Summary
The cotton rat (Sigmodon hispidus) belongs to the rodent family of Cricetidae and provides a powerful model to study the pathogenesis of human respiratory viruses and measles virus. Recent studies in other rodent models have suggested a role for invariant natural killer T (iNKT) cells in antiviral immunity and vaccination against respiratory virus infections. Using new experimental tools, we provide the first evidence for a functional CD1d cell molecule (crCD1d) and iNKT T‐cell receptor in cotton rats. The crCD1d cDNA sequence was identified and crCD1d transductants showed that monoclonal antibody WTH‐2 stains crCD1d as efficiently as mouse or rat CD1d. The expression of crCD1d was clearly weaker for thymocytes and B cells, and higher for T cells, which is different to what is found in murine species. The antigen‐presenting capacity of crCD1d was demonstrated with crCD1d–immunoglobulin dimers loaded with the glycolipid PBS57, which bound iNKT T‐cell receptors. Evidence for functional cotton rat iNKT cells was provided by detection of interferon‐γ and interleukin‐4 in cultures of splenocytes stimulated with PBS57 and α‐galactosylceramide and by specific staining of about 0·2% of splenocytes with PBS57‐loaded crCD1d dimers. Canonical AV14/AJ18 rearrangements were identified and found to contain multiple members of the AV14 (AV11) family. One of them was expressed and found to bind CD1d dimers. In summary, these data provide the first evidence for functional CD1d molecules and iNKT T‐cell receptors in cotton rats and provide the tools to analyse them both in the cotton rat model of infectious diseases.
Keywords: CD1d, cotton rat, invariant natural killer T cell
Abbreviations
- αGC
α‐galactosylceramide
- cr
cotton rat
- FBS
fetal bovine serum
- IFN‐γ
interferon‐γ
- IHL
intrahepatic lymphocyte
- IL‐2
interleukin‐2
- iNKT cells
invariant natural killer T cells
- mAb
monoclonal antibody
- RACE
rapid amplification of cDNA ends
- TCR
T‐cell receptor
Introduction
Hispid cotton rats (Sigmodon hispidus) are rodents native to the south of the USA, Central America and the north of South America and belong to the order Rodentia, family Cricetidae, subfamily Sigmodontinae.1 The cotton rat was first used in 1939 as an animal model for polio virus infections.1 Subsequently, it was demonstrated that cotton rats are susceptible to a large number of human pathogens (including influenza virus, human parainfluenza virus, respiratory syncytial virus, human metapneumovirus, measles virus2 and herpes simplex virus1) and are used for pre‐clinical testing of antiviral reagents and vaccines for respiratory syncytial virus infection.1, 3 The utility of the cotton rat model is based on its susceptibility to human infectious pathogens but is somewhat tempered by the relative paucity of reagents compared with mice and rats and by the lack of sequence information on the cotton rat genome. The major goal of this study was to develop tools for the analysis of invariant natural killer T (iNKT) cells. Invariant NKT cells are characterized by the expression of a semi‐invariant αβ T‐cell receptor (TCR) comprised of AV14/AJ18 in rats and mice (or AV24/AJ18 in humans) paired with a limited number of β‐chains such as BV8S2, BV7 and BV2 in mice (BV11 in humans).4 Highly potent glycolipids, such as α‐galactosylceramide (αGC)5 and PBS57, are important tools for iNKT cell research.6 α‐Galactosylceramide and other endogenous or exogenous iNKT‐cell‐stimulating antigens are presented by CD1d, a member of the CD1 family. CD1 molecules are non‐polymorphic glycoproteins that share structure and sequence homologies with MHC class I molecules.7 The CD1 isoforms can be divided into different groups, with group 1 containing CD1a, CD1b and CD1c, group 2 containing CD1d, and CD1e assigned to group 3.7 The latter facilitates the loading of glycolipid antigens into CD1.8 Humans express all CD1 isoforms whereas only CD1d is found in murid rodents.9 Two isoforms of CD1d exist in mice, CD1d1 and CD1d2. CD1d2 is almost exclusively expressed on thymocytes but at low levels.10 In contrast, rats possess only one CD1d gene.11 CD1a–c present antigens to clonally diverse sets of T cells and the CD1d molecule serves as an antigen‐presenting molecule to the NKT cell family.7 Most NKT cells belong to the group of type I or iNKT cells. In mice and rats, CD1d is constitutively expressed by antigen‐presenting cells, such as dendritic cells, macrophages, and B cells (especially of the marginal zone).12, 13 Cortical thymocytes that are essential for the development of iNKT cells,14 Kupffer cells and endothelial cells lining liver sinusoids also express CD1d.15 After activation, iNKT cells are considered to bridge innate and adaptive immunity and are therefore involved in a number of conditions like microbial infections,16, 17 autoimmunity,18 allergies,19 and cancer.20 The iNKT cells can have a protective role in these pathological states, or they can aggravate or even cause the disease.21 They are also known to contribute to control of virus infection. In mice, stimulation of iNKT cells leads to improved immunity against influenza virus infection. Invariant NKT cells were found to improve influenza‐specific responses and to reduce suppression by myeloid‐derived suppressor cells.22 Through studies with CD1d −/− mice, iNKT cells were also associated with an efficient CD8+ T‐cell response to respiratory syncytial virus infections.23
In this study, the sequence of cotton rat (cr) CD1d has been determined and the expression of crCD1d on different leucocyte populations was defined by the use of a cross‐reactive antibody (WTH‐2). Glycolipid‐loaded crCD1d–immunoglobulin dimers have been generated and were found to identify iNKT cells among cotton rat splenocytes. Furthermore, sequences of functional iNKT TCR‐α‐chains and of multiple members of the AV14 gene family have been identified. Altogether, these findings provide tools to address the role of CD1d and iNKT cells in cotton rats infected with human viruses.
Materials and methods
Animals
Inbred hispid cotton rats (S. hispidus) and inbred C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) were used at the age of 6–12 weeks. Animal use protocols were approved by the Institutional Animal Care and Use Committee at the Ohio State University.
Primary cell preparation
Splenocytes, thymocytes and lymph node cells were prepared by mechanical disruption of cotton rat or mouse tissue through a 70‐μm cell strainer (BD, Franklin Lakes, NJ). Organs and primary cells were kept in PBS with 0·1% fetal bovine serum (FBS).
Amplification and sequencing of crCD1d and the AV14/AJ18 rearrangement
The sequence of CD1d in the cotton rat was determined from cotton rat spleen cDNA (SuperScript™ First‐Strand Synthesis Kit for RT‐PCR; Invitrogen, Carlsbad, CA) by PCR using Platinum® Taq DNA Polymerase (Invitrogen) with primers based on alignments of hamster, mouse and rat CD1d sequences followed by nested PCR (crCD1d forward: AACCAGCTTTACCAGGGACAT, crCD1d forward nested: GCCTGTGTGGGTGATGTGGA, crCD1d reverse: TCTCCAGCCTCCACATCCAG, crCD1d reverse nested: ATCCACATCACCCACACAGG). The resulting partial sequence was used to design primers for rapid amplification of cDNA ends (RACE) ‐PCR to determine the upstream 5′ and the downstream 3′ end (crCD1d RACE 5′ reverse: ATTCTCAGAGTACACTTCACATCCTACA, crCD1d RACE 3′ forward: AAGGCCATAAGCAATTGGTATGTCATGT). The AV14/AJ18 rearrangement was sequenced following the same strategy used for crCD1d. The first partial sequence was amplified from cotton rat intrahepatic lymphocyte (IHL) cDNA with primers based on sequence alignments of human, rat and mouse. The 5′ and 3′ end was then amplified from RACE‐ready spleen cDNA with the following primers: crAV14 RACE 5′ reverse: GCATCTTCATCCAGAGCTGCTGAGTATC, crAC RACE 3′ forward: AAGGCCATAAGCAATTGGTATGTCATGT.
The GeneRacer Kit® with SuperScript III RT® (Invitrogen) was used according to the manufacturer's instructions.
Alignments were calculated with the clustal omega software and the GenBank references of the sequences used are as follows: crCD1d KM_267558, Chinese hamster (Cricetulus griseus) XM_007644702.1, rat CD1d NM_017079.1, mouse CD1d NM_007639.3 and human CD1d NM_001766.3. For the alignment of different iNKT α‐chains, accession numbers were as follows: cotton rat KT367785, rat ABC69268.1, mouse AAA40180.1 and human ABC72374.1. No published predicted or confirmed canonical rearrangement of AV14 and AJ18 was available for Chinese hamster. Here, a rearrangement was designed from genomic homologous sequences (AV14, including signal peptide: NW_003615069.1, AJ18: NW_003614213.1, AC: NW_003614213.1). The coding sequence of the Chinese hamster constant region was put together using conserved splice acceptor and donor sequences to predict splice sites.
Cloning of the AV14/AJ18 rearrangement and genomic AV14
The cotton rat iNKT α‐chain amplified from IHL cDNA was subsequently cloned into pEGN vector using the restriction sites 5′ EcoRI and 3′ BamHI (primers: crAV14EcoRI_fwd: GGGCTAGAATTCAGTAGAACAACAATGGAGAAG and crACBamHI_rev: ATGCGGATCCTCAACTGGACCATAGCCGCAGCGTCATGAG). After transformation of competent Escherichia coli, plasmid DNA of 17 clones was prepared and sequences were analysed. The same strategy was applied for crAV14 from gDNA (primers: crAV14_gDNA_EcoRI_fwd: GCATGAATTCAGAGCCCCAAGTTCCTGACT and crAV14_gDNA_BamHI_rev: GCATGGATCCGGCAGTGTCCTCAAACTGGG) and sequences from 20 clones were compared. Alignments were calculated with clustal omega.
Expression of iNKT TCRs containing cotton rat α‐chains
The pEGN crAV14 clone 1 was used to retrovirally transduce BW r/m CD28 mouse thymus hybridoma cells, which are TCR‐negative BW58 cells transduced with chimeric rat/mouse CD28.24, 25 The β‐chain used was a rat BV8S4‐like β‐chain containing CDR2+4.24, 26
CD1d expression in human lymphoblast cell line Raji
Cotton rat CD1d was cloned into pEGZ plasmid using the restriction sites 5′ SwaI and 3′ BamHI, and CD1d was expressed in Raji cells using retroviral transduction, as described for rat and mouse CD1d.24, 27 Raji crCD1d cells were sorted on high green fluorescent protein (GFP) expression and used for flow cytometry analysis.
Stimulation assays
For stimulation of hybridoma cells with CD1d dimers, 4 μg/ml of each, CD1d dimers and anti‐rat CD28 monoclonal antibody (mAb) JJ319 (Exbio, Vestec, Czech Republic) were diluted in PBS. PBS57‐loaded and unloaded dimers were combined to reach a total concentration of 4 μg/ml. This resulted in the following compositions of PBS57‐loaded/unloaded dimers: 4/0, 2/2, 1/3, 0·5/3·5, 0·25/3·75, 0·125/3·875 and 0/4 μg/ml. Anti‐mouse CD3ε mAb (BD Pharmingen, San Diego, CA) together with JJ319 (4 μg/ml each) was used as a positive control. Fifty microlitres per well was used to coat wells of U‐bottom 96‐well suspension culture plates and plates were incubated at 4° overnight and afterwards washed three times with PBS. Then, 5 × 104 rat iNKT TCR‐expressing mouse T‐cell hybridoma cells, BW r/m CD28 EGN rAV14 S6 93A S65T CDR2+4 L14V,24, 28 were added in RPMI‐1640 medium [Gibco, Grand Island, NY; supplemented with 10% FBS, 1 mm sodium pyruvate, 0·05 mm glutamine, 0·1 mm non‐essential amino acids, 5 mm β‐mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml)], and cultured for 22 hr at 37° in 5% CO2. Supernatants were analysed with a mouse interleukin‐2 (IL‐2) sandwich ELISA (BD OptEIA™ mouse IL‐2 ELISA Kit).
Primary cell stimulations
Cotton rat splenocytes were prepared and 1 × 106 cells per sample were used together with the respective amount of glycolipid. The αGC (KRN7000, 50 μg/ml) or PBS57 (50 μg/ml) was used in 10‐fold serial dilutions starting from 100 to 0·1 ng/ml. Concanavalin A C201 type IV (Sigma, St Louis, MO) was used as a positive control at 2 μg/ml and splenocytes cultured in media only served as a negative control. The cells were cultured for 24 hr in RPMI‐1640 [supplemented with 10% FBS, 1 × Penicillin/Streptomycin (Gibco), 1× GlutaMAX (Gibco), and 5 mm β‐mercaptoethanol], using 96‐well plates (U‐bottom), at 37°, 5% CO2. Supernatants were analysed with cotton rat interferon‐γ (IFN‐γ) and IL‐4 ELISA (R&D Systems, Minneapolis, MN) undiluted, fivefold diluted or 25‐fold diluted.
Dimers
Cotton rat CD1d–murine IgG (mIgG) dimers were produced as previously described for mouse and rat CD1d dimers.29, 30, 31 The extracellular domains of crCD1d were cloned into pXIg vector with Platinum® Taq DNA Polymerase High Fidelity (Invitrogen) and primers containing restriction sites for MluI and XhoI, respectively (pXIg 5′ MluI forward: GGTCCACGCGTCGCAGCAAAAGAATTCCACCTTC, pXIg 3′ XhoI reverse: GGTCACTCGAGCCAATAGAGGATGATGTCTTGGT). Loading of CD1d dimers was performed with a 40 × molar excess of αGC or its analogue PBS576 and 8% DMSO for 24 hr at 37° or as negative control with DMSO alone.
Preparation of a mAb reactive with cotton rat Fc
The IgG was isolated from S. hispidus serum (a kind gift from Kevin Yim, Sigmovir Biosystems, Rockville, MD) by protein A chromatography. The purified IgG was dialysed into PBS and digested with immobilized papain. The Fc fraction was isolated on a protein A–agarose column and eluted with 0·1 m citrate buffer, pH 3·3. The Fc‐containing fractions were pooled, concentrated using a 10 000 MW centrifugal filter, dialysed into PBS and sterilized by filtration. Eight‐week‐old BALB/c female mice received a subcutaneous injection (0·2 ml) of 10 μg of cotton rat Fc in 50% complete Freund's adjuvant. On days 16 and 56 the mice were injected with 5 μg of cotton rat Fc in 50% incomplete Freund's adjuvant. Three days before hybridoma formation, one mouse received an intravenous injection of 2 μg of cotton rat Fc in sterile PBS. Splenocytes from the immunized mouse were fused with SP2/0 cells using standard techniques. Hybridomas producing anti‐Fc antibodies were identified by ELISA using cotton rat Fc, IgM and IgA as the target antigen. Positive cultures were expanded, retested, cryopreserved and cloned. One clone, identified as 14‐106FF1 IF4, was further expanded and grown in ExCell medium (Sigma; catalogue no. H4281) supplemented with 4 mm l‐glutamine and 0·1% FBS for in vitro antibody production. The antibody was purified using protein A chromatography and the purified antibody was sterilized by filtration.
Flow cytometry
Either 1 × 105 cells from a cell line or 5 × 105 primary cells per sample were used for flow cytometry analysis. All antibodies were used with appropriate isotype controls. CD1d‐specific antibodies were anti‐rat/mouse WTH‐1 and WTH‐227 and generated in the laboratory of TH, and anti‐mouse 1B1 phycoerythrin (PE)13 from Becton Dickinson (Franklin Lakes, NJ). Purified H2E‐specific anti‐mouse/rat I‐Ek mAb (14‐4‐4S; Affymetrix, Santa Clara, CA) was used to stain MHC class II molecules with a pre‐adsorbed (10% normal cotton rat serum for 1 hr at 4°) F(ab′)2 fragment goat anti‐mouse IgG (H+L) R‐PE (GαM R‐PE) as secondary antibody (Jackson ImmunoResearch, West Grove, PA). A rabbit anti‐cotton rat IgG antibody (Virion Systems, Rockville, MD) was used to stain IgG and was detected with a pre‐adsorbed (10% cotton rat serum for 1 hr) goat anti‐rabbit IgG (H+L) FITC secondary antibody (GαR FITC). CD3 stainings were performed intracellularly with a rat anti‐human CD3ε FITC mAb (clone CD3‐12; AbD Serotech, Raleigh, NC) using the Leucoperm™ fixation and permeabilization kit (AbD Serotech). CD1d dimer stainings were carried out as previously described31 and a biotinylated hamster anti‐mouse CD3ε antibody (145‐2C11; BD Pharmingen) was used to identify TCR expression of TCR transductants. CD1d dimer staining of cotton rat splenocytes followed the same protocol, using a different secondary antibody (pre‐adsorbed GαM R‐PE) and anti‐human CD3 FITC.
Measurements were performed with a FACSCalibur™ analyser and data was analysed with flowjo software. A live gate on lymphocytes was used for the evaluation of all samples. Cell sorting was performed with a FACS Aria III cell sorter (BD Biosciences).
Results
To obtain the sequence of CD1d in the cotton rat, nested PCR was performed on cDNA from cotton rat splenocytes based on primers derived from an alignment of human, mouse and rat CD1d sequences. The resulting partial sequence of cotton rat CD1d (crCD1d) was used to design primers for the determination of the upstream 5′ and the downstream 3′ end by RACE‐PCR. The complete coding sequence from four clones from spleen and 10 clones from thymus was uniform and was compared to the CD1d sequences of other species (Fig. 1a). To determine the overall conservation of crCD1d compared to human (huCD1d), mouse (mCD1d), rat (rCD1d), and Chinese hamster (chCD1d), the sequence identities were determined using alignments calculated with clustal omega software. The number of identical amino acids/nucleotides was divided by the total length of the alignment, so taking gaps into account, and the results were expressed as a rounded percentage. The similarity of sequences between species correlated with their evolutionary relationship. The crCD1d shares an amino acid (nucleotide) homology of 71% (80%) with hamster CD1d, 69% (78%) with mouse CD1d, 69% (76%) rat and 62% (73%) with human CD1d (Fig. 1b). The transmembrane and intracellular portion of CD1d was relatively diverse between species with an amino acid homology of only around 30%. In contrast, the extracellular portion that binds the antigen and interacts with the TCR was highly conserved. We found no evidence for CD1 genes other than CD1d in the cotton rat. The same is true for database analysis of C. griseus and Mesocricetus auratus, both members of the Cricetidae. Therefore, mouse CD1d and human CD1a–e were used for a blast analysis of hamster genomes. CD1d was the only homologue found in those species, whereas in primates and other non‐muroid species CD1a, b, c and e homologues are found. This suggests that the deletion of these homologues32 occurred at the latest during basal radiation of Muroidea [to which Cricetidae (e.g. cotton rat and hamster) and Muridae (e.g. mouse and rat) belong] between 24·5 and 25·9 million years ago.33
Figure 1.
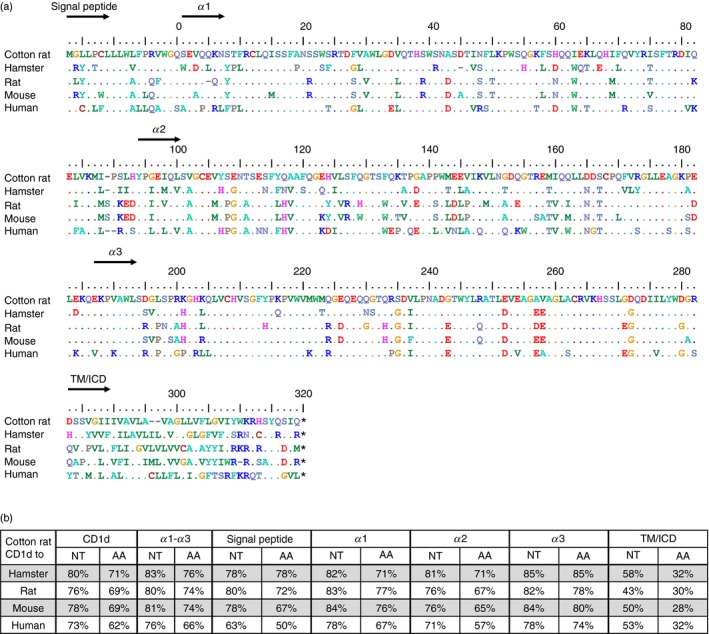
CD1d amino acid sequence alignment of different species. (a) Cotton rat CD1d (Sigmodon hispidus: KM_267558) was compared to Chinese hamster (Cricetulus griseus, GenBank: XM_007644702.1), rat (GenBank: KM_267558), mouse (GenBank: NM_007639.3), and human CD1d (GenBank: NM_001766.3). The alignment was calculated with clustal omega. Dots indicate identical amino acids, dashes indicate gaps, and asterisks indicate stop codons. The different regions are depicted in this order: signal peptide, extracellular regions α1, α2, α3 and transmembrane/intracellular regions. (b) Identical nucleotides or amino acids were counted and the overall identity was calculated as the ratio of identical nucleotides/amino acids to the length of the alignment, therefore including gaps, and are given in rounded percentages.
Identification of mAbs reactive with crCD1d
Different mAbs specific for mouse and rat CD1d were tested for cross‐reactivity with crCD1d. For this purpose, the mouse anti‐rat mAbs WTH‐1 and WTH‐2, as well as the rat anti‐mouse mAb 1B1, were tested.27, 34 WTH‐1 and WTH‐2 were produced from B‐cell hybridomas derived from CD1d‐deficient mice immunized with rat CD1d‐transfected cells and bind to mouse and rat CD1d with the same avidity,27, 34 whereas 1B1 mAb binds only mouse CD1d.12 The three mAbs were used to stain the human B‐cell lymphoma Raji, transduced with CD1d of mouse, rat, or cotton rat using a vector with enhanced GFP (EGFP) expressed 3′ of an internal ribosomal entry site. Hence, EGFP expression correlated directly with CD1d expression. To directly compare the avidity of the mAbs for the different CD1d molecules, the cells were stained with WTH‐1, WTH‐2 or 1B1. The cells were then analysed and the depicted histograms were generated from cells of the same EGFP intensity (Fig. 2). Staining with WTH‐1 mAb confirmed equal recognition of this mAb for cell surface‐expressed CD1d of either mouse or rat,27 whereas crCD1d could not be detected. WTH‐2 stained crCD1d with the same intensity as mouse and rat CD1d. This identical staining intensity for the different species was also found for lower, non‐saturating concentrations of the mAb. As expected, 1B1 bound to mouse but not rat CD1d whereas staining intensity of crCD1d was about 15 times lower than for mouse CD1d. In summary, WTH‐2 stains crCD1d with the same intensity as mouse and rat CD1d and can be used to directly compare CD1d expression levels between these species.
Figure 2.
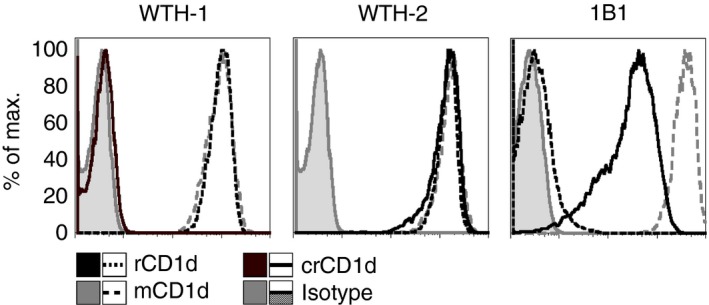
WTH‐2 and 1B1 but not WTH‐1 monoclonal CD1d antibodies can be used to stain cotton rat CD1d. Representative data from one of two experiments in total are shown. The biotinylated monoclonal mouse anti‐rat antibodies WTH‐1 and WTH‐2 (each 125 ng/ml), detected with streptavidin‐allophycocyanin, and rat anti‐mouse 1B1 phycoerythrin (2 μg/ml) were used to stain Raji cells transduced with rat, mouse, or cotton rat CD1d. Staining intensities are compared to isotype controls.
Organ‐specific CD1d expression in cotton rat primary cells
CD1d is constitutively expressed on haematopoietic cells of rats and mice,25 and the pattern and level of expression are remarkably similar in LEW rats and C57BL/6 mice. However, crCD1d expression patterns and levels determined with mAb WTH‐2 differ considerably from those observed in mice (Fig. 3a). In accordance with previous studies,27 mice constitutively expressed CD1d at approximately the same levels in thymus, lymph nodes and spleen. When comparing CD1d expression in mice and cotton rats, the expression pattern was most similar for thymus, where a rather homogeneous CD1d expression is seen in both species, but staining intensity in cotton rat was only 20–25% of what is seen in mouse. CD1d expression in lymph nodes of C57BL/6 mice was also rather homogenous, whereas in cotton rat a small CD1d‐negative population (< 10%) and two positive populations were found. Of the two positive populations, one (about 30% of all cells) expressed less CD1d than mouse lymphocytes, and one population (almost 60% of total cells) more than mouse lymphocytes. CD1d expression in mouse spleen was homogeneous with the exception of a small population of CD1d very high cells (3%) (foothill in histogram of C57BL/6 spleen), which consists mainly of marginal zone B cells.27 In the cotton rat spleen, a small population of CD1d‐negative cells (< 10%) and two larger populations of CD1d intermediate (38%) and high cells (40%) were found. Additionally, similar to mouse spleen, a foothill of CD1d very high cells (12%) could be seen, which might also contain marginal zone B cells. This assumption is supported by the fact that this small peak of the histogram does not occur in lymph node stainings.
Figure 3.
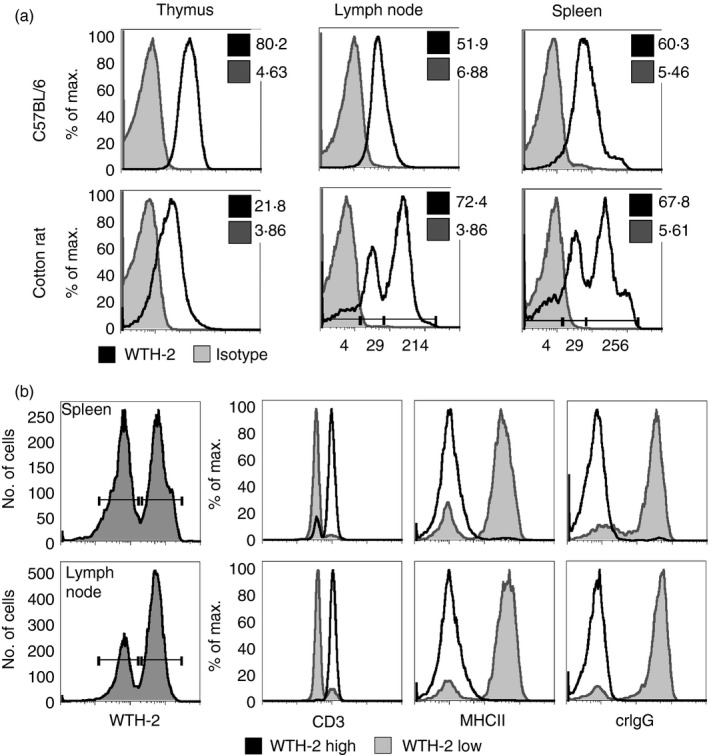
Organ‐specific CD1d expression in cotton rat primary cells. (a) Representative data for a total of three experiments are shown. Cells (5 × 105) isolated from mouse tissue or cotton rat tissues were stained with the monoclonal antibody (mAb) WTH‐2 FITC (2 μg/ml) or an isotype control. Geometric means of isotype control (tinted grey) or CD1d staining (black) are indicated in each graph. Geometric means of different expression levels (cotton rat lymph node and spleen) are additionally indicated below the histogram. (b) Primary cells (5 × 105) where stained with biotinylated WTH‐2 (3·6 μg/ml) and antibodies against CD3 (2 μg/ml), MHC class II (5 μg/ml, GαM R‐PE) and cotton rat IgG (10 μg/ml, GαR FITC). WTH‐2 positive cells from cotton rat spleen and lymph node were divided into low and high expression and CD3, MHC class II and cotton rat IgG expression of those two populations are compared in histograms. Representative data for two independent experiments are shown.
Two distinct CD1d expressing populations in cotton rats were further characterized by antibodies against CD3ε, MHC class II (14‐4‐4S, specific for H2E and RT1D), and crIgG (Fig. 3b). Cells expressing high levels of CD1d (as shown by WTH‐2 staining) were mostly CD3 positive whereas almost all CD3‐negative cells expressed CD1d at lower levels. The opposite was true for MHC II and crIgG stainings, where CD1d high cells did not express MHC II or crIgG. Evidently, cotton rat T cells express higher levels of CD1d than non‐T cells. This is in stark contrast to the lower CD1d expression of CD3‐positive cells compared with CD3‐negative cells of rat and mouse.27 In conclusion, the expression patterns of CD1d in cotton rats are therefore remarkably different from rats and mice.
Cotton rat CD1d presents glycolipids and binds iNKT TCR
In contrast to mouse, rat and human CD1d, bovine CD1d was found to lack the capacity to present typical iNKT TCR ligands such as αGC.35 To test the capacity of crCD1d to present glycolipids and to bind to iNKT TCR, crCD1d dimers were produced. For this purpose, the extracellular domains of crCD1d were cloned into the pXIg vector that contains a mIgG heavy chain,36 and transfected into J558L cells, which express a λ light chain.36 J558L cell clones were then selected for high production of crCD1d–mIgG fusion proteins with a mouse IgG‐specific ELISA.36 The purified and concentrated dimers were loaded with PBS57 as an antigen or incubated with DMSO as a negative control.6 To test whether the CD1d molecule in cotton rats is able to present glycolipids to iNKT cells, a stimulation assay was performed with mouse T‐cell hybridoma cells expressing a transfected rat iNKT TCR. This cell line was cultured in the presence of immobilized rat or cotton rat CD1d–mIgG dimers PBS57‐loaded/unloaded and an anti‐CD28 antibody (Fig. 4a). As shown in Fig. 4(a), PBS57‐loaded CD1d dimers induce IL‐2 production of the responder cells in a concentration‐dependent manner, whereas unloaded dimers alone did not stimulate IL‐2 secretion. The CD1d–mIgG dimers were also used to stain the same iNKT TCR transductant in a flow cytometry assay (Fig. 4b). Vehicle‐loaded dimers were used as a negative control and rat CD1d–mIgG dimers served as a positive control. In comparison to rat CD1d dimers, crCD1d bound with the same or slightly higher affinity while generating less background staining.
Figure 4.
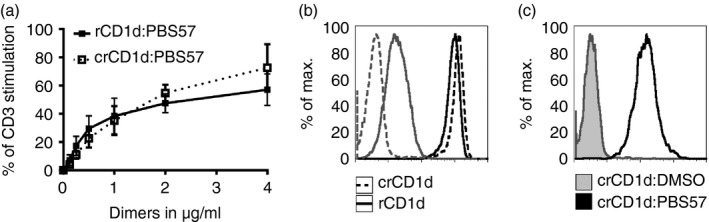
Cotton rat (cr) CD1d presents glycolipids and binds invariant natural killer T (iNKT) T‐cell receptor (TCR). (a) CD1d dimers loaded with the glycolipid PBS57 were diluted with CD1d vehicle‐loaded dimers (DMSO) to CD1d : PBS57 concentrations of 4, 2, 1, 0·5, 0·25, 0·125 and 0 μg/ml. Monoclonal antibody (mAb) anti‐mouse CD3 was used as a positive control (4 μg/ml) and anti‐rat CD28 mAb (4 μg/ml) was added to every sample. The dilutions were coated on a 96‐well plate overnight. Cells (5 × 104) expressing a rat iNKT TCR were added and the stimulation assay was cultured for 22 hr. Supernatants were analysed by measuring mouse interleukin‐2 (IL‐2) by ELISA. The results were calculated as a percentage of the IL‐2 production following stimulation with anti‐mouse CD3. Three independent experiments were carried out in total, mean + SD were calculated with graphpad prism. (b) crCD1d dimers loaded with PBS57 (4 μg/ml, black) were used to stain cells expressing rat iNKT TCR and detected with donkey anti‐mouse secondary antibody. Dimers loaded with the vehicle DMSO (grey) served as a negative control. Geometric means are as follows: rat CD1d : DMSO 17, rat CD1d : PBS57 851, crCD1d : DMSO 4, crCD1d : PB57 1192. The staining is representative for three experiments. (c) The iNKT α‐chain 1 (pEGN crAV14 clone 1) was used to transduce mouse BW cells, together with a rat β‐chain. The transductants were stained with crCD1d dimers loaded with PBS57 (black) and detected with donkey anti‐mouse secondary antibody. Loading with the vehicle DMSO (tinted grey) served as a negative control. Geometric means are as follows: crCD1d : DMSO 3, crCD1d : PBS57 138. The staining is representative for three experiments.
Cotton rat splenocytes produce cytokines in response to typical iNKT cell antigens
A hallmark of iNKT cells is the strong production of IFN‐γ and IL‐4 in response to typical antigens such as the glycolipids αGC and PBS57. Primary splenocytes were stimulated with αGC or PBS57 for 24 hr and IFN‐γ and IL‐4 production was measured with an ELISA (Fig. 5a). Both glycolipids induced a dose‐dependent production of both cytokines. Induction of cytokines in vivo was also tested. Upon intraperitoneal injection of KRN7000 (0·1 μg/g bodyweight) of two groups of five cotton rats each, no increased serum levels of IL‐4 and IFN‐γ could be detected after 2, 4 and 6 hr.
Figure 5.
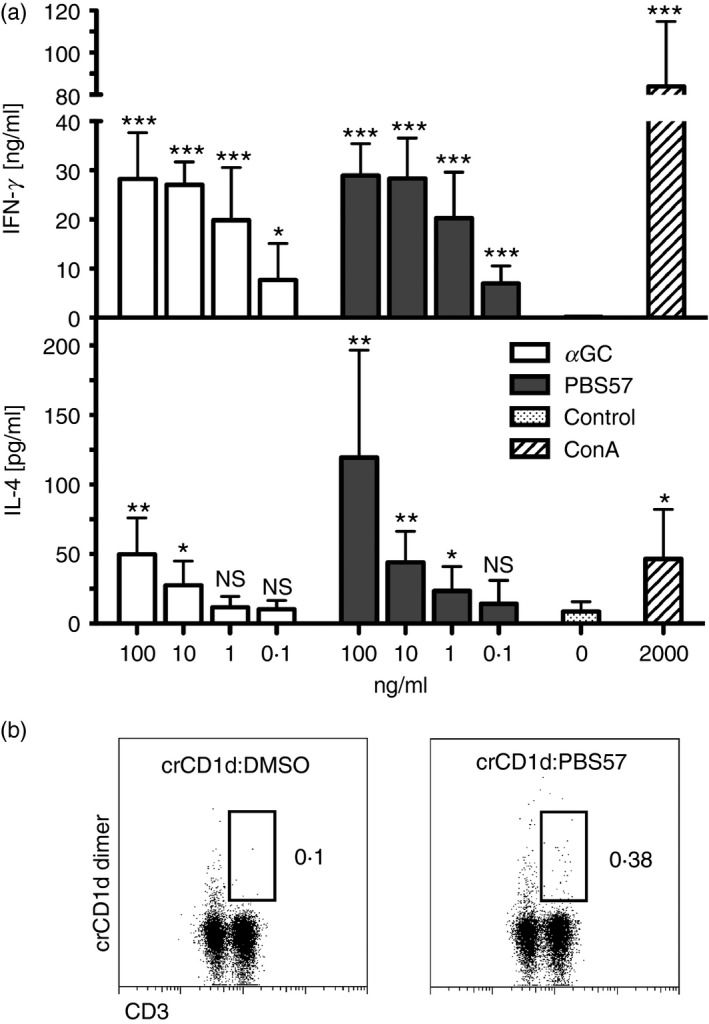
Cotton rat splenocytes produce cytokines in response to typical glycolipid antigens and invariant natural killer T (iNKT) cells can be detected with cotton rat (cr) CD1d dimers. (a) Splenocytes (1 × 106) were stimulated with different concentrations of the glycolipids α‐galactosylceramide (αGC) or PBS57 ranging from 100 to 0·1 ng/ml. Media was used as a negative control, 2 μg/ml concanavalin A as positive control. Stimulations were carried out in duplicates and cytokine production was measured with cotton rat interferon‐γ (IFN‐γ) and interleukin‐4 (IL‐4) ELISA. Three experiments were carried out in total and mean + SD were calculated using graphpad prism. Statistical analysis is indicated above columns in comparison to negative control as ns: P > 0·05,*P < 0·05, ** P < 0·005, *** P < 0·0005, unpaired Student's t‐test. (b) Splenocytes were stained with antigen‐loaded or control crCD1d dimers (detected with GαM R‐PE) followed by intracellular staining of CD3. Gates on CD3‐positive, crCD1d‐dimer‐positive cells were set to indicate NKT cells. The experiment is representative for three individual experiments, the overall frequency of iNKT cells was 0·23% ± 0·05.
Detection of cotton rat iNKT cells in primary cells with crCD1d dimers
To extend our studies to potentially detect iNKT cells ex vivo we used crCD1d dimers to identify a possible iNKT cell population in cotton rat splenocytes (Fig. 5b). These dimers were detected with a secondary antibody and a double staining with anti‐CD3 mAb was used to identify T cells and subsequently CD3‐positive, CD1d dimer‐positive iNKT cells. A small population of 0·28% of live cells (vehicle control versus antigen‐loaded dimers) was detected via indirect staining with CD1d dimers in one experiment and mean iNKT cell frequency of 0·23% ± 0·05 from three experiments in total could be calculated. Cross‐reactivity of rat CD1d dimers with cotton rat cells was observed; however, there was a less pronounced background staining with crCD1d dimers compared with stainings with rat dimers (data not shown).
Canonical AV14/AJ18 rearrangements in the cotton rat
The cotton rat AV14/AJ18 rearrangement was obtained using the same strategy applied to crCD1d. In F344 rats, iNKT cells are more frequent in the liver than the spleen,31 therefore, IHL cDNA was used for the initial amplification of a partial sequence of this α‐chain. The 5′ and 3′ end was then obtained using the RACE‐PCR protocol. The overall conservation of the cotton rat iNKT rearrangement was determined using clustal omega to calculate an alignment with Chinese hamster, rat, mouse and human canonical rearrangements (Fig. 6a). The CDR1–3 region and the hypervariable region 4 (HV4) are based on published data and indicated in the alignment.28 The number of identical amino acids/nucleotides was divided by the total length of the alignment, so taking gaps into account, and the results were expressed as a percentage. Comparable to the sequence analysis of CD1d, the cotton rat AV14/AJ18 rearrangement shared an amino acid (nucleotide) homology of 80% (86%) with the predicted hamster rearrangement, 75% (83%) with rat, 74% (82%) with mouse and only 66% (75%) with the homologous human rearrangement (Fig. 6b). However, the human AV14 shows 65% amino acid identity with cotton rat AV14, which is comparable to rodents (rat: 65% and mouse: 68%) and AJ18 is even more conserved between species.
Figure 6.
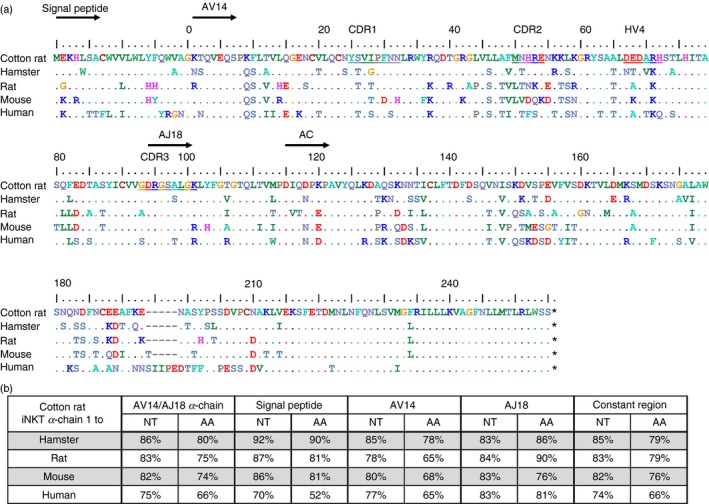
Invariant natural killer T (iNKT) α‐chain amino acid sequence alignment of different species. (a) A cotton rat α‐chain (pEGN crAV14 clone 1, GenBank: KT367785) was compared to Chinese hamster (Cricetulus griseus designed from genomic sequences, GenBank: NW_003615069.1 and NW_003614213.1), rat (GenBank: ABC69268.1), mouse (GenBank: AAA40180.1), and human α‐chains (GenBank: ABC72374.1). The alignment was calculated with clustal omega. Dots indicate identical amino acids, dashes indicate gaps, and asterisks indicate stop codons. The different regions are indicated and depict the signal peptide, AV14, AJ18 and the constant region of the T‐cell receptor α‐chain, in this order. The regions of CDR1, CDR2, hypervariable region 4 (HV4), and CDR3 are underlined in the top row of the alignment.
Cotton rats possess multiple AV14 family members
To address the question whether cotton rats, like rats, possess multiple AV14‐family members, cloning and sequencing of AV14/AJ18 rearrangements on mRNA level and AV14 on genomic level were performed. Cloning and sequencing of α‐chains obtained from cotton rat IHL cDNA resulted in the discovery of six different members of the AV14‐family on the genomic levels and three different cDNAs corresponding each to a genomic rearrangement (Fig. 7a). The transcribed AV14‐family members of the cotton rat show differences in the CDR1, CDR2, hypervariable region 4 and at position 78 of AV14. Functionality of one family member (pEGN crAV14 clone 1) could be shown by staining of iNKT TCR transductants with crCD1d–immunoglobulin dimers (Fig. 4b).
Figure 7.
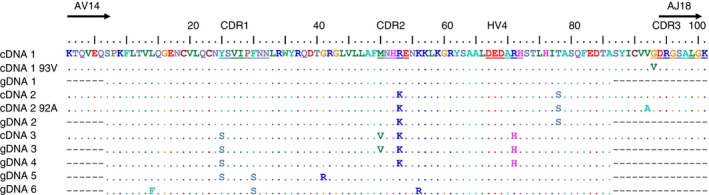
Cotton rats possess multiple AV14‐family members with canonical invariant natural killer T (iNKT) T‐cell receptor (TCR) rearrangements. (a) Cloning of intrahepatic lymphocyte iNKT TCR α‐chains amplified from cDNA into pEGN was used to identify different AV14‐family members. The alignment was calculated with clustal omega. Dots indicate identical amino acids. The different regions are indicated and depict the protein sequences of AV14 and the first part of AJ18. The CDR1–3 regions and hypervariable region 4 (HV4) are underlined in the top row of the alignment. The frequencies of the different members in the pool of sequenced clones were as follows: cDNA1 5/17, cDNA1 93V 1/17, gDNA1 2/20, cDNA2 3/17, cDNA2 92A 1/17, gDNA2 4/20, cDNA3 7/17, gDNA3 1/20, gDNA4 8/20, gDNA5 2/20, gDNA6 3/20.
Discussion
The glycolipid antigen‐presenting molecule CD1d was cloned from cotton rat tissue and analysed for tissue expression and functionality. Nucleotide and amino acid sequences were clearly more similar to those of rodents than of humans. According to Steppan et al., the split between the murid and cricetid groups containing rat and mice or hamster and cotton rat, respectively, happened about 23·3–24·9 million years ago, whereas the split between the Arvicolinae‐Cricetinae (hamster) and Sigmodontineae‐Netominae (cotton rat) occurred 18·7–19·6 million years ago. Consistent with this timeline, the overall similarity between CD1d of hamster and cotton rat was slightly higher than between cotton rat and rats or mice, respectively. The cloned CD1d gene was expressed in human B‐cell lymphoma. Staining revealed a slight cross‐reactivity to crCD1d with the rat anti‐mouse CD1d mAb 1B1. WTH‐1 and WTH‐2 antibodies generated in CD1d knock‐out mice reacted quite differently. WTH‐1 mAb did not bind to crCD1d at all but readily detected rat and mouse CD1d, which is consistent with the location of the WTH‐1 epitope in the loop linking the α1 and α2 domains.27 The aspartic acid at positions 92 of rat or 93 of mouse CD1d is especially important for the binding to WTH‐1. This aspartic acid is replaced by a histidine in cotton rat CD1d. As this substitution is not found for hamster, it might be interesting to test WTH‐1 reactivity in this species, although differences in adjacent amino acids could also affect WTH‐1 reactivity. The WTH‐2 epitope of mouse and rat is not yet mapped but it appears to be conserved because this mAb binds CD1d of mouse, rat and cotton rat equally well and it will be worthwhile to test its reactivity for other rodent species. CD1d cell surface expression of cells from lymphoid organs was analysed with mAb WTH‐2. Occurrence of CD1 genes and their expression differ considerably between species. This may in part reflect the fact that specialized CD1 molecules bind distinct antigens and present them to different T‐cell populations. Furthermore, so far unknown functions beyond antigen presentation are likely to exist, given some unique patterns of expression such as the massive expression of CD1d in exocrine acinar cells of rats.27 With respect to CD1d, expression on haematopoietic cells also shows some differences between distantly related species such as mouse and rat on the one hand and humans on the other, but between murine species it appears to be nearly identical.27 As suggested above, cotton rats likely possess CD1d but no other members of the CD1 family. Therefore, it is especially interesting to discuss possible functional consequences of differences in CD1d expression between haematopoietic cell populations of rat and mouse versus cotton rat. In the case of thymocytes, the expression of CD1d was considerably lower than in mouse and rat. As iNKT cells are positively selected on immature thymocytes and TCR avidity affects selection and functionality of the emerging iNKT cells, it will be interesting to learn whether this somewhat lower expression results in a different affinity or density of cotton rat iNKT TCR or unique functional features of cotton rat iNKT cells. The same applies to different expression levels of B and T lymphocytes as well as the cross‐talk of iNKT cells with these cells. The production of crCD1d dimers allowed us to test the functionality of crCD1d in terms of binding and presentation of typical iNKT TCR antigens. In both instances, crCD1d was clearly functional, as loading of CD1d with a classical iNKT TCR antigen leads to binding to iNKT TCR transductants and activation of these cells. A cotton rat TCR α‐chain with a typical iNKT TCR α‐chain rearrangement was cloned and expressed together with rat and mouse β‐chains (unpublished data) and was found to bind αGC‐loaded crCD1d dimers, corroborating the existence of canonical iNKT TCR in cotton rat and the specificity of crCD1d dimers. Cloning and sequencing of α‐chains obtained from cotton rat IHL cDNA resulted in the discovery of six different members of the AV14‐family on the genomic DNA level and three different cDNAs corresponding each to a genomic rearrangement, showing that occurrence and use of multiple AV14‐family members by rat iNKT TCR28, 31, 37, 38 is not a peculiarity of rat iNKT TCR. In some rearrangements glycine 93 or valine 92 were substituted by valine or alanine, respectively. This is analogous to substitutions that, in rat and mouse, respectively, were found to affect ligand binding.28, 39 Furthermore, the cytokine production of splenocytes in response to typical iNKT cell antigens such as αGC and PBS57 indicates the existence of a typical iNKT cell population in this species. The levels of IFN‐γ were about 100‐fold higher and for IL‐4 twice as high as levels in F344 rats.31 This may in part reflect the higher iNKT cell frequency, which is about 10‐fold compared with F344 rats but it may also be the result of indirect effects, especially in the case of IFN‐γ, which may have been produced by NK cells after activation by iNKT cells.40 However, no IL‐4 and IFN‐γ could be detected in the serum of cotton rats injected intraperitoneally with αGC. This lack of response resembles clinical data, where no increased serum levels of IL‐4 were detected after intravenous injection of escalating doses of KRN7000, a synthetic αGC, and increased serum‐IFN‐γ was detected for only one of ten patients with cancer who had high iNKT cell numbers before treatment.41 This increase was found only at a single time‐point (8 hr) out of seven time‐points within the first 2 days after drug administration. Hence, the lack of a strong systemic serum IFN‐γ response, which is different to what has been found in mice,42, 43 might make cotton rats an especially suitable model to test iNKT cell action, e.g. in vaccination against human pathogens.
In summary, the results and reagents presented in this paper should prove to be valuable in further analysis of cotton rat immune function, especially when analysing the complex function of iNKT cells in infectious disease models. In the mouse, a role of iNKT cells has been demonstrated for infections such as influenza and hepatitis B virus infection. In the cotton rat, these studies could be extended to a variety of human respiratory viruses so combining the natural susceptibility of this species with our novel reagents for iNKT cell analysis.
Contribution of authors
ASF planned, performed and analysed experiments, and wrote the manuscript. DP and LS performed and analysed experiments. RS provided reagent. SN and TH conceived the study, planned and analysed experiments, and wrote the manuscript.
Disclosure
The authors declare no commercial or financial conflict of interest.
Acknowledgements
We thank Dr Paul Savage (Brigham Young University, Provo, UT, USA) for providing the glycolipid PBS57 and C. Linden for the technical assistance. This project was funded by Deutsche Forschungsgemeinschaft HE 2346/6‐1 and the Baron von Swaine Stipendium/Universitätsbund‐Würzburg awarded to *ASF.
References
- 1. Niewiesk S, Prince G. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim 2002; 36:357–72. [DOI] [PubMed] [Google Scholar]
- 2. Boukhvalova MS, Prince GA, Blanco JC. The cotton rat model of respiratory viral infections. Biologicals 2009; 37:152–9. PubMed PMID: 19394861. Pubmed Central PMCID: 2882635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faith RE, Montgomery CA, Durfee WJ, Aguilar‐Cordova E, Wyde PR. The cotton rat in biomedical research. Lab Anim Sci 1997; 47:337–45. [PubMed] [Google Scholar]
- 4. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25:297–336. PubMed PMID: 17150027. Epub 2006/12/08. eng. [DOI] [PubMed] [Google Scholar]
- 5. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K et al CD1d‐restricted and TCR‐mediated activation of vα14 NKT cells by glycosylceramides. Science 1997; 278:1626–9. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A et al A modified α‐galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods 2006; 312:34–9. [DOI] [PubMed] [Google Scholar]
- 7. Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol 2007; 7:929–41. PubMed PMID: ISI:000251139700011. English. [DOI] [PubMed] [Google Scholar]
- 8. de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia‐Alles LF, Malm D et al Assistance of microbial glycolipid antigen processing by CD1e. Science 2005; 310:1321–4. [DOI] [PubMed] [Google Scholar]
- 9. Kasmar A, Van Rhijn I, Moody DB. The evolved functions of CD1 during infection. Curr Opin Immunol 2009; 21:397–403. PubMed PMID: 19541469. Pubmed Central PMCID: 2725205. Epub 2009/06/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YH, Wang B, Chun T, Zhao L, Cardell S, Behar SM et al Expression of CD1d2 on thymocytes is not sufficient for the development of NK T cells in CD1d1‐deficient mice. J Immunol 1999; 162:4560–6. [PubMed] [Google Scholar]
- 11. Ichimiya S, Kikuchi K, Matsuura A. Structural analysis of the rat homologue of CD1. Evidence for evolutionary conservation of the CD1D class and widespread transcription by rat cells. J Immunol 1994; 153:1112–23. PubMed PMID: 7517972. Epub 1994/08/01. eng. [PubMed] [Google Scholar]
- 12. Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL et al Mouse CD1 is mainly expressed on hemopoietic‐derived cells. J Immunol 1997; 159:1216–24. [PubMed] [Google Scholar]
- 13. Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen‐presenting cells and marginal zone B cells. J Immunol 1998; 160:3121–7. [PubMed] [Google Scholar]
- 14. Bendelac A. Positive selection of mouse NK1+ T cells by CD1‐expressing cortical thymocytes. J Exp Med 1995; 182:2091–6. PubMed PMID: 7500054. Pubmed Central PMCID: 2192225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ et al Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005; 3:e113. PubMed PMID: 15799695. Pubmed Central PMCID: 1073691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 2010; 22:79–86. [DOI] [PubMed] [Google Scholar]
- 17. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 2007; 5:405–17. [DOI] [PubMed] [Google Scholar]
- 18. Novak J, Lehuen A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine 2011; 53:263–70. [DOI] [PubMed] [Google Scholar]
- 19. Meyer EH, DeKruyff RH, Umetsu DT. iNKT cells in allergic disease. Curr Top Microbiol Immunol 2007; 314:269–91. [DOI] [PubMed] [Google Scholar]
- 20. Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013; 13:101–17. [DOI] [PubMed] [Google Scholar]
- 22. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO et al Invariant NKT cells reduce the immunosuppressive activity of influenza A virus‐induced myeloid‐derived suppressor cells in mice and humans. J Clin Investig 2008; 118:4036–48. PubMed PMID: 19033672. Pubmed Central PMCID: 2582442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol 2002; 76:4294–303. PubMed PMID: 11932395. Pubmed Central PMCID: 155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pyz E, Naidenko O, Miyake S, Yamamura T, Berberich I, Cardell S et al The complementarity determining region 2 of BV8S2 (Vβ8.2) contributes to antigen recognition by rat invariant NKT cell TCR. J Immunol 2006; 176:7447–55. PubMed PMID: 16751390. Epub 2006/06/06. eng. [DOI] [PubMed] [Google Scholar]
- 25. Luhder F, Huang Y, Dennehy KM, Guntermann C, Muller I, Winkler E et al Topological requirements and signaling properties of T cell‐activating, anti‐CD28 antibody superagonists. J Exp Med 2003; 197:955–66. PubMed PMID: 12707299. Pubmed Central PMCID: 2193880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreiss M, Asmuss A, Krejci K, Lindemann D, Miyoshi‐Akiyama T, Uchiyama T et al Contrasting contributions of complementarity‐determining region 2 and hypervariable region 4 of rat BV8S2+ (Vβ8.2) TCR to the recognition of myelin basic protein and different types of bacterial superantigens. Int Immunol 2004; 16:655–63. [DOI] [PubMed] [Google Scholar]
- 27. Monzon‐Casanova E, Steiniger B, Schweigle S, Clemen H, Zdzieblo D, Starick L et al CD1d expression in paneth cells and rat exocrine pancreas revealed by novel monoclonal antibodies which differentially affect NKT cell activation. PLoS ONE 2010; 5:e13089. doi: 10.1371/journal.pone.0013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paletta D, Fichtner AS, Hahn AM, Starick L, Beyersdorf N, Monzon‐Casanova E et al The hypervariable region 4 (HV4) and position 93 of the α chain modulate CD1d‐glycolipid binding of iNKT TCRs. Eur J Immunol 2015; 45:2122–33. [DOI] [PubMed] [Google Scholar]
- 29. Schneck JP. Monitoring antigen‐specific T cells using MHC‐Ig dimers. Immunol Invest 2000; 29:163–9. [DOI] [PubMed] [Google Scholar]
- 30. Schumann J, Voyle RB, Wei BY, MacDonald HR. Cutting edge: influence of the TCR V beta domain on the avidity of CD1d: α‐galactosylceramide binding by invariant Vα14 NKT cells. J Immunol 2003; 170:5815–9. PubMed PMID: 12794105. Epub 2003/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 31. Monzon‐Casanova E, Paletta D, Starick L, Muller I, Sant'Angelo DB, Pyz E et al Direct identification of rat iNKT cells reveals remarkable similarities to human iNKT cells and a profound deficiency in LEW rats. Eur J Immunol. 2013;43:404–15. PubMed PMID: 23165932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dascher CC, Brenner MB. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol 2003; 24:412–8. [DOI] [PubMed] [Google Scholar]
- 33. Steppan S, Adkins R, Anderson J. Phylogeny and divergence‐date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol 2004; 53:533–53. [DOI] [PubMed] [Google Scholar]
- 34. Pyz E. Identification of rat NKT cells and molecular analysis of their surface receptor mediated activation. Würzburg: University of Würzburg, 2004: p. 91. [Google Scholar]
- 35. Wang J, Guillaume J, Pauwels N, Van Calenbergh S, Van Rhijn I, Zajonc DM. Crystal structures of bovine CD1d reveal altered αGalCer presentation and a restricted A' pocket unable to bind long‐chain glycolipids. PLoS ONE 2012; 7:e47989. PubMed PMID: 23110152. Pubmed Central PMCID: 3479135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dal Porto J, Johansen TE, Catipovic B, Parfiit DJ, Tuveson D, Gether U et al A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc Natl Acad Sci USA 1993; 90:6671–5. PubMed PMID: 8341685. Pubmed Central PMCID: 46994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuura A, Kinebuchi M, Chen HZ, Katabami S, Shimizu T, Hashimoto Y et al NKT cells in the rat: organ‐specific distribution of NK T cells expressing distinct Vα14 chains. J Immunol 2000; 164:3140–8. [DOI] [PubMed] [Google Scholar]
- 38. Kinebuchi M, Matsuura A. Rat T‐cell receptor TRAV11 (Vα14) genes: further evidence of extensive multiplicity with homogeneous CDR1 and diversified CDR2 by genomic contig and cDNA analysis. Immunogenetics 2004; 55:756–62. [DOI] [PubMed] [Google Scholar]
- 39. Bedel R, Berry R, Mallevaey T, Matsuda JL, Zhang J, Godfrey DI et al Effective functional maturation of invariant natural killer T cells is constrained by negative selection and T‐cell antigen receptor affinity. Proc Natl Acad Sci USA 2014; 111:E119–28. PubMed PMID: 24344267. Pubmed Central PMCID: 3890789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol 2000; 30:985–92. [DOI] [PubMed] [Google Scholar]
- 41. Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M et al A phase I study of the natural killer T‐cell ligand α‐galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 2002; 8:3702–9. [PubMed] [Google Scholar]
- 42. Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M et al Cutting edge: activation of NK T cells by CD1d and α‐galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol 1999; 163:2373–7. [PubMed] [Google Scholar]
- 43. Burdin N, Brossay L, Kronenberg M. Immunization with α‐galactosylceramide polarizes CD1‐reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol 1999; 29:2014–25. [DOI] [PubMed] [Google Scholar]


