Abstract
Polymorphs of 4-aminoquinaldine (4-AQ) have been predicted in silico and experimentally identified and characterised. The two metastable forms, AH (anhydrate) II and AH III, crystallise in the trigonal space group and are less densely packed than the thermodynamically most stable phase AH I° (P21/c). AH II can crystallise and exist both, as a solvent inclusion compound and as an unsolvated phase. The third polymorph, AH III, is exclusively obtained by desolvation of a carbon tetrachloride solvate. Theoretical calculations correctly estimated the experimental 0K stability order, confirmed that AH II can exist without solvents, gave access to the AH III structure, and identified that there exists a subtle balance between close packing and number of hydrogen bonding interactions in the solid state of anhydrous 4-AQ. Furthermore, the prevalence of void space and solvent inclusion in structures is discussed.
1. Introduction
Screening for different solid forms (polymorphs, hydrates, solvates) is an essential step during drug development.1 This is because the crystal form dictates fundamental properties such as stability, solubility (bioavailability), mechanical properties, etc.,2-4 and thus, suitable solid form(s) need to be identified and thoroughly characterised before they can be processed into high quality (drug) products. Controlling the solid state form is, therefore, of considerable interest since it provides a possibility to tune product properties without changing the chemistry of the molecule.5 Solid form screens are typically attempted by crystallising the compound from a broad range of solvents or solvent mixtures under different conditions (e.g. rate of cooling, crystallisation temperature, solvent evaporation, precipitation with anti-solvent).6-8 Templates, additives or impurities have been shown to result in the formation of new, sometimes otherwise elusive, solid forms,9-12 making it practically not feasible to cover the whole range of techniques that may lead to alternate phases.13
Given this and the recent successes in computationally generating the crystal energy landscapes (crystal structure prediction, CSP),13-28 in silico screening, might be used as an assurance that all practically important forms have been found in an experimental screening programme, i.e. to minimise the risk of late-appearing crystal forms.29 Based on the CSP results not only the propensity for polymorphism but also solvent inclusion (framework structures30,31), the potential for disorder32,33 and local short-range order in the amorphous state34 can be deduced. Another motivation for pursuing CSP studies is to increase the access to structural data. Finally, lattice energy estimations provide an alternative method to define the stability hierarchy of solid forms.35,36
During solvent crystallisation it can happen that solvent molecules co-crystallise with the host molecules, leading to the formation of solvates. The solvent molecules can simply fill channels or voids within the crystal lattice or interact with the host molecule through hydrogen bonding or other stabilising intermolecular interactions. The existence of a solvate depends on pressure, temperature, and amount of solvent present in the storage atmosphere.37 Changes in the latter factors can induce a phase transformation to an alternative solid form (e.g., unsolvated crystalline phase, amorphous phase). When the solvent molecule becomes entrapped in the solid to some extent, in sub-stoichiometric amounts, and cannot be removed by suitable drying conditions the term “residual solvents” is used.38 The solvent molecules are then considered as “impurities” and regulated by the ICH guideline Q3C (residual solvents in pharmaceuticals).39 Probably the most famous example showing “solvent inclusion” (< 5 wt.%) is carbamazepine (CBZ) form II, where the solvent plays an important role in the crystallisation of the form II, by stabilising its crystal structure.40,41
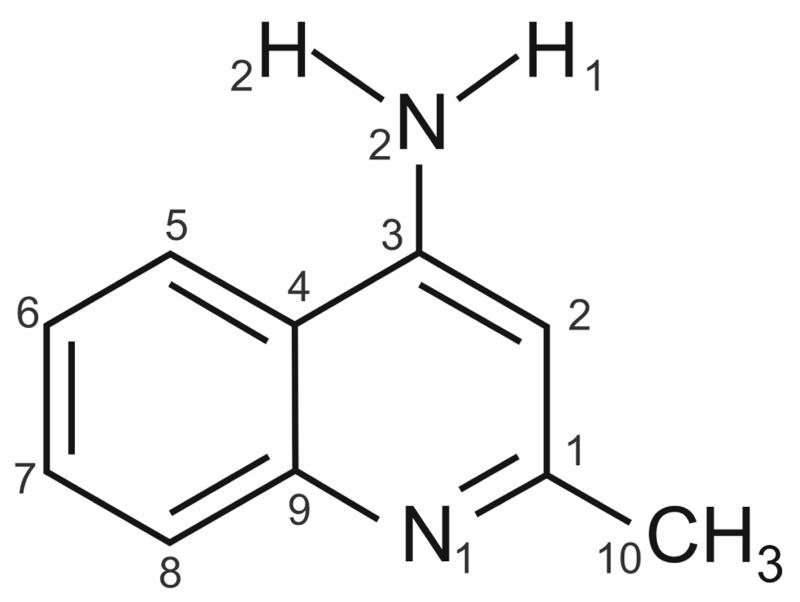
The small organic molecule 4-aminoquinaldine (4-amino-2-methylquinolidine, 4-AQ, Fig. 1) was subjected to a multidisciplinary solid form screening programme with the aim to contrast and evaluate experimental and computational search methods, as well as measured and computed energy differences between polymorphs. The investigated compound belongs to the pharmaceutically and biologically important class of quinolone derivatives and can be seen as a model compound for a co-crystal former. Surprisingly, hardly any information on solid forms of this compound can be found in the literature. Dimorphism of 4-AQ has already been reported in 196942 and only the crystal structure of the monohydrate has been reported (Cambridge Structural Database43 Refcode: LOBSOL44). In addition, experimental and computed Infrared and Raman spectra have been published by Krishnakumar and Xavier45 and Arjunan et al.46 The first of these reports gives the experimental spectral data for the anhydrate (AH) I°, and the second for the monohydrate. The water molecules have been ignored in these simulations.
Fig. 1.
Molecular diagram of 4-amino-2-methylquinolidine (4-aminoquinaldine).
The aim of this study was to develop a consistent picture of the solid state properties, structural and thermodynamic features of the three anhydrate polymorphs of 4-AQ (including a novel phase) and its carbon tetrachloride solvate. This was possible only by combining of a broad range of analytical techniques (hot-stage microscopy, differential scanning calorimetry, thermogravimetric analysis, X-ray diffractometry and Infrared spectroscopy) complemented with crystal structure prediction.
2. Experimental Section
2.1. Materials and Solid Form Screen
4-AQ was purchased from Aldrich (Lot#STBD1705V, purity 98%). The substance was recrystallised for purification from a hot saturated ethanol solution at 8 °C. The 29 solvents used for the polymorphism screen were all of analytical quality and all organic solvents were purchased from Aldrich or Fluka.
The solid form screen encompassed a solvent crystallisation screening, sublimation and desolvation studies. The most commonly used solvents/solvent mixtures, covering different classes based on molecular descriptors (hydrogen bonding capability, polarity, dielectric constant and dipole moment) have been chosen.47 Solvent evaporation, cooling crystallisation, anti-solvent addition and liquid assisted grinding experiments were employed for the solvent screening and are detailed in ESI† Section 1.1.
2.2. Infrared Spectroscopy (IR)
FT-IR spectra were recorded with a Bruker IFS 25 spectrometer connected to a Bruker IR microscope I with a 15x-Cassegrain-objective (Bruker Analytische Messtechnik GmbH, Ettlingen, Germany). The samples were prepared on ZnSe discs and the following measurement conditions were applied: spectral range 4000 to 600 cm−1, resolution 4 cm−1, 64 scans per spectrum.
2.3. X-ray Powder Diffractometry (XRPD)
The X-ray powder diffraction patterns were obtained using an X’Pert PRO diffractometer (PANalytical, Almelo, The Netherlands) equipped with a θ/θ coupled goniometer in transmission geometry, programmable XYZ stage with well plate holder, Cu-Kα1,2 radiation source with a focussing mirror, a 0.5° divergence slit and a 0.02° Soller slit collimator on the incident beam side, a 2 mm antiscattering slit and a 0.02° Soller slit collimator on the diffracted beam side and a solid state PIXcel detector. The patterns were recorded at a tube voltage of 40 kV, tube current of 40 mA, applying a step size of 2θ = 0.013° with 80 s per step in the 2θ range between 2° and 40°.
2.4. Single Crystal X-ray Diffractometry
Single crystals of AH I° were obtained from sublimation experiments carried out between two glass slides, separated by a spacer ring of 5 mm thickness, on a Kofler hot bench at 130 °C. AH II crystals were prepared by slow solvent evaporation at 40 °C from a carbon tetrachloride solution saturated at room temperature. Essential crystal data are collected in Table 1. The data for AH I° (Mo radiation; λ = 0.71073 Å) were collected on a Rigaku AFC12 goniometer driven by the CrystalClear-SM Expert 3.1 b27 software (Rigaku, 2012) and equipped with an enhanced sensitivity (HG) Saturn724+ detector mounted at the window of an FR-E+ Super Bright Mo rotating anode generator with HFVarimax optics.48 The data for AH II (Mo radiation; λ = 0.71073 Å) were recorded on an Oxford Diffraction Gemini-R Ultra diffractometer operated by CrysAlis software.49 The structures were solved by direct methods (SIR201150 or SHELXL201351) and refined by full-matrix least squares on F2 using SHELXL2013 and the program package WinGX.52 Polar hydrogen atoms were located in difference maps, and those bonded to carbon atoms were fixed in idealised positions and their displacement parameters were set to 1.2Ueq (for CH) or 1.5Ueq (for the CH3 group) of the parent C atom, while the H atoms of the NH2 group were refined freely. The hydrogen atoms in the −CH3 group of AH I° were found to be statistically disordered over two positions with occupancies of 0.5:0.5 and of AH II with occupancies of 0.63:0.37 and 0.59:0.41, respectively.
Table 1.
Crystal data and structure refinement details.
| Compound | AH I° | AH II (CCl4) |
|---|---|---|
| Chemical formula | C10H10N2 | C10H10N2 · 0.085 (CCl4) |
| M /g mol−1 | 158.20 | 342.03 |
| Crystal system | monoclinic | trigonal |
| Space group | P21/c | |
| Z/Z′ | 4/1 | 18/2 |
| a/Å | 5.2162(4) | 28.4074(8) |
| b/Å | 12.3693(9) | |
| c/Å | 13.1192(9) | 11.9126(5) |
| β/° | 99.065(3) | |
| Unit cel volume /Å3 | 835.89(11) | 8325.3(6) |
| Temperature / K | 293(2) | 173(2) |
| Density / g cm−3 | 1.257 | 1.228 |
| No. of reflections measured | 4759 | 11536 |
| No. of independent ref. | 1502 | 3315 |
| R int | 0.027 | 0.032 |
| Parameters | 128 | 275 |
| Final R1 values (I > 2σ(I)) | 0.037 | 0.056 |
| Final wR(F2) values (all data) | 0.106 | 0.164 |
| CCDC no. | 1043184 | 1043185 |
2.5. Hot-stage Microscopy (HSM)
For hot-stage thermomicroscopic investigations a Reichert Thermovar polarisation microscope, equipped with a Kofler hot-stage (Reichert, A), was used. Photographs were taken with an Olympus DP71 digital camera (Olympus, D).
2.6. Differential Scanning Calorimetry (DSC)
DSC thermograms were recorded on a DSC 7 or Diamond DSC (Perkin-Elmer Norwalk, Ct., USA) controlled by the Pyris 7.0 software. Using a UM3 ultramicrobalance (Mettler, Greifensee, Switzerland), samples of approximately 2 - 6 mg were weighed into perforated or sealed aluminium pans. The samples were heated using rates in between 2 and 20 °C min−1, with dry nitrogen as the purge gas (purge: 20 ml min−1). The two instruments were calibrated for temperature with pure benzophenone (mp 48.0 °C) and caffeine (236.2 °C), and the energy calibration was performed with indium (mp 156.6 °C, heat of fusion 28.45 Jg−1). The errors on the stated temperatures (extrapolated onset temperatures) and enthalpy values were calculated at the 95% confidence intervals (CI) and are based on at least five measurements.
2.7. Thermogravimetric Analysis (TGA)
TGA was carried out with a TGA7 system (Perkin-Elmer, Norwalk, CT, USA) using the Pyris 2.0 software. Approximately 2 - 5 mg of sample was weighed into a platinum pan. Two-point calibration of the temperature was performed with ferromagnetic materials (Alumel and Ni, Curie-point standards, Perkin-Elmer). Heating rates of 0.5 to 20 °C min−1 were applied and dry nitrogen was used as a purge gas (sample purge: 20 mL min−1, balance purge: 40 mL min−1).
2.8. Computational Generation of the Anhydrate Crystal Energy Landscape
The anhydrate crystal energy landscape was generated using the planar 4-AQ molecule, obtained from the potential energy surface calculations with Gaussian09.53 Using the program CrystalPredictor2.0,54-56 150,000 Z′=1 anhydrate structures were randomly generated in 48 space groups (ESI† Section 2.2.1) and 75,000 Z′=2 anhydrate structures in (chosen based on experimental evidence). Each crystal structure was relaxed to a local minimum in the intermolecular lattice energy, calculated from the FIT57 exp-6 repulsion-dispersion potential and atomic charges which had been fitted to electrostatic potential around the PBE0/6-31G(d,p) charge density using the CHELPG scheme.58 All structures within 20 kJ mol−1 of the lowest energy structure (2520 Z′=1 and 390 Z′=2 structures) were reminimised using DMACRYS59 with a more realistic, distributed multipole model60 for the electrostatic forces which had been derived using GDMA261 to analyze the PBE0/6-31G(d,p) charge density.
The optimal proton positions of the amino group (i.e. pyramidal, deviation from planarity) and methyl group in all crystal structures within 15 kJ mol−1 of the global minimum (147 Z′=1 and 31 Z′=2 structures) were determined using the CrystalOptimizer database method.62 This was done by minimising the lattice energy (Elatt), calculated as the sum of the intermolecular contribution (Uinter) and the conformational energy penalty paid for distortion of the molecular geometry to improve the hydrogen bonding geometries. Conformational energy penalties (ΔEintra, with respect to the pyramidal global conformational energy minimum) and isolated molecule charge densities were computed at the PBE0/6-31G(d,p) level, for each conformation considered in the minimisation of Elatt.
The most stable structures (60 Z′=1 and 3 Z′=2 structures) were used as starting points for periodic electronic structure calculations. The DFT-D calculations were carried out with the CASTEP plane wave code63 using the Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA) exchange-correlation density functional64 and ultrasoft pseudopotentials,65 with the addition of a semi-empirical dispersion correction, either the Tkatchenko and Scheffler (TS)66 or Grimme06 (G06)67 model. For more details see ESI† Section 2.2.2.
PIXEL calculations68-70 were also performed on the low energy structures to estimate the repulsive (ER), dispersion (ED), electrostatic (Coulombic, EC) and polarisation (also called induction, EP) contributions from individual pairs of molecules within a crystal. The charge density for the crystal was constructed from the MP2/6-31G(d,p) ab initio charge density of the isolated molecule as extracted from the computed PBE-TS crystal structure. The electron density was described using medium cube settings and a step size of 0.08 Å, with the pixels condensed into superpixels with a condensation level n=4.
3. Results
3.1. Solid Form Screening
The experimental screen for solid forms resulted in three anhydrate polymorphs (AH I° – III), a monohydrate (MH),44 a carbon tetrachloride solvate (SCCl4) and amorphous 4-AQ. Either the MH or a mixture of MH and AH I° was obtained in the vast majority of evaporation experiments performed at room temperature (RT). AH I° was obtained from most experiments performed at 40 °C. AH II was formed in slow evaporation experiments from 1-butanol, carbon tetrachloride and tetrahydrofurane performed at 40 °C or concomitantly with AH I° from chloroform at RT. In addition, SCCl4/AH III emerged from tetrachloride in room temperature evaporation experiments. From dimethyl formamide and dimethyl sulfoxide a different compound emerged at 40 °C which was not further characterised. Cooling crystallisation from different solvents or precipitation experiments with toluene or water resulted in MH or AH I° while CCl4 yielded SCCl4. Depending on the water activity of the used solvent either MH or AH I° emerged from liquid assisted grinding experiments. For more details see ESI† Section 1.
Dehydration experiments starting from the MH resulted in AH I°, whereas desolvation of SCCl4 led to AH III. Finally, in sublimation experiments exclusively AH I° was obtained and recrystallisation form the melt at temperatures < 60 °C resulted in AH II.
3.2. Single Crystal Structures
The structures of AH I° and AH II (CCl4), crystallised from carbon tetrachloride, were determined. The 4-AQ molecule features only the aromatic amino group as a potential donor for two hydrogen bonds and the pyridine nitrogen as a hydrogen bond acceptor.
3.2.1. Anhydrate I°
This polymorph crystallises in the monoclinic space group P21/c with Z′=1 (Table 1). The 4-AQ molecule is essentially planar with the amino group adopting a slightly pyramidal conformation to optimise the geometry of the hydrogen bond. This is in contrast to the planar amino group in the monohydrate structure.44 The 4-AQ molecules are interlinked through N2–H2⋯N1 hydrogen bonds into a 71 chain motif, which propagates in direction of the c axis and exhibits glide plane symmetry (Fig. 2a). Hence, only one of the two polar protons is engaged in a strong intermolecular interaction. The chains are stacked, forming π⋯π, along the crystallographic a axis. Adjacent stacks of chains are related by inversion and linked through weak C–H⋯π contacts.
Fig. 2.
(a,b) Packing and hydrogen bonding motif present in 4-AQ AH I°. Hydrogen bonds shown as red dotted lines in (a). Only one orientation of the disordered – CH3 group shown.
3.2.2. Anhydrate II
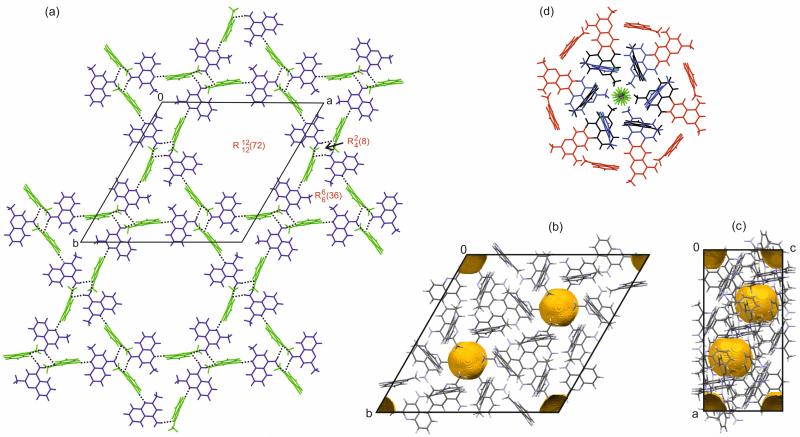
The second 4-AQ polymorph crystallises in the trigonal space group with Z′=2 (Table 1). The single crystal structure data clearly revealed the presence of residual solvent, CCl4, located in cavities. The solvent molecule is disordered around the site at (0,0,0) and refined with the maximum possible occupancy of a 4-AQ:CCl4 ratio of 1:1/12. Similar to AH I° the 4-AQ molecules are essentially planar with only one amino proton deviating slightly from the plane defined of the aromatic ring system. Three distinct hydrogen bonded ring motifs involving both independent 4-AQ molecules can be identified, i.e. forming a tetrameric, forming a hexameric, and forming a 12-membered 4-AQ ring motif. The two symmetry independent 4AQ molecules are denoted A and B hereafter. The two polar protons of molecule A are engaged in NA–H⋯NB hydrogen bonds to the pyridine N1 of molecule B. Only one of the polar protons of molecule B forms a strong hydrogen bond, NB–H2⋯NA, to the pyridine N1 of molecule A. The ring motif is formed by the two NA–H1/2⋯NB hydrogen bonds. Alternating NA–H1⋯NB and NB–H2⋯NA hydrogen bonds form the hexameric ring motif and alternating NA–H2⋯NB and NB–H2⋯NA hydrogen bonds the 12-membered hydrogen bonded ring. Thus, in contrast to the AH I° structure, three of the four N–H protons are involved in strong intermolecular interactions. Altogether, the 4-AQ molecules form hydrogen bonded layers, which lay parallel to the ab-plane (Fig. 3a). Layers of this kind are related by inversion, leading to voids around the roto-inversion axes (Fig. 3b and c). The hydrophobic cavities can accommodate solvent molecules (Fig. 3d), but no strong intermolecular interactions are formed between the host and guest molecules. Thus, the solvent molecules only fill the structural voids. In addition, C–H⋯π interactions stabilise AH II.
Fig. 3.
Packing and hydrogen bonding motifs of 4-AQ AH II: (a) Graph set notation and hydrogen bonds shown in red and as black dotted lines, respectively. Symmetry independent 4-AQ molecules are coloured differently: blue – molecules A and green – molecules B. (b, c) Unit cell and voids visualisation (2.0 Å probe radius) of the “solvent stripped” AH II (CCl4) structure. The yellow surfaces represent the boundaries of calculated voids in the structure and correspond to the location of the CCl4 molecule (c) in AH II (CCl4). Disorder of –CH3 group for clarity not shown. (d) Stacked and ring motifs with disordered CCl4 in the centre. Note that the 4-AQ molecules involved in the same ring motif are coded in one colour.
3.2.3. Monohydrate
The MH structure44 has the space group symmetry Pna21, with Z′ = 1. The structure is densely packed and all hydrogen bonding donor and acceptor groups are involved in strong intermolecular interactions. A further characteristics of the structure are π⋯π of 4-AQ molecules.
3.3. Identification and Characterisation of 4-AQ Anhydrates and Carbon Tetrachloride Solvate
3.3.1. Fourier Transform Infrared Spectroscopy
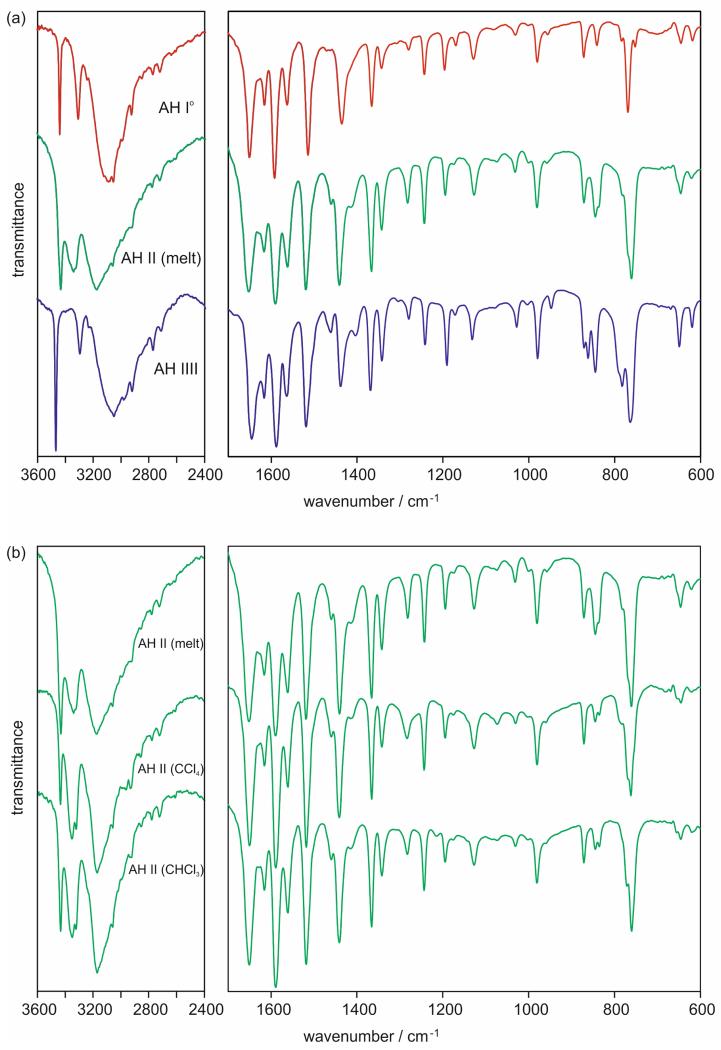
The 4-AQ MH, AH I°, AH III and SCCl4 forms showed high reproducibility in their analytical data. In contrast, AH II, obtained using different crystallisation conditions, i.e the melt [AH II (melt)] or different solvents [(AH II (CCl4) and AH II(CHCl3)], showed variability in its spectral, X-ray diffraction and thermo-analytical data. Exemplarily, the FT-IR spectra of the three anhydrates, including different AH II batches, are given in Fig. 4 and selected IR band positions are listed in Table 2. The three anhydrates can be easily distinguished based on their νNH2 and νCH stretching vibrations. Furthermore, the δNH2 and δCH3 band positions can be used to identify the phases. AH II is likely to be a Z′=2 phase for AH II (νNH2 bands split for AH II) and AH I° and AH III Z′=1 phases.
Fig. 4.
FT-IR spectra of (a) the 4-AQ anhydrates and (b) 4-AQ AH II samples crystallised from the melt or solvents (carbon tetrachloride or chloroform).
Table 2.
Selected FT-IR band positions for 4-AQ anhydrates.
| AH I° | AH II (melt)a | AH II (CCl4)a | AH II (CHCl3)a | AH III | |
|---|---|---|---|---|---|
| νNH2 | 3438 | 3431 | 3434 | 3433 | 3466 |
| 3307 | 3339/ | 3352/ | 3349/ | 3294 | |
| 3329 | 3322 | 3323 | |||
| νCH, | 3170/ | 3171/ | 3170/ | ||
| νCH3 | 3055/ | 3060/ | 3058/ | 3058/ | 3051/ |
| 2994/ | 2993/ | 2995/ | 2996/ | 2981/ | |
| 2925 | 2923 | 2929 | 2931 | 2921 | |
| δNH2 | 1650 | 1652 | 1650 | 1651 | 1645 |
| νC=C | 1615 | 1616 | 1615 | 1616 | 1616 |
| 1562 | 1561 | 1561 | 1561 | 1563 | |
| 1514 | 1519 | 1518 | 1518 | 1518 | |
| νC=N | 1592 | 1590 | 1589 | 1589 | 1587 |
| δCH3 | 1435 | 1440 | 1441 | 1441 | 1438 |
For AH II three different crystallisation products are compared, i.e. crystallisation from the melt, carbon tetrachloride and chloroform.
A closer analysis was necessary for AH II (which will be discussed later). Figure 4b contrasts three different AH II samples, which were crystallised either from the melt, carbon tetrachloride or chloroform. Overall, their IR spectra are similar but show distinguishable features in the νCH3 and δCH3 region, in particular between AH II (melt) on the one hand and AH II (CCl4) and AH II(CCl4) on the other hand. Band positions within the fingerprint region do not differ (beyond the resolution of the instrument) with the exception of the regions of νCCl vibrations (840 – 740 cm−1). Nevertheless, based on the similarity of the IR spectra (Figure 4b) it can be suggested that all three crystallisation products are the same solid form and solvent molecules are located beside apolar protons in AH II (CCl4) and AH II (CCl3). All 4-AQ intermolecular interactions can be expected to be the same from the spectroscopic AH II data.
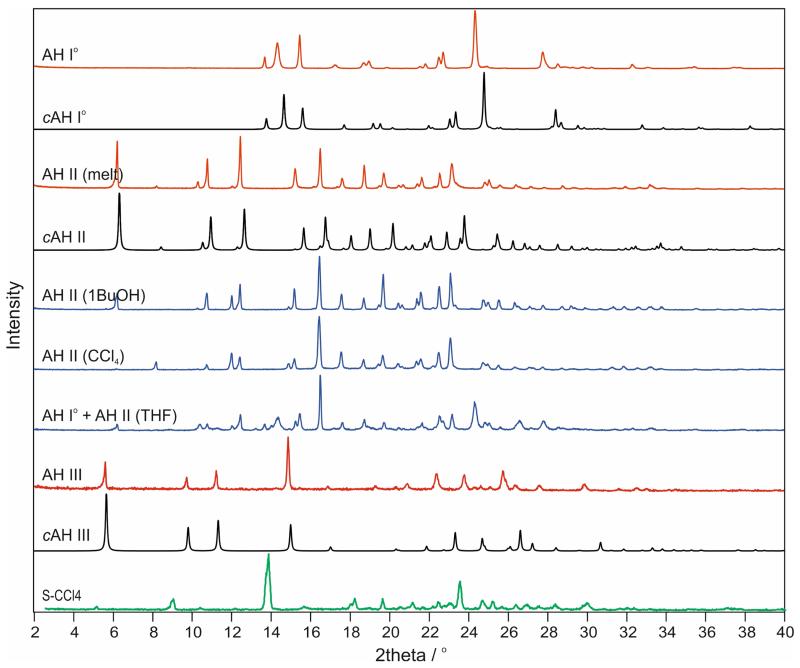
3.3.2. X-ray Powder Diffraction
The XRPD patterns of the AH II samples crystallised from the melt or from a solution differ only slightly in their peak positions but significantly in the intensities of their low-angle reflections (Fig. 5). Pawley fits of the different AH II samples, using the cell information of AH II (CCl4), confirmed the same space group symmetry, , with similar lattice parameters (ESI† Figures S3-S5). Peak shifts can be attributed to small changes in lattice parameters (Δa and Δc < 0.5%) and differences in intensities may be related to additional residual solvent (see section 3.4.2).
Fig. 5.
Experimental X-ray powder diffraction patterns (AH I°, AH II, AH III, SCCl4) obtained at room temperature compared with simulated patterns (λ = 1.5418 Å) for computationally generated anhydrate structures (Section 3.5). For AH II data for samples obtained from different crystallisation experiments are shown.
The XRPD patterns of SCCl4 and its desolvation product AH III show some resemblance as well, despite significant changes in peak positions and intensities. This may imply a structural relationship between the two phases which could explain why AH III was only obtained from desolvation of SCCl4 (see section 3.5.3).
3.4. Thermal Characterisation
3.4.1. Solvent-Free Forms: Anhydrates I° – III and amorphous 4-AQ
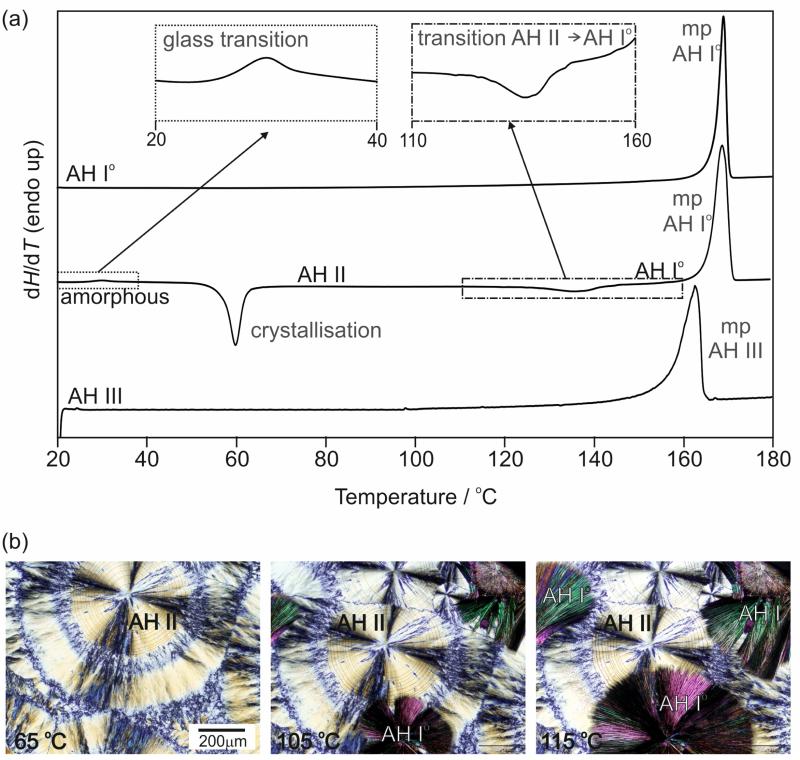
The anhydrate polymorphs were analysed with DSC (Fig. 6a) and HSM (Fig. 6b). The DSC curve of AH I° shows only one thermal event, the melting, which occurs at 167.3 ± 0.1 °C (onset temperature) with an enthalpy of fusion, ΔfusHI°, of 25.4 ± 0.1 kJ mol−1 (Table 3). Similarly, the DSC trace of AH III shows one event, melting at 157.1 ± 0.3 °C (onset temperature) with ΔfusHIII of 20.0 ± 0.5 kJ mol−1. Quench cooling of the melt results in amorphous 4-AQ. The glass transition of the latter phase is observed around room temperature (25 °C) and spontaneous nucleation of AH II (Fig. 6b, first picture) occurs at temperatures > 45 °C. Upon further heating of AH II, an exothermic phase transformation to AH I° occurs between 100 and 140 °C, which can also clearly be seen with HSM (Fig. 6b, second and third photograph) and indicates a monotropic relationship between AH I° and AH II. The HSM observations are essentially in agreement with the descriptions given by Kofler and Kolsek.42 The transition enthalpy, ΔtrsHII-I°, was determined by us to be –3.3 ± 0.1 kJ mol−1. The final event in the DSC curve (Fig. 6a, second curve) corresponds to the melting of AH I°. Concomitant nucleation of forms AH I° and AH II is observed when the melt is annealed at temperatures ≥ 70 ° C. If AH II is contaminated with AH I°, the solid-solid phase transformation is accelerated and occurs within days at room conditions. Furthermore, the HSM investigations revealed strong sublimation of 4-AQ, with the sublimed plate-shaped crystals being AH I° (ESI† Figure S1).
Fig. 6.
(a) DSC curves (heating rate 10 °C min−1) of 4-AQ anhydrate polymorphs; mp – melting point. The inserts show the glass transition and AH II → AH I° phase transformation. (b) Microphotographs showing the AH II → AH I° phase transformation in the temperature range 105 to 115 °C.
Table 3.
Physicochemical data for 4-AQ polymorphs.
| Modification | AH I° | AH II (melt) | AH III |
|---|---|---|---|
| Tfus / °Ca | |||
| HSM | 167 | 156 – 157.5 | |
| DSC (onset) ± 95 c.i. | 167.3 ± 0.1 | 157.1 ± 0.3 | |
| ΔfusHb ± 95 c.i. / kJ mol−1 | 25.4 ± 0.1 | 22.1 ± 0.2f | 20.0 ± 0.5 |
| Ttrsc / °C | |||
| HSM | 100 – 140 | ||
| DSC (onset) ± 95 c.i. | 116 ± 3 | ||
| ΔtrsHd to AH I° ± 95 c.i. / kJ mol−1 | −3.3 ± 0.1 | −5.4 ± 0.6g | |
| Elatt / kJ mol−1 (PBE-G06) | −132.18 | −129.00 | −127.35 |
| Order of thermodynamic stability | |||
| DSC | a (highest) | b | c |
| Elatt, −273 °C | a (highest) | b | c |
| density ordere | a (highest) | c | b |
3.4.2. Solvent Containing Forms: Anhydrate II and Carbon tetrachloride Solvate
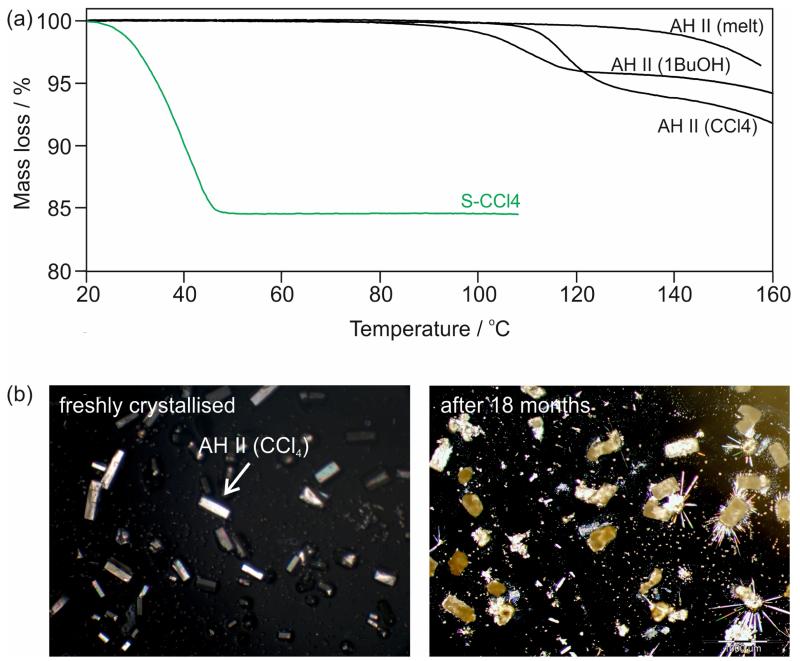
Thermal analytical techniques have provided experimental evidence for solvent inclusion in AH II. The release of solvent can be observed by heating a sample immersed in high viscosity silicon oil. DSC traces exhibit a small endothermic event around 120 °C (not shown in Fig. 6), within the temperature range of the AH II → AH I° phase transition. The observation of an endothermic rather than an exothermic event can be interpreted in terms of an expulsion of solvent molecules. The TGA curves of AH II crystallised from solution reveal a clear mass loss of 4.5 – 6% prior to sublimation, depending on the nature of the solvent (Fig. 7a). The measured mass loss corresponds to substoichiometric ratios, e.g. ~1/12 mol of carbon tetrachloride or 1-butanol per mol 4-AQ. The melt crystallised AH II sample shows no mass loss except for sublimation at temperatures > 130 °C.
Fig. 7.
(a) TGA curves (heating rate 5 °C min−1) of 4-AQ AH II crystallised from the melt, 1-butanol or carbon tetrachloride and SCCl4. (b) Microphotographs of (left) freshly crystallised AH II from carbon tetrachloride and (right) of the same sample stored for 18 months at ambient conditions. The elongated plates (AH I°) grown on top of the crystals of the stored sample formed due to sublimation.
It was not possible to “desolvate” the solvent containing AH II samples to produce solvent-free AH II. The release of the solvent molecules always resulted in the destruction of the crystal lattice. Thus, it was concluded that solvent molecules were entrapped at isolated sites of the AH II structure during crystallisation and are not freely accessible anymore once the crystals are formed.
The SCCl4 solvate is unstable and desolvation to AH III is completed within days at ambient conditions. Exposure to the lowest relative humidity (RH) levels leads to a fast desolvation at room temperature with the appearance of cracks and darkening of the crystals in non-polarised light. The measured mass loss of 15.6 ± 0.7% derived from TGA (Fig. 7a) corresponds to 0.19 ± 0.01 mol of carbon tetrachloride per mol of 4-AQ.
3.5. Crystal Energy Landscape
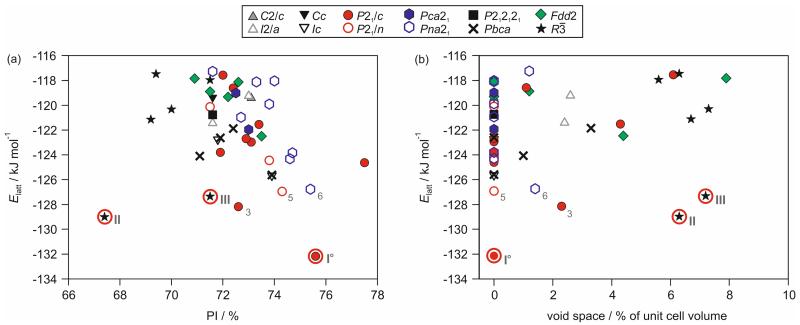
The experimental forms AH I° and AH II correspond to the lowest and second lowest energy structures on the crystal energy landscape (Fig. 8), with rmsd15 values of 0.22 Å and 0.19 Å for AH I° and AH II, respectively (ESI† Table S7). The combination of the applied search routine and lattice energy minimisation method produced a sufficiently realistic crystal energy landscape. This allowed the complementary interpretation of the experimental results using the computed low energy structures. All structures within 10 kJ mol−1 of the global minimum, 19 structures, were used for interpreting the possible 4-AQ solid form diversity.
Fig. 8.
Crystal energy landscape for 4-AQ anhydrates, classified by space group. Each symbol denotes a crystal structure. Experimental structures are encircled and labelled with Roman numbers. Arabic numbers are used for the most stable hypothetical structures and the numbers correspond to their stability rank (ESI† Table S6). In (a) the packing index (PI) and in (b) the void space calculated using a 1.0 Å probe radius are plotted against lattice energies (Elatt).
3.5.1. Structural Diversity on the Crystal Energy Landscape
The computationally generated 4-AQ structures (Fig. 8) exhibit a planar conformation with the C–C–N2–H1/2 dihedrals deviating by less than 25° from the plane of the aromatic rings. Thus, the structures differ solely in their packing arrangements and can be seen as (hypothetical) “packing polymorphs”.72 As expected, only two distinct hydrogen bonding interactions are formed among the computed structures, N2–H1⋯N1 and N2–H2⋯N1, respectively. Due to the molecular geometry of 4-AQ and the range of possible symmetry operations only one of the donor groups can be engaged in hydrogen bonding in Z′=1 structures. In case of a Z′=2 structure it is possible that both amino protons form hydrogen bonds (Fig. 3a, molecule A), although at cost of close packing (AH II on Fig. 8a). Using the H2 as a donor allows more packing possibilities amongst the most stable structures, 13 of the lowest structures form N2–H2⋯N1 hydrogen bonds, three N2–H1⋯N1, and only one shows both types. The global minimum structure, AH I°, shows the N2–H2⋯N1 connectivity, the second lowest structure, AH II, both possibilities and the fourth lowest energy structure is the most stable structure with a N2–H1⋯N1 hydrogen bond. In addition to the hydrogen bonds also C–H⋯π and π⋯π close contacts (Fig. 9) are essential for the stability of the 4-AQ structures (ESI† Table S8).
Fig. 9.
2D Fingerprint plots derived from Hirshfeld surfaces73,74 of (a) 4-AQ AH I°, (b) AH II, (c) third lowest energy structure on Error! Reference source not found. and (d) AH III (rank 4 structure). The large number of points at high values of de and di in (b) and (d) are indicative of voids in the structures. Numbers correspond to PIXEL68-70 energies of the strongest intermolecular interactions (ESI† Table S8), with N–H⋯N hydrogen bonds indicated with solid arrows, C–H⋯πC short contacts with dotted arrows and the regions of π⋯π interactions encircled.
3.5.2. Void Space Analysis
A striking feature of Fig. 8a is the broad range of packing indices among the most stable structures, spanning 67.4 to 77.5 %. Analyzing the void space of the computationally generated structures revealed that eight of the 19 most stable structures are not densely packed, with some of the structures showing significant void space (Fig. 8b). Amongst those structures are the solvent-free AH II and AH III, the fourth lowest energy structure (Fig. 9b and d).
3.5.3. Proposed Anhydrate III Structure
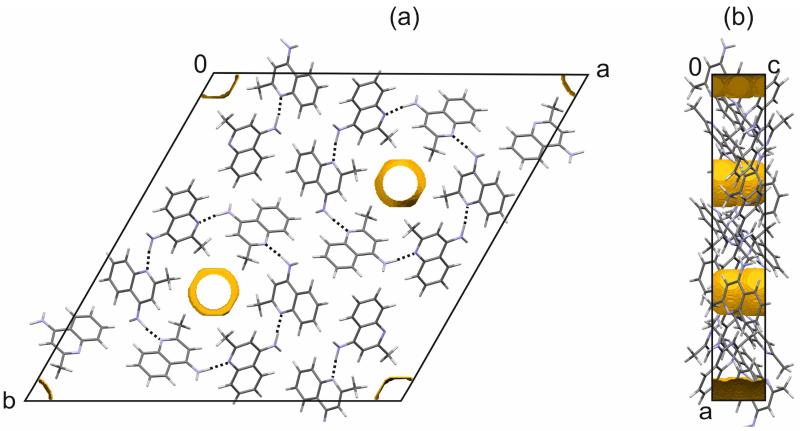
AH III, estimated to be 5.4 ± 0.6 kJ mol−1 less stable than AH I° (Table 3), was obtained exclusively by desolvating SCCl4. Only a low crystallinity product was observed, which did not allow us to solve the structure from X-ray powder diffraction data. The experimental XRPD data indexed to a trigonal unit cell (a = 31.511(4) Å, c = 4.6658(8) Å) and space group using the first 20 peaks with DICVOL04 on statistical assessment of systematic absences75 and the DASH76 and Topas Academic V577 packages. IR spectroscopy indicated the presence of a Z′ = 1 structure (Fig. 4). By comparing the experimental XRPD pattern to the simulated powder patterns of the computationally generated low energy structures (Fig. 8) it should be possible to identify AH III. The experimental XRPD pattern of AH III matches the pattern simulated from the fourth most stable structure (, Z′ = 1, a = 32.265 Å, c = 4.425 Å). Equally, the experimental AH I° and AH II XRPD patterns match with those simulated from the corresponding computationally derived structures. Thus, the fourth lowest energy structure corresponds to AH III.
In AH III the 4-AQ molecules form a hydrogen bonded ring motif mediated through N2–H1⋯N1 interactions and roto-inversion symmetry (Fig. 10a). Adjacent rings interact through C–H⋯π intermolecular interactions (Fig. 9c) and are stacked in direction of the c axis. The AH III structure shows significant void space (Fig. 8b), which is located in hydrophobic channels in direction of the c axis (Fig. 10). The occurrence of a channel structure agrees with the fact that AH III is observed on route of desolvation. The XRPD patterns of AH III and SCCl4 show resemblance. Distinct shifts to lower diffraction angles of the peak positions in SCCl4 (Fig. 5) imply, as expected, a bigger unit cell for the solvate. Changes in diffraction intensities of the peaks, in particular , on which the roto-inversion axis is located in AH III, may indicate the presence of CCl4 along the axis in SCCl4 if the space group is maintained. Based on the IR spectra of SCCl4 and AH III we can exclude isostructurality as there are significant differences in the region of the νNH2 and νCH stretching vibrations (ESI† Figure S9). This is also supported by the HSM investigations which showed darkening of the crystals (“pseudomorphosis” 78) rather than only the appearance of cracks on the surfaces of the crystals. Thus, AH III and SCCl4 may belong to the same crystal system, which would facilitate a phase transformation, but differ in packing (hydrogen bonding). The rings motif of the AH III structure may only be accessible via the SCCl4.
Fig. 10.
(a) Hydrogen bonding and (a,b) unit cell and voids visualisation (2.0 Å probe radius) of the computationally generated AH III structure. The yellow surfaces represent the boundaries of calculated voids in the structure.
Overall, the experimental 4-AQ structures, AH I°-III and MH, show very little structural similarity to each other. The only common building block is a one-dimensional stack of 4-AQ molecules, which is present in AH I, AH III and MH.
3.6. Thermodynamic and Kinetic Stability of 4-AQ Anhydrates
The DSC and slurry experiments of the three anhydrates revealed that AH I° is the thermodynamically most stable polymorph. Since AH I° melts at a higher temperature and shows a higher enthalpy of fusion (Table 3) than AH III the two polymorphs are monotropically related.79 The transformation of AH III to AH I° occurs within days at RT. Similarly, AH II is metastable relative to AH I° and from the exothermic transition to the stable form between 100 and 140 °C (see Fig. 6) we can also conclude that AH II/AH I° is a monotropic pair of polymorphs. Phase pure solvent-free AH II transforms within two to three weeks to AH I° at RT and much faster at higher temperatures (Fig. 6b). In contrast, AH II samples obtained by solvent crystallisation are more stable than the solvent-free AH II samples but still transform to AH I° and MH within less than a year if stored at room conditions. Transformation to the MH was observed for all polymorphs if stored at RH values above 40 % RH.
The thermodynamic stability order at 0 K was determined to be AH I° (most stable) > AH II > AH III (least stable). The experimental energy differences between the three polymorphs, derived from the heat of fusion (ΔfusH) and transformation (ΔtrsH) enthalpies, were measured as 3.3 ± 0.1 kJ mol−1 between AH I° and AH II and 5.4 ± 0.6 kJ mol−1 between AH I° and AH III (Table 3). These experimental values agree well with the 0 K lattice energies differences of 3.2 and 4.8 kJ mol−1 for AH I°/II and AH I°/III, respectively. The presence of a Z′=2 anhydrate and solvent inclusion may explain why the more stable AH II shows a lower density than the less stable AH III, which represents an exception to the density rule.79
4. Discussion
4.1. Solvent Inclusion in Structures
The best known example of a solid form crystallising in and showing solvent inclusion is carbamazepine (CBZ) form II.40,41 The resemblance in solid state behaviour of the two phases, CBZ form II and 4-AQ AH II, and the fact that all structures on Fig. 8 show considerable void space inspired us to closer investigate the occurrence of solvate formation/void space in known structures present in the Cambridge Structural Database43 (CSD). A search for structures containing only C, N, O and H atoms revealed that more than half of the structures (56%) have a guest molecule on the axis, with the solvent molecule either modelled or residual electron density noted by the authors who could not model the solvent molecule. The remaining 44% of the 203 unique CSD structures exhibit either a symmetric molecule on the axis or (considerable) void space, which was not commented on by the authors. These results agree with the CSD survey performed earlier by Fabbiani et al.40 The void space around the roto-inversion axis can either show the features of isolated sites, e.g. Fig. 3b&c, or channels, e.g. Fig. 10. The shape of the void space will strongly influence the stability and characteristics of the solid from. In both cases the solvent molecules may be necessary for the formation of the structure, i.e. act as a template. In case of a channel structure, the solvent content can depend on the environmental conditions and it may even be possible to exchange the guest molecules without major rearrangements of the structure. This is in contrast to the isolated void site structures where normally the guest molecule can only be released in a destructive mechanism, which results in a distinct solid form exhibiting different properties. Either type can cause practical problems. The problems with substoichiometric solvent inclusions are well known and regulated for pharmaceuticals under the term “residual solvents”.39
4.2. 4-AQ AH II and Carbamazepine Form II
Despite the similarities of the two solid state phases, forms II of CBZ and 4-AQ, they show distinct characteristics. Firstly, 4-AQ AH II is to our knowledge the only example where solvent molecules (carbon tetrachloride, chloroform, tetrahydrofurane, 1-butanol) can be entrapped in the structure of AH II. However, their presence is not necessary for the nucleation and growth of this phase, as evidenced by the melt film crystallisation of AH II. This ascertainment is supported by the computationally generated anhydrate crystal energy landscape, which revealed AH II as the second lowest energy structure only 3 kJ mol−1 above the global minimum. In the case of CBZ form II was found on the corresponding CBZ crystal energy landscape, albeit higher in energy.80,81 Cruz-Cabeza et al. rationalised that the computed high energy structure corresponds to the CBZ form II framework and that solvent inclusion stabilises this phase.41 However, the inclusion of solvent molecules also seems to increase the barrier for the AH II to AH I° phase transformation in 4-AQ, i.e. AH II samples containing residual solvent were stable for a longer time than solvent-free AH II samples (see section 3.6). The two phases differ considerably in their overall stability. The investigation of 25 years old in house samples of CBZ II (containing carbon tetrachloride, toluene, butanol or ethyl acetate as guest molecules) revealed that only a part of the sample transformed to CBZ form III after this long storing time at ambient conditions. In contrast, all 4-AQ AH II samples transformed to AH I° after storing the sample under the same conditions for only 18 months.
4.3. Carbon Tetrachloride Solvate as an Intermediate Phase for Producing AH III
The formation of a specific solvate form may be the only route to an otherwise elusive phase, e.g. premofloxacin,82 cinacalect HCl,83 prilocaine HCl,84 DB7 (3-(4-dibenzo[b,f][1,4]oxepin-11-yl-piperazin-1-yl)-2,2-dimethylpropanoic acid).27 Thus, the formation of solvates not only expands the solid form landscape of a compound but also the possible screening techniques, i.e. desolvation studies, as recently discussed for two model pharmaceuticals.27 The awareness of the occurrence of solvent adducts, as 4-AQ SCCl4, increased immensely over the last decade. Solvate formation is rather common among organic (drug) molecules as shown by the steadily growing number of scientific publications and patent applications dealing with this topic. The formation of a highly unstable intermediate solvates can be easily overlooked, especially if the analytics is not performed on “wet” but dried samples. This then can lead to incorrect conclusions about the influence of the solvent on the nucleation of a specific solid form and our general understanding about templating effects.
The 4-AQ SCCl4 can be seen as both, an intermediate solvate form and, to our knowledge, as the only gateway to AH III. This solvate seems to be the necessary precursor for the formation of the N2–H1⋯N1 hydrogen bonded ring motifs, preventing the formation of the close packed chain motif found for 4-AQ which would be otherwise preferred. In contrast to examples with flexible molecules,85 the solvate formation of 4-AQ cannot be ascribed to the inability of its molecules to pack efficiently, but may be interpreted as a possibility to expand the observed hydrogen bonding and packing motifs. The presence of carbon tetrachloride does not automatically lead to the formation of the solvate, but the degree of supersaturation and the crystallisation temperature determine whether SCCl4 or AH II (CCl4) is formed. The solvate crystallises preferentially from supersaturated solutions and at room temperature, as indicated by TGA experiments (Fig. 7a).
4.4. Computational and Experimental Solid Form Screening
The interpretation of computationally generated low energy structures can provide information about possible and energetically feasible intermolecular interactions and packing arrangements in a compound, which would not be accessible from experiments. The lattice energy, often in combination with the packing index/density, is normally used to select the most likely alternative structures. In the case of 4-AQ, Fig. 8 clearly indicates that packing efficiency/density does not directly correlate with stability. The molecular shape of 4-AQ dictates that a close packing can be achieved, but with only half of the hydrogen bonding donors involved in strong intermolecular interactions. The number of hydrogen bonding interactions can be increased by increasing the number of independent 4-AQ molecules (cf., AH II), but at the cost of packing efficiency. By adding additional H-bonding acceptor/donor groups, as in MH (LOBSOL),44 the frustration between packing efficiency and number of strong intermolecular interactions is resolved. Thus, for solvent-free 4-AQ forms there is a subtle balance between close packing and the number of strong intermolecular interactions (i.e. hydrogen bonds). Furthermore, the planarity of the aromatic 4-AQ molecule favours the formation of π⋯π or C–H⋯π interactions, which significantly contribute to the lattice energy of every structure on Fig. 8 (ESI† Table S8).
Crystal energy landscapes can guide experimental investigations to find alternative polymorphs, as demonstrated for CBZ form V11 or creatine.28 When one considers the outcome of the crystal structure predictions illustrated in Fig. 8, which reproduces the structures and stability order of the experimental forms reasonably well, it appears not to be unlikely that additional 4-AQ polymorphs can be generated experimentally. The third (3, ESI† Table S6), fifth (5) and sixth (6) lowest energy structures are still within 6 kJ mol−1 of AH I°, and based on energy, the most probable candidates when the effects of thermal motion are neglected. Structures 3 and 6 show no packing similarity86 with the experimental polymorphs. The strongest intermolecular interactions are in both cases the hydrogen bonded chains involving either H2 (3) or H1 (6 as a donor. Nucleation of 3 would require an environment that prevents the formation of π⋯π interactions, as in the more stable packing AH II. In 6 π⋯π interactions strongly contribute to the stability of the structure (ESI† Figure S14b), as in AH III, the polymorph showing the same N2–H1⋯N1 hydrogen bonding connectivity. One could assume that the right crystallisation conditions for those forms have not been found/applied yet. However, both hypothetical structures may not have enough void space to be templated by solvent molecules. Structure 5 shows packing similarity with AH I°. The two structures are built up of the same π⋯π stacked chain motifs, differing only slightly in the stacking of the 2D building blocks, the C–H⋯π/C short contacts. With AH I° being the thermodynamically most stable form (as experimentally confirmed), structure 5 is unlikely to be observed as a long-lived metastable polymorph as its transformation to AH I° would be facile.
The combination of experimental and computational results gives us reassurance that AH I° is the thermodynamically most stable form, and that low density structures play a crucial role in the solid form landscape of 4-AQ.
5. Conclusions
Crystal structure prediction for a small organic model compound, 4-aminoquinaldine, was successfully performed as part of an interdisciplinary study. The computationally generated anhydrate crystal energy landscape provided insight into the range of possible and stable packing arrangements and explained the unique solid state behaviour of 4-AQ. The complex crystallisation behaviour of this compound is determined by the interplay between dense packing and a number of possible strong intermolecular interactions (hydrogen bonds). Analysing the void space of the computed structures rationalised the solvent inclusion behaviour.
The thermodynamically most stable anhydrate, AH I°, a densely packed structure, was found as the global minimum of the computed crystal energy landscape. The two metastable forms, AH II and AH III (both ), were amongst the lowest energy structures (ΔElatt relative to AH I° < 5 kJ mol−1), rank two and four, respectively, but showed both considerable void space. The solvent accessible volume accounts for the fact that the formation of the metastable anhydrates can be templated by solvent inclusion. The isolated void space present in AH II can be filled either with solvent molecules (4.5 – 6 wt.%), or, in contrast to other known examples showing solvent inclusion in structural voids, be empty. The presence of the solvent molecules is not essential for the formation of this phase but contributes to the stability of AH II, i.e. slow down the phase transformation to AH I°. The third polymorph, AH III, was exclusively obtained by desolvating the carbon tetrachloride solvate and exhibits void space in channels. Its structural information could be derived by comparing the experimental X-ray powder diffraction pattern to simulated patterns of the computationally generated anhydrate structures. Based on the structural features the role of the solvate as a precursor is rationalised.
As demonstrated in this study, carefully conducted CSP can help to understand the complexity of the solid form landscape of a molecule. Analysing void space has been shown to be a useful tool for identifying phases that might be templated or stabilised by solvent inclusion.41 We think that studies like this will help to advance computational methodologies for predicting thermodynamically feasible crystal structures and identifying putative alternative polymorphs.
Supplementary Material
Acknowledgements
The authors are grateful to Profs. C. C. Pantelides and C. S. Adjiman (Imperial College London) for the use of the CrystalPredictor and CrystalOptimizer programs, to Prof. S. L. Price (University College London) for the use of the DMACRYS program, and to V. Illing for experimental assistance. DEB gratefully acknowledges funding by the Hertha Firnberg Programme of the Austrian Science Fund (FWF, project T593-N19). This work was supported by the Austrian Ministry of Science BMWF as part of the UniInfrastrukturprogramm of the Focal Point Scientific Computing at the University of Innsbruck.
Footnotes
Electronic Supplementary Information (ESI) available: Conditions and outcomes of the manual solvent crystallisation screen; Pawley Fits, Infrared spectrum for SCCl4; photographs; crystallographic information (cifs); potential energy surface scans; representation of the experimental structure; computationally generated AH crystal energy landscape; 2D Hirshfeld fingerprint plots for computed low energy structures; PIXEL energies. See DOI: 10.1039/b000000x/
Notes and references
- 1. Brittain HG. In: Brittain HG. Polymorph in Pharmaceutical Solids. Second Edition. Informa Healthcare; 2009. pp. 1-23. [Google Scholar]
- 2.Hilfiker R. Polymorphism: In the Pharmaceutical Industry. Wiley-VCH; Germany: 2006. [Google Scholar]
- 3.Bernstein J. Polymorphism in Molecular Crystals. Clarendon Press; Oxford: 2002. [Google Scholar]
- 4. Byrn SR, Pfeiffer RR, Stowell JG. Solid-State Chemistry of Drugs. 2nd ed. SSCI, Inc.; West Lafayette, Indiana: 1999. [Google Scholar]
- 5.Bernardes CES, da Piedade MEM. Cryst. Growth Des. 2012;12:2932–2941. [Google Scholar]
- 6.Llinas A, Goodman JM. Drug Discover. Today. 2008;13:198–210. doi: 10.1016/j.drudis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Aaltonen J, Alleso M, Mirza S, Koradia V, Gordon KC, Rantanen J. Eur. J. Pharm. Biopharm. 2009;71:23–37. doi: 10.1016/j.ejpb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Florence AJ, Johnston A, Fernandes P, Shankland N, Shankland K. J. Appl. Crystallogr. 2006;39:922–924. [Google Scholar]
- 9.Zencirci N, Gelbrich T, Kahlenberg V, Griesser UJ. Cryst. Growth Des. 2009;9:3444–3456. doi: 10.1021/acs.cgd.0c00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokes SP, Seaton CC, Eccles KS, Maguire AR, Lawrence SE. Cryst. Growth Des. 2014;14:1158–1166. [Google Scholar]
- 11.Arlin JB, Price LS, Price SL, Florence AJ. Chem. Commun. 2011;47:7074–7076. doi: 10.1039/c1cc11634g. [DOI] [PubMed] [Google Scholar]
- 12.Eccles KS, Deasy RE, Fabian L, Braun DE, Maguire AR, Lawrence SE. CrystEngComm. 2011;13:6923–6925. [Google Scholar]
- 13.Braun DE, Karamertzanis PG, Arlin JB, Florence AJ, Kahlenberg V, Tocher DA, Griesser UJ, Price SL. Cryst. Growth Des. 2011;11:210–220. doi: 10.1021/cg101162a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun DE, Bhardwaj RM, Florence AJ, Tocher DA, Price SL. Cryst. Growth Des. 2013;13:19–23. doi: 10.1021/cg301506x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson GA, Kendrick J, Wolfangel C, Leusen FJ. Cryst. Growth Des. 2012;12:3964–3976. [Google Scholar]
- 16.Kendrick J, Stephenson GA, Neumann MA, Leusen FJ. Cryst. Growth Des. 2013;13:581–589. [Google Scholar]
- 17.Gorbitz CH, Dalhus B, Day GM. Phys. Chem. Chem. Phys. 2010;12:8466–8477. doi: 10.1039/c004055j. [DOI] [PubMed] [Google Scholar]
- 18.Braun DE, Ardid-Candel M, D’Oria E, Karamertzanis PG, Arlin JB, Florence AJ, Jones AG, Price SL. Cryst. Growth Des. 2011;11:5659–5669. doi: 10.1021/cg101162a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun DE, Bhardwaj RM, Arlin JB, Florence AJ, Kahlenberg V, Griesser UJ, Tocher DA, Price SL. Cryst. Growth Des. 2013;13:4071–4083. doi: 10.1021/cg4009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun DE, Karamertzanis PG, Price SL. Chem. Commun. 2011;47:5443–5445. doi: 10.1039/c1cc10762c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Cabeza AJ, Day GM, Jones W. Chem. Eur. J. 2008;14:8830–8836. doi: 10.1002/chem.200800668. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Cabeza AJ, Karki S, Fabian L, Friscic T, Day GM, Jones W. Chem. Commun. 2010;46:2224–2226. doi: 10.1039/b922955h. [DOI] [PubMed] [Google Scholar]
- 23.Bardwell DA, Adjiman CS, Arnautova YA, Bartashevich E, Boerrigter SX, Braun DE, Cruz-Cabeza AJ, Day GM, la Valle RG, Desiraju GR, van Eijck BP, Facelli JC, Ferraro MB, Grillo D, Habgood M, Hofmann DW, Hofmann F, Jose K, V, Karamertzanis PG, Kazantsev AV, Kendrick J, Kuleshova LN, Leusen FJ, Maleev AV, Misquitta AJ, Mohamed S, Needs RJ, Neumann MA, Nikylov D, Orendt AM, Pal R, Pantelides CC, Pickard CJ, Price LS, Price SL, Scheraga HA, van de Streek J, Thakur TS, Tiwari S, Venuti E, Zhitkov IK. Acta Crystallogr., Sect. B. 2011;67:535–551. doi: 10.1107/S0108768111042868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazantsev AV, Karamertzanis PG, Adjiman CS, Pantelides CC, Price SL, Galek PT, Day GM, Cruz-Cabeza AJ. Int. J. Pharm. 2011;418:168–178. doi: 10.1016/j.ijpharm.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Bhardwaj RM, Price LS, Price SL, Reutzel-Edens SM, Miller GJ, Oswald IDH, Johnston B, Florence AJ. Cryst. Growth Des. 2013;13:1602–1617. [Google Scholar]
- 26.Ismail SZ, Anderton CL, Copley RC, Price LS, Price SL. Cryst. Growth Des. 2013;13:2396–2406. [Google Scholar]
- 27.Braun DE, McMahon JA, Koztecki LH, Price SL, Reutzel-Edens SM. Cryst. Growth Des. 2014;14:2056–2072. [Google Scholar]
- 28.Braun DE, Orlova M, Griesser UJ. Cryst. Growth Des. 2014;14:4895–4900. doi: 10.1021/cg501159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemburkar SR, Bauer J, Deming K, Spiwek H, Patel K, Morris J, Henry R, Spanton S, Dziki W, Porter W, Quick J, Bauer P, Donaubauer J, Narayanan BA, Soldani M, Riley D, McFarland K. Org. Process Res. Dev. 2000;4:413–417. [Google Scholar]
- 30.Cruz-Cabeza AJ, Day GM, Jones W. Chem. -Eur. J. 2009;15:13033–13040. doi: 10.1002/chem.200901703. [DOI] [PubMed] [Google Scholar]
- 31.Pyzer-Knapp EO, Thompson HPG, Schiffmann F, Jelfs KE, Chong SY, Little MA, Cooper AI, Day GM. Chem. Sci. 2014;5:2235–2245. [Google Scholar]
- 32.Copley RCB, Barnett SA, Karamertzanis PG, Harris KDM, Kariuki BM, Xu MC, Nickels EA, Lancaster RW, Price SL. Cryst. Growth Des. 2008;8:3474–3481. [Google Scholar]
- 33.Braun DE, Tocher DA, Price SL, Griesser UJ. J. Phys. Chem. B. 2012;116:3961–3972. doi: 10.1021/jp211948q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habgood M, Lancaster RW, Gateshki M, Kenwright AM. Cryst. Growth Des. 2013;13:1771–1779. [Google Scholar]
- 35.Gelbrich T, Braun DE, Ellern A, Griesser UJ. Cryst. Growth Des. 2013;13:1206–1217. doi: 10.1021/cg301639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun DE, Gelbrich T, Kahlenberg V, Griesser UJ. Mol.Pharmaceutics. 2014;11:3145–3163. doi: 10.1021/mp500334z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernardes CES, Piedade MF, Minas da Piedade ME. Cryst. Growth Des. 2010;10:3070–3076. [Google Scholar]
- 38.Griesser UJ. In: Polymorphism: In the Pharmaceutical Industry. Hilfiker Rolf., editor. Wiley-VCH; Germany: 2006. pp. 211–233. [Google Scholar]
- 39.ICH Q3C Impurities: Guideline for Residual Solvents. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; Geneva, Switzerland. 1997. [Google Scholar]
- 40.Fabbiani FPA, Byrne LT, McKinnon JJ, Spackman MA. CrystEngComm. 2007;9:728–731. [Google Scholar]
- 41.Cruz-Cabeza AJ, Day GM, Motherwell WDS, Jones W. Chem. Commun. 2007:1600–1602. doi: 10.1039/b701299c. [DOI] [PubMed] [Google Scholar]
- 42.Kofler A, Kolsek J. Mikrochim. Acta. 1969:408–435. [Google Scholar]
- 43.Allen FH. Acta Crystallogr., Sect. B. 2002;58:380–388. doi: 10.1107/s0108768102003890. [DOI] [PubMed] [Google Scholar]
- 44.Tai XS, Xu J, Feng YM, Liang ZP. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2008;64:o1026, o1026–1-o1026, o1026–6. doi: 10.1107/S1600536808013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnakumar V, Xavier RJ. Chem. Phys. 2005;312:227–240. [Google Scholar]
- 46.Arjunan V, Saravanan I, Ravindran P, Mohan S. Spectrochim. Acta, Part A. 2009;74A:375–384. doi: 10.1016/j.saa.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Gu CH, Li H, Gandhi RB, Raghavan K. Int. J. Pharm. 2004;283:117–125. doi: 10.1016/j.ijpharm.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Coles SJ, Gale PA. Chem. Sci. 2012;3:683–689. [Google Scholar]
- 49.Oxford Diffraction Oxford Diffraction Ltd . Abingdon, Oxford, England: 2003. [Google Scholar]
- 50.Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, Giacovazzo C, Mallamo M, Mazzone A, Polidori G, Spagna R. J. Appl. Crystallogr. 2012;45:357–361. [Google Scholar]
- 51.Sheldrick GM. Acta Crystallogr., Sect. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 52.Farrugia LJ. J. Appl. Crystallogr. 2012;45:849–854. [Google Scholar]
- 53.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb JMA, Cheeseman R, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Gaussian Inc.; Wallingford CT: 2009. [Google Scholar]
- 54.Karamertzanis PG, Pantelides CC. J Comput Chem. 2005;26:304–324. doi: 10.1002/jcc.20165. [DOI] [PubMed] [Google Scholar]
- 55.Karamertzanis PG, Pantelides CC. Mol. Phys. 2007;105:273–291. [Google Scholar]
- 56.Habgood M, Sugden I, Kazantsev AV, Adjiman CS, Pantelides CC. JCTC. 2015 doi: 10.1021/ct500621v. (accepted) [DOI] [PubMed] [Google Scholar]
- 57.Coombes DS, Price SL, Willock DJ, Leslie M. J. Phys. Chem. 1996;100:7352–7360. [Google Scholar]
- 58.Breneman CM, Wiberg KB. J. Comput. Chem. 1990;11:361–373. [Google Scholar]
- 59.Price SL, Leslie M, Welch GWA, Habgood M, Price LS, Karamertzanis PG, Day GM. Phys. Chem. Chem. Phys. 2010;12:8478–8490. doi: 10.1039/c004164e. [DOI] [PubMed] [Google Scholar]
- 60.Stone AJ. J. Chem. Theory Comput. 2005;1:1128–1132. doi: 10.1021/ct050190+. [DOI] [PubMed] [Google Scholar]
- 61.Stone AJ. GDMA: A Program for Performing Distributed Multipole Analysis of Wave Functions Calculated Using the Gaussian Program System [2.2] University of Cambridge Cambridge; United Kingdom: 2010. [Google Scholar]
- 62.Kazantsev AV, Karamertzanis PG, Adjiman CS, Pantelides CC. J. Chem. Theory Comput. 2011;7:1998–2016. doi: 10.1021/ct100597e. [DOI] [PubMed] [Google Scholar]
- 63.Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MJ, Refson K, Payne MC. Z. Kristallogr. 2005;220:567–570. [Google Scholar]
- 64.Perdew JP, Burke K, Ernzerhof M. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 65.Vanderbilt D. Phys. Rev. B. 1990;41:7892–7895. doi: 10.1103/physrevb.41.7892. [DOI] [PubMed] [Google Scholar]
- 66.Tkatchenko A, Scheffler M. Phys. Rev. Lett. 2009;102:073005-1–073005/4. doi: 10.1103/PhysRevLett.102.073005. [DOI] [PubMed] [Google Scholar]
- 67.Grimme S. J. Comput. Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- 68.Gavezzotti A. New J. Chem. 2011;35:1360–1368. [Google Scholar]
- 69.Gavezzotti A. J. Phys. Chem. B. 2002;106:4145–4154. [Google Scholar]
- 70.Gavezzotti A. J. Phys. Chem. B. 2003;107:2344–2353. [Google Scholar]
- 71.Etter MC, MacDonald JC, Bernstein J. Acta Crystallogr., Sect. B. 1990;46:256–262. doi: 10.1107/s0108768189012929. [DOI] [PubMed] [Google Scholar]
- 72.Braun DE, Gelbrich T, Kahlenberg V, Laus G, Wieser J, Griesser UJ. New J. Chem. 2008;32:1677–1685. [Google Scholar]
- 73.Spackman MA, Jayatilaka D. CrystEngComm. 2009;11:19–32. [Google Scholar]
- 74.Wolff SK, Grimwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA. CrystalExplorer [3.1] University of Western Australia; Perth: 2012. [Google Scholar]
- 75.Markvardsen AJ, David WIF, Johnson JC, Shankland K. Acta Crystallogr., Sect. A. 2001;57:47–54. doi: 10.1107/s0108767300012174. [DOI] [PubMed] [Google Scholar]
- 76.David WIF, Shankland K, van de Streek J, Pidcock E, Motherwell WDS, Cole JC. J. Appl. Crystallogr. 2006;39:910–915. [Google Scholar]
- 77.Coelho AA. Topas Academic V5 Version 5 Coelho Software Brisbane. 2012.
- 78.Kuhnert-Brandstaetter M, Proell F. Mikrochim. Acta. 1983;3:287–300. [Google Scholar]
- 79.Burger A, Ramberger R. Mikrochim. Acta. 1979;2:273–316. [Google Scholar]
- 80.Florence AJ, Johnston A, Price SL, Nowell H, Kennedy AR, Shankland N. J. Pharm. Sci. 2006;95:1918–1930. doi: 10.1002/jps.20647. [DOI] [PubMed] [Google Scholar]
- 81.Cruz-Cabeza AJ, Day GM, Motherwell WDS, Jones W. Cryst. Growth Des. 2006;6:1858–1866. [Google Scholar]
- 82.Schinzer WC, Bergren MS, Aldrich DS, Chao RS, Dunn MJ, Jeganathan A, Madden LM. J. Pharm. Sci. 1997;86:1426–1431. doi: 10.1021/js970063o. [DOI] [PubMed] [Google Scholar]
- 83.Braun DE, Kahlenberg V, Gelbrich T, Ludescher J, Griesser UJ. CrystEngComm. 2008;10:1617–1625. [Google Scholar]
- 84.Schmidt AC, Niederwanger V, Griesser UJ. J. Therm. Anal. Calorim. 2004;77:639–652. [Google Scholar]
- 85.Berzins A, Skarbulis E, Rekis T, Actins A. Cryst. Growth Des. 2014;14:2654–2664. [Google Scholar]
- 86.Gelbrich T, Threlfall TL, Hursthouse MB. CrystEngComm. 2012;14:5454–5464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.