Figure 3.
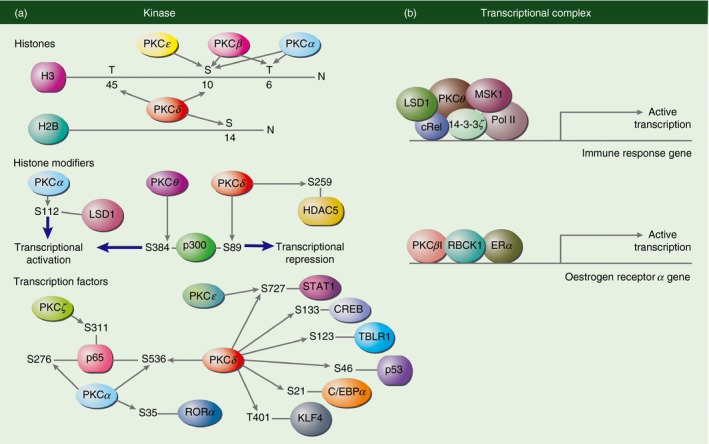
Protein kinase C (PKC) has a role as chromatin regulator in the nucleus. (a) PKC can act as a kinase to phosphorylate histones, histone modifiers or transcription factors at specific serine and/or threonine residues leading to transcriptional activation or repression. Both PKC α and PKC β can phosphorylate histone H3 at threonine (T)6 and serine (S)10 while PKC ε phosphorylates S10 only. PKC δ phosphorylates H3S10, H3T45 and H2BS14. PKC α phosphorylates S112 on lysine specific demethylase 1 (LSD1) leading to transcriptional activation. While PKC θ phosphorylates S384 on p300 causes gene activation, PKC δ phosphorylation on S389 of p300 leads to transcriptional repression. PKC δ can also phosphorylate S259 on histone deacetylase 5 (HDAC5). As for transcription factors, PKC α phosphorylates S276 and S536 on p65 and S35 on retinoic acid‐related orphan nuclear receptor α (ROR α). PKC ζ phosphorylates S311 on p65. PKC δ phosphorylates T401 on Krüppel‐like factor 4 (KLF4), S21 on CCAAT/enhancer‐binding protein α (C/EBP α), S46 on p53, S123 on transducin β‐like 1X‐linked receptor 1 (TBLR4), S133 on cAMP response element‐binding protein (CREB) and S727 on signal transducer and activator of transcription 1 (STAT1). PKC ε can also phosphorylate STAT1 at S727. (b) PKC can also form a transcriptional complex on a gene promoter. For example, PKC θ forms a transcriptional complex with LSD1, mitogen and stress‐activated protein kinase 1 (MSK1), RNA polymerase II (Pol II), 14‐3‐3ζ and cRel on immune response gene promoters to lead to transcriptional activation. PKC βI is recruited to the oestrogen receptor a promoter as part of a regulatory complex with RanBP‐type and C3HC4‐type zinc finger containing 1 (RBCK1) and oestrogen receptor α (ER α) to regulate ER α promoter gene expression.
