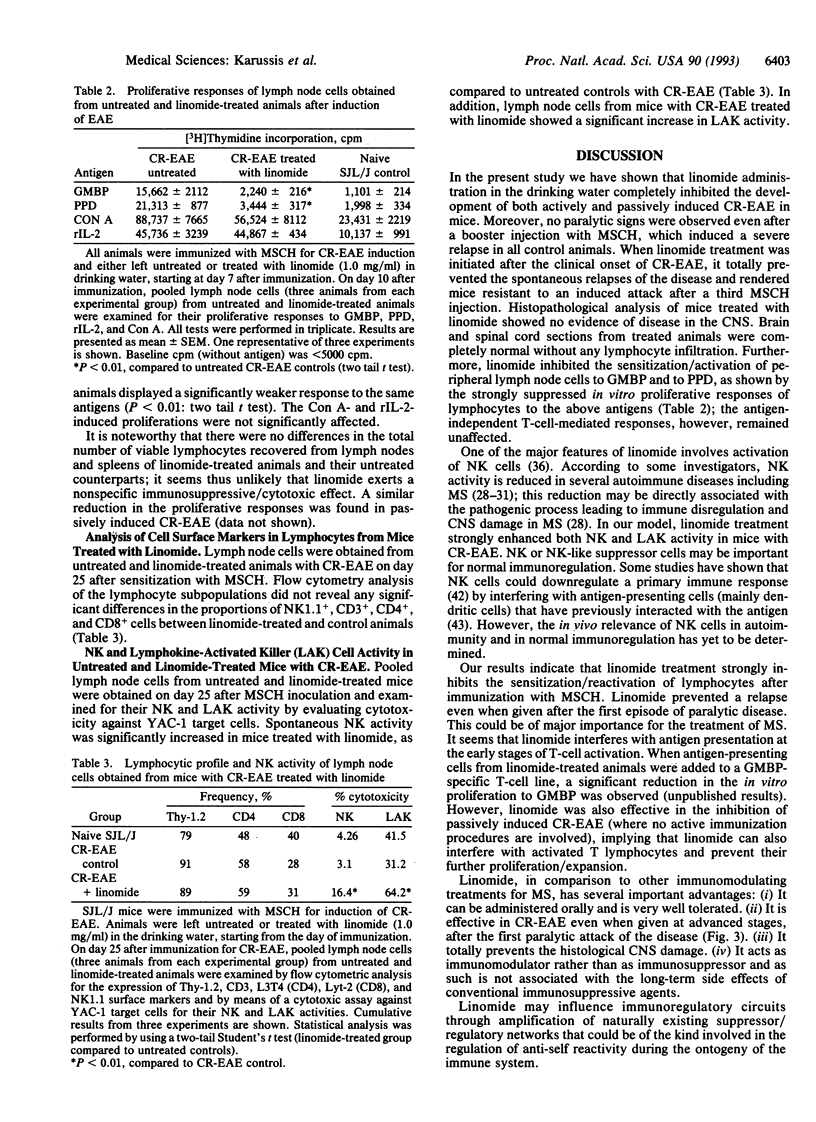
Linomide is a synthetic immunomodulator that enhances natural killer cell activity and significantly activates several lymphocytic cell subpopulations in both experimental animals and humans. In this study we examined the effect of linomide (80 mg per kg per day in drinking water) on mice with chronic-relapsing experimental autoimmune encephalomyelitis (CR-EAE), a T-cell-mediated organ-specific autoimmune disease that resembles human multiple sclerosis. None of the mice (n = 17) that were treated with linomide from day 7 after disease induction developed any clinical or histopathological signs of CR-EAE, as compared to 19 of 20 untreated controls that were severely paralyzed and had extensive demyelinating lesions in the central nervous system. Linomide-treated animals were also resistant to an induced attack by a booster injection with a murine spinal cord homogenate. When administered to mice exhibiting severe clinical signs of paralysis, linomide inhibited both spontaneous and induced relapses. Linomide treatment protected mice from passively induced CR-EAE as well, when given from the day of injection with myelin-basic-protein-specific lymphocytes. Lymphocytes obtained from linomide-treated mice had a reduced in vitro proliferative response to the myelin basic protein and to the tuberculin purified protein derivative, whereas the mitogenic response to concanavalin A was not affected. Natural killer cell and lymphokine-activated killer cell activities were enhanced. These results suggest that linomide regulates autoimmunity in the absence of systemic immunosuppression. Since linomide is very well tolerated in experimental animals and humans, it might be used in the treatment of multiple sclerosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Lando Z. Detection of autoimmune cells proliferating to myelin basic protein and selection of T cell lines that mediate experimental autoimmune encephalomyelitis (EAE) in mice. J Immunol. 1983 Mar;130(3):1205–1209. [PubMed] [Google Scholar]
- Bernard C. C., Carnegie P. R. Experimental autoimmune encephalomyelitis in mice: immunologic response to mouse spinal cord and myelin basic proteins. J Immunol. 1975 May;114(5):1537–1540. [PubMed] [Google Scholar]
- Bernard C. C., Leydon J., Mackay I. R. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 1976 Sep;6(9):655–660. doi: 10.1002/eji.1830060912. [DOI] [PubMed] [Google Scholar]
- Brown A. M., McFarlin D. E. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981 Sep;45(3):278–284. [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- FIELD E. J., MILLER H. Experimental allergic encephalomyelitis: comparison of protective effects of prednisolone and corticotrophin. Br Med J. 1962 Mar 24;1(5281):843–844. doi: 10.1136/bmj.1.5281.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer C., Chow L. H., Borel J. F. Preventive and therapeutic effects of cyclosporin and valine2-dihydro-cyclosporin in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Immunology. 1988 Feb;63(2):219–223. [PMC free article] [PubMed] [Google Scholar]
- Greig M. E., Gibbons A. J., Elliott G. A. A comparison of the effects of melengestrol acetate and hydrocortisone acetate on experimental allergic encephalomyelitis in rats. J Pharmacol Exp Ther. 1970 May;173(1):85–93. [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Andreu J. L., Revilla Y., Viñuela E., Martinez C. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature. 1990 Jul 19;346(6281):271–274. doi: 10.1038/346271a0. [DOI] [PubMed] [Google Scholar]
- Kalland T., Alm G., Stålhandshe T. Augmentation of mouse natural killer cell activity by LS 2616, a new immunomodulator. J Immunol. 1985 Jun;134(6):3956–3961. [PubMed] [Google Scholar]
- Karussis D. M., Slavin S., Lehmann D., Mizrachi-Koll R., Abramsky O., Ben-Nun A. Prevention of experimental autoimmune encephalomyelitis and induction of tolerance with acute immunosuppression followed by syngeneic bone marrow transplantation. J Immunol. 1992 Mar 15;148(6):1693–1698. [PubMed] [Google Scholar]
- Komarek A., Dietrich F. M. Chemical prevention of experimental allergic encephalomyelitis in rats: a quantitative evaluation of steroids and various non-steroid drugs. Arch Int Pharmacodyn Ther. 1971 Oct;193(2):249–257. [PubMed] [Google Scholar]
- Larsson E. L., Joki A., Stålhandske T. Mechanism of action of the new immunomodulator LS2616 on T cell responses. Int J Immunopharmacol. 1987;9(4):425–431. doi: 10.1016/0192-0561(87)90016-6. [DOI] [PubMed] [Google Scholar]
- Levine S., Sowinski R. Suppression of the hyperacute form of experimental allergic encephalomyelitis by drugs. Arch Int Pharmacodyn Ther. 1977 Dec;230(2):309–318. [PubMed] [Google Scholar]
- Lider O., Reshef T., Beraud E., Ben-Nun A., Cohen I. R. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 1988 Jan 8;239(4836):181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- Lublin F. D. Immunomodulation of relapsing experimental allergic encephalomyelitis. Neurology. 1984 Dec;34(12):1615–1617. doi: 10.1212/wnl.34.12.1615. [DOI] [PubMed] [Google Scholar]
- Lublin F. D., Maurer P. H., Berry R. G., Tippett D. Delayed, relapsing experimental allergic encephalomyelitis in mice. J Immunol. 1981 Mar;126(3):819–822. [PubMed] [Google Scholar]
- Merrill J., Jondal M., Seeley J., Ullberg M., Sidén A. Decreased NK killing in patients with multiple sclerosis: an analysis on the level of the single effector cell in peripheral blood and cerebrospinal fluid in relation to the activity in the disease. Clin Exp Immunol. 1982 Feb;47(2):419–430. [PMC free article] [PubMed] [Google Scholar]
- Mokhtarian F., McFarlin D. E., Raine C. S. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984 May 24;309(5966):356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- Neighbour P. A., Grayzel A. I., Miller A. E. Endogenous and interferon-augmented natural killer cell activity of human peripheral blood mononuclear cells in vitro. Studies of patients with multiple sclerosis, systemic lupus erythematosus or rheumatoid arthritis. Clin Exp Immunol. 1982 Jul;49(1):11–21. [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y., Drobish D. G. Cyclophosphamide: effect on experimental allergic encephalomyelitis in Lewis rats. Science. 1969 Jul 11;165(3889):191–192. doi: 10.1126/science.165.3889.191. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Rosenthale M. E., Datko L. J., Kassarich J., Schneider F. Chemotherapy of experimental allergic encephalomyelitis (EAE). Arch Int Pharmacodyn Ther. 1969 Jun;179(2):251–275. [PubMed] [Google Scholar]
- Schuller-Levis G. B., Kozlowski P. B., Wisniewski H. M. Cyclosporin A treatment of an induced attack in a chronic relapsing model of experimental allergic encephalomyelitis. Clin Immunol Immunopathol. 1986 Aug;40(2):244–252. doi: 10.1016/0090-1229(86)90027-9. [DOI] [PubMed] [Google Scholar]
- Shah P. D., Gilbertson S. M., Rowley D. A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985 Aug 1;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Solomon D., Rouse R. V., Steinman L. Identification of T cell subsets and B lymphocytes in mouse brain experimental allergic encephalitis lesions. J Immunol. 1982 Oct;129(4):1649–1651. [PubMed] [Google Scholar]
- Steiner I., Brenner T., Mizrachi-Kol R., Abramsky O. Development of experimental allergic encephalomyelitis during steroid administration. Outcome of neurological immune-mediated disorders under immunosuppressive therapy. Isr J Med Sci. 1991 Jul;27(7):365–368. [PubMed] [Google Scholar]
- Tarkowski A., Gunnarsson K., Nilsson L. A., Lindholm L., Stålhandske T. Successful treatment of autoimmunity in MRL/1 mice with LS-2616, a new immunomodulator. Arthritis Rheum. 1986 Nov;29(11):1405–1409. doi: 10.1002/art.1780291115. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Gunnarsson K., Stålhandske T. Effects of LS-2616 administration upon the autoimmune disease of (NZB x NZW) F1 hybrid mice. Immunology. 1986 Dec;59(4):589–594. [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Milo R., Arnon R., Sela M. Synthetic copolymer 1 inhibits human T-cell lines specific for myelin basic protein. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Vranes Z., Poljaković Z., Marusić M. Natural killer cell number and activity in multiple sclerosis. J Neurol Sci. 1989 Dec;94(1-3):115–123. doi: 10.1016/0022-510x(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Keith A. B. Chronic relapsing experimental allergic encephalomyelitis: an experimental model of multiple sclerosis. Ann Neurol. 1977 Feb;1(2):144–148. doi: 10.1002/ana.410010207. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]



