Abstract
We have conducted radioastronomical observations of 9 dark clouds with the IRAM 30m telescope. We present the first identification in space of the ketenyl radical (HCCO) toward the starless core Lupus-1A and the molecular cloud L483, and the detection of the related molecules ketene (H2CCO) and acetaldehyde (CH3CHO) in these two sources and 3 additional dark clouds. We also report the detection of the formyl radical (HCO) in the 9 targeted sources and of propylene (CH2CHCH3) in 4 of the observed sources, which extends significantly the number of dark clouds where these molecules are known to be present. We derive a beam-averaged column density of HCCO of ~ 5 × 1011 cm−2 in both Lupus-1A and L483, which means that the ketenyl radical is just ~10 times less abundant than ketene in these sources. The non-negligible abundance of HCCO found implies that there must be a powerful formation mechanism able to counterbalance the efficient destruction of this radical through reactions with neutral atoms. The column densities derived for HCO, (0.5-2.7) ×1012 cm−2, and CH2CHCH3, (1.9-4-2) ×1013 cm−2, are remarkably uniform across the sources where these species are detected, confirming their ubiquity in dark clouds. Gas phase chemical models of cold dark clouds can reproduce the observed abundances of HCO, but cannot explain the presence of HCCO in Lupus-1A and L483 and the high abundances derived for propylene. The chemistry of cold dark clouds needs to be revised in the light of these new observational results.
Keywords: astrochemistry, line: identification, ISM: clouds, ISM: molecules, radio lines: ISM
1. Introduction
Organic molecules are ubiquitous in interstellar clouds. The most complex and saturated ones are found in clouds around young stellar objects, where they are most likely formed on the surface of dust grains and released to the gas phase by thermal evaporation (Herbst & van Dishoeck 2009). In cold dark clouds, the chemical composition is characterized by highly unsaturated carbon chains of the families of polyynes and cyanopolyynes and relatively simple oxygen-bearing organic molecules, whose synthesis relies to a large extent on gas phase chemical processes (Agúndez & Wakelam 2013). However, the widespread ocurrence of methanol (CH3OH) in cold dark clouds, and the more recent detections of other complex and saturated organic molecules such as propylene (CH2CHCH3), methyl formate (CH3OCOH), dimethyl ether (CH3OCH3), methoxy (CH3O), and formic acid (HCOOH) in some dark clouds (Marcelino et al. 2007; Öberg et al. 2010; Bacmann et al. 2012; Cernicharo et al. 2012) have put on the table the role of grain surface reactions and non-thermal desorption processes in these cold and quiescent environments.
Many of the oxygen-bearing organic molecules observed in dark clouds can be described by the general formula HxCnO, with n = 1 (CO, HCO, H2CO, CH3O, and CH3OH), n = 2 (C2O, H2CCO, and CH3CHO), and n = 3 (C3O and HCCCHO). The formation of most of them is reasonably well explained by gas phase chemical reactions, with notable exceptions as in the case of CH3OH (Agúndez & Wakelam 2013). A better understanding of the chemistry of dark clouds must necessarily entail deep observational studies able to enlarge both the number of sources chemically characterized and the inventory of molecules identified. The recent discovery of a starless core in the Lupus molecular cloud with a chemical richness comparable to that of the widely studied cloud TMC-1 (Sakai et al. 2009, 2010), provides an excellent opportunity to bring new observational constraints to the chemistry of dark clouds.
In this Letter we present the first detection in space of the ketenyl radical (HCCO), a missing link in the series HxC2O, in the starless core Lupus-1A and the molecular cloud L483. We also report observations of the more hydrogenated derivatives H2CCO and CH3CHO, the HCO radical, and the highly saturated hydrocarbon CH2CHCH3 in various molecular clouds.
2. Observations
The observations were carried out with the IRAM 30m telescope from September to November 2014 during an observational campaign aimed at searching for negative ions in molecular sources. The observed sources are Lupus-1A (Sakai et al. 2010), L483 (Agúndez et al. 2008), L1521F (Crapsi et al. 2005), the Serpens South complex at the HC7N emission peak 1a (Friesen et al. 2013), L1495B, L1389, L1172, L1251A, and L1512 (Cordiner et al. 2013). The coordinates of the sources were taken from the references quoted above. We used the EMIR receivers in single sideband mode with image rejections >10 dB to cover selected frequency ranges from 83 to 105 GHz (HPBW in the range 23-29″). System temperatures ranged from 80 to 150 K. The highest values were obtained for Lupus-1A due to the low elevation (<20°) reached by this source at the IRAM 30m latitude. We used the FFTS backend in its narrow mode, providing a bandwidth of 1.8 GHz and a spectral resolution of 50 kHz, which translates to velocity resolutions of 0.14-0.18 km s−1 at the observed frequencies. We used the frequency switching technique with a frequency throw of 7.8 MHz to minimize the effects of the standing waves between the secondary mirror and the receivers on the spectral baselines. Pointing and focus were regularly checked every 1-2 h by observing nearby planets or quasars.
3. Results
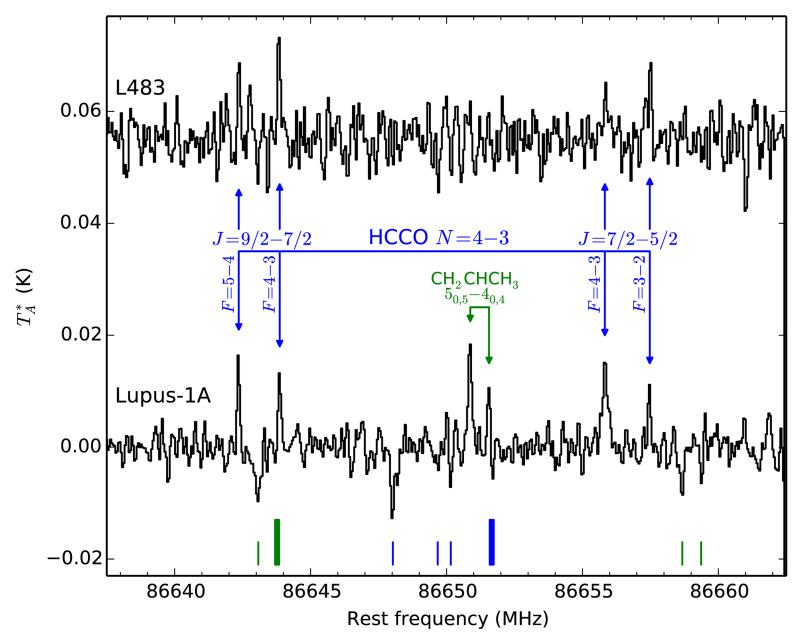
The spectrum of Lupus-1A around 86.65 GHz shows a quartet of emission lines whose frequencies coincide precisely with the strongest fine and hyperfine components of the N = 4 – 3 rotational transition of the HCCO radical (see Fig. 1 and Table 1). The lines, with antenna temperatures of 0.010-0.015 K, are detected at ~10σ confidence after 7 h of ‘on source’ telescope time. The same quartet of lines is detected in L483 at a lower confidence level of ~5σ (see Fig. 1 and Table 1).
Fig. 1.
Spectra of Lupus-1A and L483 around 86.65 GHz showing the quartet of emission lines assigned to HCCO and the doublet assigned to CH2CHCH3 in Lupus-1A. The rest frequency scale corresponds to a systemic velocity VLSR = +5.0 km s−1 (Lupus-1A) and +5.3 km s−1 (L483). The rms is 0.0022 K (Lupus-1A) and 0.0034 K (L483) per 50 kHz channel. The bottom vertical marks indicate the position of the frequency switching negative artifacts, located at ±7.8 MHz from each line. Note that in the Lupus-1A spectrum, the intensity of the HCCO J = 9/2 – 7/2 F = 4 – 3 and the A CH2CHCH3 50,5 – 40,4 lines, whose frequencies differ by 7.7 MHz, is artificially reduced due to an overlap with frequency switching negative artifacts (thick bottom marks).
Table 1.
Observed line parameters of the N = 4 – 3 transition of HCCO
| Transition J′ F′ – J″ F″ |
Frequency (MHz) |
VLSR (km s−1) |
Δv (km s−1) |
(K km s−1) |
|---|---|---|---|---|
| Lupus-1A | ||||
| 9/2 5 – 7/2 4 | 86642.357 | +5.05(4) | 0.36(8) | 0.006(1) |
| 9/2 4 – 7/2 3 | 86643.862 | +5.01(5) | 0.42(10) | 0.006(1)a |
| 7/2 4 – 5/2 3 | 86655.825 | +4.96(5) | 0.71(14) | 0.011(2) |
| 7/2 3 – 5/2 2 | 86657.477 | +5.05(5) | 0.35(10) | 0.004(1) |
|
| ||||
| L483 | ||||
| 9/2 5 – 7/2 4 | 86642.357 | +5.27(4) | 0.35(9) | 0.006(1) |
| 9/2 4 – 7/2 3 | 86643.862 | +5.39(4) | 0.42(8) | 0.009(1) |
| 7/2 4 – 5/2 3 | 86655.825 | +5.20(5) | 0.47(15) | 0.005(1) |
| 7/2 3 – 5/2 2 | 86657.477 | +5.22(4) | 0.36(11) | 0.006(1) |
Numbers in parentheses are 1σ uncertainties in units of the last digits.
Line parameters obtained by gaussian fits to the line profiles.
Intensity is reduced due to overlap with frequency switching artifact.
The ketenyl radical has a planar structure, with a practically linear CCO backbone and the H atom lying out of the linear axis. The radical has a 2A″ ground electronic state and a complex rotational structure whose NKa,Kc levels split in a fine (electronic spin-rotation interaction) and hyperfine (H nuclear spin) structure described by the quantum numbers J and F, respectively (Endo & Hirota 1987; Ohshima & Endo 1993; Sattelmeyer et al. 2004). We obtained the line frequencies from the Cologne Database for Molecular Spectroscopy1 (Müller et al. 2005), where only the levels Ka = 0 are considered, in which case the effective hamiltonian is similar to that of a linear molecule with a 2Σ state. The total dipole moment, which should be only marginally larger than the component along the a axis, has been calculated as 1.59 D by Szalay et al. (1996) and as 1.68 D by Jerosimić (2007). We adopt this latter value for μa.
In Lupus-1A, we derive a kinetic temperature of 14 ± 2 K from observations of CH3CCH J = 5 – 4 (K = 0, 1, 2). At the position of IRAS15398–3359, away from Lupus-1A, Sakai et al. (2009) obtain 12.6 ± 1.5 K. In the Lupus-1A core, Sakai et al. (2010) derive a rotational temperature of 7.3 ± 1 K for C6H, while we derive 8.2 ± 0.8 K and 5.5 ± 0.8 K for C4H and C3N, respectively, from observations of a couple of doublets of lines. Since the dipole moment of HCCO is in between those of C4H and C3N we expect a rotational temperature in between 5.5 and 8.2 K. Adopting a rotational temperature of 7 K, we find a beam-averaged column density of 5.4 × 1011 cm−2 for HCCO (only Ka = 0 levels) in Lupus-1A. In the dense core L483, adopting a rotational temperature of 10 K, close to the gas kinetic temperature (Fuller & Myers 1993; Anglada et al. 1997), we derive a beam-averaged column density of 4.3 × 1011 cm−2. From a previous unsuccessful search for HCCO in interstellar clouds, Turner & Sears (1989) reported an upper limit to its column density of 2.9 × 1010 cm−2 in TMC-1, although from their observational details we derive an upper limit of 5 × 1011 cm−2 in TMC-1, i.e., close to the values derived in Lupus-1A and L483. The difference may arise from the different dipole moment and spectroscopic parameters adopted by these authors and us. In the sources in which we obtained sensitive observations around 86.65 GHz (L1495B, L1521F, and Serpens South 1a) we derive 3σ upper limits to the column density of HCCO around 1011 cm−2, for a rotational temperature of 10 K (see Table 2).
Table 2.
Column densities (in cm−2) and column density ratios
|
N(HCCO) |
N(H2CCO) |
N(HCO) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | N(H2) | Ref | N(HCCO) | N(H2) | N(H2CCO) | N(HCCO) | N(CH3CHO) | N(HCO) | N(H2) | N(CH2CHCH3) |
| Lupus-1A | 1.5 × 1022 | (1) | 5.4 × 1011 | 3.6 × 10−11 | 4.3 × 1012 | 8 | 2.3 × 1012 | 2.5 × 1012 | 1.7 × 10−10 | 2.6 × 1013 |
| L483 | 3.0 × 1022 | (2) | 4.3 × 1011 | 1.4 × 10−11 | 4.4 × 1012 | 10 | 3.2 × 1012 | 2.7 × 1012 | 9.0 × 10−11 | < 1.0 × 1013 |
| L1495B | 1.2 × 1022 | (3) | < 1.3 × 1011 | < 1.1 × 10−11 | 2.2 × 1012 | > 17 | 5.8 × 1011 | 6.7 × 1011 | 5.6 × 10−11 | 2.8 × 1013 |
| L1521F | 1.35 × 1023 | (4) | < 1.0 × 1011 | < 7.4 × 10−13 | 2.8 × 1012 | > 28 | 7.9 × 1011 | 1.7 × 1012 | 1.3 × 10−11 | 1.9 × 1013 |
| Serpens South 1a | 2.0 × 1022 | (5) | < 1.7 × 1011 | < 8.5 × 10−12 | 3.9 × 1012 | > 23 | 1.0 × 1012 | 1.6 × 1012 | 8.0 × 10−11 | 4.2 × 1013 |
| L1389 | 1.0 × 1023 | (6) | – | – | – | – | – | 2.0 × 1012 | 2.0 × 10−11 | – |
| L1172 | 6.0 × 1021 | (7) | – | – | – | – | – | 6.3 × 1011 | 1.1 × 10−10 | – |
| L1251A | 5.1 × 1021 | (8) | – | – | – | – | – | 6.0 × 1011 | 1.2 × 10−10 | – |
| L1512 | 5.7 × 1021 | (3) | – | – | – | – | – | 4.6 × 1011 | 8.1 × 10−11 | – |
Upper limits are given at 3σ confidence. A blank value indicates that observations were not sensitive enough.
References.–
This study;
quoted by Cordiner et al. (2013);
The column densities of H2 in the observed sources, which are needed to convert molecular column densities into fractional abundances relative to H2, are compiled in Table 2. Sakai et al. (2009) estimate a column density of H2 of 3 × 1022 cm−2 toward IRAS15398–3359, located away from Lupus-1A, based on the similar dust continuum flux at 850 and 450 μm of the IRAS source and L1527. In our observations we have detected the J = 1 – 0 line of 13C18O in Lupus-1A, with a velocity-integrated antenna temperature of 0.037±0.006 K km s−1, which translates to a column density of 5.7 × 1013 cm−2 assuming a rotational temperature of 10 K. Adopting the 16O/18O ratio of 500 within the local interstellar medium (Zinner 1996), the relationship between N(13CO) and AV recommended by Tothill et al. (2009) for the Lupus molecular cloud, and the canonical relationship between AV and N(H2) of Bohlin et al. (1978), we derive N(H2) = 1.5 × 1022 cm−2 in Lupus-1A. This value could be somewhat higher if the densities are high enough to result in a significant depletion of CO. In the dense core L483, Anglada et al. (1997) derive N(H2) = 1.4 × 1022 cm−2 from NH3 observations, while Tafalla et al. (2000) derive N(H2) = 3×1022 cm−2 from C17O observations. The much higher value of 9.3 × 1023 cm−2 derived by Jørgensen et al. (2002) from dust continuum flux measurements may correspond to a more compact region of high column density than probed by molecular observations. The fractional abundance relative to H2 of the HCCO radical is then a few 10−11 in both Lupus-1A and L483.
We have observed in Lupus-1A, L483, L1495B, L1521F, and Serpens South 1a, a couple of additional members of the series HxC2O: ketene (H2CCO) and the more saturated species acetaldehyde (CH3CHO), two molecules that have been previously found in various cold dark clouds (Matthews et al. 1985; Irvine et al. 1989; Ohishi & Kaifu 1998; Öberg et al. 2010; Bacmann et al. 2012; Cernicharo et al. 2012). The line parameters are listed in Tables 3 and 4 and the column densities are given in Table 2. The column densities of ketene and acetaldehyde in the five sources where they have been detected are quite uniform, in the range (0.6-4.4) ×1012 cm−2, and similar to those found in TMC-1 (see, e.g., Table 4 of Agúndez & Wakelam 2013). We find that the ketenyl radical is 8-10 times less abundant than ketene in Lupus-1A and L483, while in L1495B, L1521F, and Serpens South 1a, as well as in TMC-1, HCCO is more than 10 times less abundant than H2CCO.
Table 3.
Observed line parameters of H2CCO
| Transition Frequency (MHz) |
JKa,Kc = 51,5 – 41,4 100094.514 |
JKa,Kc = 50,5 – 40,4 101036.630 |
JKa,Kc = 51,4 – 41,3 101981.429 |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Source |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
| Lupus-1A | +5.06(2) | 0.32(2) | 0.043(2) | +5.06(3) | 0.41(7) | 0.036(5) | +5.04(3) | 0.27(4) | 0.037(5) |
| L483 | +5.28(1) | 0.46(2) | 0.069(2) | – | – | – | – | – | – |
| L1495B | +7.62(1) | 0.30(1) | 0.035(1) | – | – | – | – | – | – |
| L1521F | +6.39(1) | 0.39(1) | 0.044(1) | – | – | – | – | – | – |
| Serpens South 1a | +7.58(1) | 0.64(2) | 0.062(2) | – | – | – | – | – | – |
Numbers in parentheses are 1σ uncertainties in units of the last digits. Line parameters obtained by gaussian fits to the line profiles.
Table 4.
Observed line parameters of CH3CHO
| Transition Frequency (MHz) |
JKa,Kc = 21,2 – 10,1 E 83584.279 |
JKa,Kc = 21,2 – 10,1 A 84219.748 |
JKa,Kc = 51,4 – 41,3 E 98863.313 |
JKa,Kc = 51,4 – 41,3 A 98900.944 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Source |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
| Lupus-1A | +5.02(9) | 0.76(19) | 0.008(2) | +4.98(3) | 0.30(6) | 0.007(1) | +5.04(2) | 0.33(4) | 0.009(1) | +5.03(2) | 0.30(4) | 0.008(1) |
| L483 | – | – | – | +5.15(6) | 0.53(12) | 0.012(2) | +5.27(2) | 0.78(7) | 0.037(2) | +5.30(2) | 0.54(6) | 0.027(2) |
| L1495B | – | – | – | – | – | – | +7.57(6) | 0.56(14) | 0.006(1) | +7.40(10) | 0.77(19) | 0.005(1) |
| L1521F | – | – | – | – | – | – | +6.46(4) | 0.51(11) | 0.007(1) | +6.47(4) | 0.47(9) | 0.008(1) |
| Serpens South 1a | – | – | – | – | – | – | +7.41(6) | 0.62(11) | 0.009(2) | +7.53(6) | 0.66(15) | 0.010(2) |
Numbers in parentheses are 1σ uncertainties in units of the last digits. Line parameters obtained by gaussian fits to the line profiles.
The formyl radical (HCO) has been detected in the 9 targeted sources through the 4 strongest components of the 10,1–00,0 transition, lying at 86 GHz. In 4 of the sources, only one component was detected because the other three were not covered by the observations (see Table 5). The HCO lines are relatively intense in all observed sources, with antenna temperatures in the range 0.03-0.2 K. The presence of the formyl radical in dark clouds was first reported by Jiménez-Serra et al. (2004) in L1448 and later on by Cernicharo et al. (2012) in B1 and TMC-1. We derive column densities of HCO which are remarkably uniform across the observed sources, with values in the range (0.5-2.7) ×1012 cm−2 (see Table 2), similar to the value derived in B1 (Cernicharo et al. 2012) and slightly above that found in L1448 (Jiménez-Serra et al. 2004). It is also worth noting that the fractional abundances of HCO relative to H2 are quite uniform, in the range 10−11-10−10, across all sources (see Table 2).
Table 5.
Observed line parameters of HCO NKa,Kc = 10,1 – 00,0
| Transition Frequency (MHz) |
J = 3/2 – 1/2 F = 2 – 1 86670.76 |
J = 3/2 – 1/2 F = 1 – 0 86708.36 |
J = 1/2 – 1/2 F = 1 – 1 86777.46 |
J = 1/2 – 1/2 F = 0 – 1 86805.78 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Source |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
| Lupus-1A | +5.23(1) | 0.38(2) | 0.069(1) | +5.34(1) | 0.44(2) | 0.052(1)a | +5.22(1) | 0.38(2) | 0.047(1) | +5.27(3) | 0.35(3) | 0.017(1) |
| L483 | +5.46(1) | 0.45(2) | 0.077(2) | +5.44(1) | 0.68(2) | 0.084(2)a | +5.42(2) | 0.46(2) | 0.047(2) | +5.50(3) | 0.58(5) | 0.021(2) |
| L1495B | +7.78(1) | 0.42(3) | 0.021(1) | +7.64(1) | 0.46(2) | 0.036(1)a | +7.76(2) | 0.33(4) | 0.010(1) | +7.74(3) | 0.31(6) | 0.005(1) |
| L1521F | +6.61(1) | 0.43(2) | 0.053(1) | +6.50(1) | 0.68(2) | 0.066(1)a | +6.58(1) | 0.41(2) | 0.032(1) | +6.63(2) | 0.33(5) | 0.007(1) |
| Serpens South 1a | +7.65(2) | 0.66(4) | 0.038(2) | +7.58(1) | 0.63(1) | 0.149(2)a | +7.62(2) | 0.68(4) | 0.030(1) | +7.50(8) | 1.15(17) | 0.015(2) |
| L1389 | −4.53(1) | 0.45(3) | 0.060(3) | – | – | – | – | – | – | – | – | – |
| L1172 | +2.91(6) | 0.61(11) | 0.019(3) | – | – | – | – | – | – | – | – | – |
| L1251A | −3.79(6) | 0.47(11) | 0.018(4) | – | – | – | – | – | – | – | – | – |
| L1512 | +7.26(2) | 0.24(7) | 0.014(2) | – | – | – | – | – | – | – | – | – |
Numbers in parentheses are 1σ uncertainties in units of the last digits. Line parameters obtained by gaussian fits to the line profiles.
Uncertain intensity due to severe overlap with the J = 15 – 14 transition of C3S at 86708.374 MHz.
In the same spectrum of Lupus-1A that shows the quartet of lines of HCCO (see Fig. 1), there is a doublet of lines arising from propylene (CH2CHCH3), a highly saturated hydrocarbon which has been previously found only in TMC-1 (Marcelino et al. 2007). Propylene has been detected in Lupus-1A and three additional dense clouds: L1495B, L1521F, and Serpens South 1a (see Table 6). In some of the sources, only the strongest transitions were clearly detected above the noise level. As in TMC-1, the column densities derived in the 4 sources are a few 1013 cm−2 (see Table 2) and are consistent with a A/E ratio of 1. The fractional abundances relative to H2 are in the range (0.14-2.3) ×10−9.
Table 6.
Observed line parameters of CH2CHCH3
| Lupus-1A | L1495B | L1521F | Serpens South 1a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Transition | Frequency (MHz) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
VLSR (km/s) |
Δv (km/s) |
(K km/s) |
| 51,5 – 41,4 A | 84151.670 | +5.00(4) | 0.33(11) | 0.005(1) | +7.55(2) | 0.25(8) | 0.006(1) | +6.33(4) | 0.34(9) | 0.006(1) | – | – | – |
| 50,5 – 40,4 A | 86651.566 | +5.05(5) | 0.36(9) | 0.004(1)a | +7.50(2) | 0.28(4) | 0.009(1) | +6.28(2) | 0.17(8) | 0.005(1) | +7.46(6) | 0.58(12) | 0.011(2) |
| 52,4 – 42,3 A | 87134.576 | – | – | – | +7.64(3) | 0.26(7) | 0.004(1) | – | – | – | – | – | – |
| 60,6 – 50,5 A | 103689.979 | +4.99(7) | 0.52(16) | 0.006(2) | +7.55(3) | 0.44(8) | 0.008(1) | +6.25(4) | 0.44(8) | 0.006(1) | – | – | – |
| 51,5 – 41,4 E | 84151.52b | – | – | – | +7.67b | 0.25(7) | 0.006(1) | +6.40b | 0.44(20) | 0.005(2)c | – | – | – |
| 50,5 – 40,4 E | 86650.873 | +5.03(4) | 0.56(10) | 0.011(2) | +7.61(2) | 0.33(6) | 0.010(1) | +6.34(3) | 0.28(5) | 0.005(1) | +7.57(4) | 0.68(10) | 0.017(2) |
| 52,4 – 42,3 E | 87137.941 | – | – | – | +7.70(4) | 0.44(9) | 0.006(1) | – | – | – | – | – | – |
| 60,6 – 50,5 E | 103689.117 | +5.19(8) | 0.77(16) | 0.009(2) | +7.64(2) | 0.27(4) | 0.006(1) | +6.41(3) | 0.30(5) | 0.006(1) | – | – | – |
Numbers in parentheses are 1σ uncertainties in units of the last digits. Line parameters obtained by gaussian fits to the line profiles.
Intensity diminished due to overlap with frequency switching artifact.
Rest frequency of transition 51,5 – 41,4 of E species constrained to 84151.52 ± 0.03 MHz by fixing VLSR.
Line blended.
4. Discussion
After the astronomical search by Turner & Sears (1989), the ketenyl radical has not attracted much attention in astrochemistry, although it has been of some interest in combustion chemistry (Baulch et al. 1992) and in the study of Titan’s atmosphere (Dobrijevic et al. 2014). No reaction involving this species is included in any of the main chemical kinetics databases of use in interstellar chemistry, UMIST (McElroy et al. 2013) and KIDA (Wakelam et al. 2015). The detection of HCCO in Lupus-1A makes it worth discussing its chemistry in interstellar clouds.
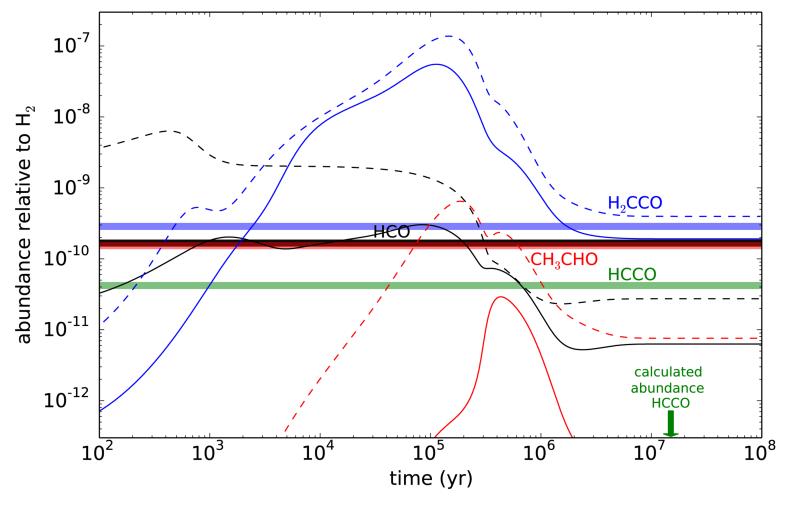
By including various formation and destruction reactions of HCCO from combustion chemistry and the literature in chemical kinetics in a standard chemical model of a cold dark cloud (see, e.g., Agúndez & Wakelam 2013) we find that the main formation route to HCCO is the reaction between OH and C2H. This reaction is exothermic and has an estimated rate constant of 3 × 10−11 cm3 s−1 (Frenklach et al. 1992). The reaction between atomic O and C2H2 is also exothermic in the channel yielding HCCO, although its reaction barrier is too large to be relevant at low temperatures (Balucani et al. 2012). A possible precursor of HCCO is ketene. The reaction of ketene with OH is a potential source of HCCO radicals as this reaction is rapid (Brown et al. 1989), although it seems to have a low yield of HCCO (Grußdorf et al. 1994). The ketenyl radical does not react with stable hydrocarbons or H2 but it does react fast with radicals and atoms (Dobrijevic et al. 2014). In dark clouds, the destruction of HCCO is dominated by the reactions with H, O, and N atoms, whose rate constants are of the order of 10−10 cm3 s−1 (Baulch et al. 1992; Dobrijevic et al. 2014). The abundance of HCCO calculated by the chemical model remains about six orders of magnitude below that calculated for H2CCO (see also Occhiogrosso et al. 2013), i.e., well below the values observed in Lupus-1A and L483. It is clear that some key formation mechanism is missing. In the chemical model, ketene, which is predicted with an abundance much larger than observed at an early time of ~105 yr and in good agreement with the observed value at late times (see Fig. 2), is mainly formed by the reaction of atomic O and the C2H3 radical and the dissociative recombination of the CH3CO+ ion with electrons. If we assume that these routes contribute also to the formation of HCCO, then its abundance increases significantly, but still remains 3-4 orders of magnitude below that of H2CCO.
Fig. 2.
Calculated abundances as a function of time for a standard dark cloud model (parameters from Agúndez & Wakelam 2013). Solid lines use the KIDA kida.uva.2014 ratefile (Wakelam et al. 2015) and dashed lines the UMIST RATE12 ratefile (McElroy et al. 2013). In both cases a subset of reactions involving HCCO is introduced, although its calculated abundance remains below the range plotted. The horizontal thick lines indicate the observed abundances in Lupus-1A.
A powerful formation mechanism of HCCO is needed to counterbalance the efficient depletion of this radical by reactions with neutral atoms. An interesting possibility are the reactions of O atoms with CnH radicals (n ≥ 3), which are assumed to yield CO as this is the most exothermic channel (Smith et al. 2004), although the channel leading to HCCO is also exothermic. It is also worth noting that recent studies (Hudson & Loeffler 2013; Maity et al. 2014) find that ketene can be efficiently formed in various types of ices upon irradiation with energetic electrons and ultraviolet photons (both of which can be formed in dark clouds following cosmic-ray impacts), which opens the possibility to the formation of HCCO as an intermediate.
In the chemical model, the HCO radical is mainly formed through the reaction of O atoms and CH2 radicals, together with the dissociative recombination of H3CO+ and H2CO+ with electrons, reaching an abundance which is in good agreement with the observed values (see Fig. 2). On the other hand, the detection of propylene in various dark clouds other than TMC-1 points to a widespread presence of this saturated hydrocarbon in cold dark clouds. This fact exacerbates the challenge to chemical models, which still lack an efficient gas phase formation mechanism for this molecule (Lin et al. 2013).
5. Summary
We have reported the first detection in space of the ketenyl radical (HCCO) in the starless core Lupus-1A and the molecular cloud L483, together with observations of the related molecules H2CCO and CH3CHO toward these two sources and some other dense clouds. Our observations also extend significantly the number of dark clouds in which the formyl radical (HCO), previously detected only in L1448, B1, and TMC-1, and propylene (CH2CHCH3), previously identified only in TMC-1, are observed. The radical HCCO is found to be just ~ 10 times less abundant than its closed shell counterpart H2CCO, more than 3 orders of magnitude more abundant than predicted by chemical models. A highly efficient formation mechanism of HCCO, yet unidentified, is needed to counterbalance the rapid depletion of this radical in cold dark clouds by reactions with neutral atoms. The discovery of ketenyl in Lupus-1A and L483 and the widespread ocurrence of propylene in dark clouds suggest that a revision of the gas phase and grain surface chemical processes at work in cold dark clouds is required.
Acknowledgements
We thank Belén Tercero for a critical reading of the manuscript, the anonymous referee for useful suggestions, and the IRAM 30m staff for their help during the observations. M.A. and J.C. thank funding support from the European Research Council (ERC Grant 610256: NANOCOSMOS) and from Spanish MINECO through grants CSD2009-00038, AYA2009-07304, and AYA2012-32032.
Footnotes
Based on observations carried out with the IRAM 30m Telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany) and IGN (Spain).
References
- Agúndez M, Cernicharo J, Guélin M, et al. A&A. 2008;478:L19. [Google Scholar]
- Agúndez M, Wakelam V. Chem. Rev. 2013;113:8710. doi: 10.1021/cr4001176. [DOI] [PubMed] [Google Scholar]
- Anglada G, Sepúlveda I, Gómez JF. A&AS. 1997;121:255. [Google Scholar]
- Bacmann A, Taquet V, Faure A, et al. A&A. 2012;541:L12. [Google Scholar]
- Balucani N, Leonori F, Casavecchia P. Energy. 2012;43:47. [Google Scholar]
- Baulch DL, Cobos CJ, Cox RA, et al. J. Phys. Chem. Ref. Data. 1992;21:411. [Google Scholar]
- Bohlin RC, Savage BD, Drake JF. ApJ. 1978;224:132. [Google Scholar]
- Brown AC, Canosa-Mas CE, Parr AD, Wayne RP. Chem. Phys. Lett. 1989;161:491. [Google Scholar]
- Cernicharo J, Marcelino N, Roueff E, et al. ApJ. 2012;759:L43. [Google Scholar]
- Cordiner MA, Buckle JV, Wirström ES, et al. ApJ. 2013;770:48. [Google Scholar]
- Crapsi A, Caselli P, Walmsley CM, et al. ApJ. 2005;619:379. [Google Scholar]
- Dobrijevic M, Hébrard E, Loison JC, Hickson KM. Icarus. 2014;228:324. [Google Scholar]
- Endo Y, Hirota E. J. Chem. Phys. 1987;86:4319. [Google Scholar]
- Frenklach M, Wang H, Rabinowitz MJ. Porc. Energy Combust. Sci. 1992;18:47. [Google Scholar]
- Friesen RK, Medeiros L, Schnee S, et al. MNRAS. 2013;436:1513. [Google Scholar]
- Fuller GA, Myers PC. ApJ. 1993;418:273. [Google Scholar]
- Grußdorf J, Nolte J, Temps F, Wagner H. Gg. Ber. Bunsenges. Phys. Chem. 1994;98:546. [Google Scholar]
- Herbst E, van Dishoeck EF. ARA&A. 2009;47:427. [Google Scholar]
- Hudson RL, Loeffler MJ. ApJ. 2013;773:109. [Google Scholar]
- Irvine WM, Friberg P, Kaifu N, et al. ApJ. 1989;342:871. doi: 10.1086/167643. [DOI] [PubMed] [Google Scholar]
- Jerosimić SV. J. Mol. Spectr. 2007;242:139. [Google Scholar]
- Jiménez-Serra I, Martín-Pintado J, Rodríguez-Franco A, Marcelino N. ApJ. 2004;603:L49. [Google Scholar]
- Jørgensen JK, Schöier FL, van Dishoeck EF. A&AS. 2002;389:908. [Google Scholar]
- Launhardt R, Nutter D, Ward-Thompson D, et al. ApJS. 2010;188:139. [Google Scholar]
- Lin Z, Talbi D, Roueff E, et al. ApJ. 2013;765:80. [Google Scholar]
- Maity S, Kaiser RI, Jones BM. ApJ. 2014;789:36. [Google Scholar]
- Marcelino N, Cernicharo J, Agúndez M, et al. ApJ. 2007;665:L127. [Google Scholar]
- Matthews HE, Friberg P, Irvine WM. ApJ. 1985;290:609. doi: 10.1086/163018. [DOI] [PubMed] [Google Scholar]
- McElroy D, Walsh C, Markwick AJ, et al. A&A. 2013;550:A36. [Google Scholar]
- Müller HSP, Schlöder F, Stutzki J, Winnewisser G. J. Mol. Struct. 2005;742:215. [Google Scholar]
- Myers PC, Linke RA, Benson PJ. ApJ. 1983;264:517. [Google Scholar]
- Öberg KI, Bottinelli S, Jørgensen J-K, van Dishoeck EF. ApJ. 2010;716:825. [Google Scholar]
- Occhiogrosso A, Viti S, Balucani N. MNRAS. 2013;432:3423. [Google Scholar]
- Ohishi M, Kaifu N. Faraday Discussions. 1998;109:205. [Google Scholar]
- Ohshima Y, Endo Y. J. Mol. Spectr. 1993;159:458. [Google Scholar]
- Sakai N, Sakai T, Hirota T, et al. ApJ. 2009;697:769. [Google Scholar]
- Sakai N, Shiino T, Hirota T, et al. ApJ. 2010;718:L49. [Google Scholar]
- Sato F, Mizuno A, Nagahama T, et al. ApJ. 1994;435:279. [Google Scholar]
- Sattelmeyer KW, Yamaguchi Y, Schaefer HF., III Chem. Phys. Lett. 2004;383:266. [Google Scholar]
- Smith IWM, Herbst E, Chang Q. MNRAS. 2004;350:323. [Google Scholar]
- Szalay PG, Fogarasi G, Nemes L. Chem. Phys. Lett. 1996;263:91. [Google Scholar]
- Tafalla M, Myers PC, Mardones D, Bachiller R. A&A. 2000;359:967. [Google Scholar]
- Tothill NFH, Löhr A, Parshley SC, et al. ApJS. 2009;185:98. [Google Scholar]
- Turner BE, Sears TJ. ApJ. 1989;340:900. [Google Scholar]
- Wakelam V, Loison J-C, Herbst E, et al. ApJS. 2015;217:20. [Google Scholar]
- Zinner E. ASP Conf. Ser. 99. In: Holt SS, Sonneborn G, editors. Cosmic Abundances. ASP; San Francisco, CA: 1996. p. 147. [Google Scholar]