Abstract
Mycoplasma gallisepticum is the primary etiologic agent of chronic respiratory disease in poultry, a disease largely affecting the respiratory tract and causing significant economic losses worldwide. Immunodominant proteins encoded by members of the variable lipoprotein and hemagglutinin (vlhA) gene family are thought to be important for mechanisms of M. gallisepticum-host interaction, pathogenesis, and immune evasion, but their exact role and the overall nature of their phase variation are unknown. To better understand these mechanisms, we assessed global transcriptomic vlhA gene expression directly from M. gallisepticum populations present on tracheal mucosae during a 7-day experimental infection in the natural chicken host. Here we report differences in both dominant and minor vlhA gene expression levels throughout the first week of infection and starting as early as day 1 postinfection, consistent with a functional role not dependent on adaptive immunity for driving phase variation. Notably, data indicated that, at given time points, specific vlhA genes were similarly dominant in multiple independent hosts, suggesting a nonstochastic temporal progression of dominant vlhA gene expression in the colonizing bacterial population. The dominant expression of a given vlhA gene was not dependent on the presence of 12-copy GAA trinucleotide repeats in the promoter region and did not revert to the predominate vlhA gene when no longer faced with host pressures. Overall, these data indicate that vlhA phase variation is dynamic throughout the earliest stages of infection and that the pattern of dominant vlhA expression may be nonrandom and regulated by previously unrecognized mechanisms.
INTRODUCTION
Mycoplasma gallisepticum, the primary etiologic agent of chronic respiratory disease (CRD) in poultry, is highly transmissible and causes severe inflammation of trachea, lungs, and air sacs, ultimately resulting in an overall reduction in weight gain and egg production in infected chickens. M. gallisepticum is pathogenic in other species, causing infectious sinusitis in turkeys and conjunctivitis in house finches (1, 2). Aside from primary attachment proteins GapA and CrmA, fibronectin binding proteins PlpA and Hlp3, sugar transport permease MalF, and dihydrolipoamide dehydrogenase Lpd, little is understood about specific bacterial factors affecting survival and persistence of this important avian pathogen in its natural host (3–6).
The lack of obvious homologs of traditional bacterial transcriptional regulators and signaling factors had previously led to the thought that mycoplasmas were unable to regulate transcription by conventional mechanisms. However, M. gallisepticum exhibits differential levels of gene expression in response to coincubation with MRC-5 human lung fibroblasts (7) and in response to various stress conditions (8, 9). These data suggest that changes in transcription are indeed occurring as mycoplasmas respond to external stimuli and that the regulation is, in fact, more dynamic than originally hypothesized, with genes potentially affected by multiple regulators (10).
The members of the variable lipoprotein and hemagglutinin (vlhA) family, consisting of 43 genes distributed across 5 loci and totaling just over 10% of the entire genome in strain Rlow, are potentially important for M. gallisepticum pathogenesis (11). Previous studies have indicated that M. gallisepticum vlhA expression is dependent on the presence of exactly 12 GAA trinucleotide repeats upstream of the gene (12–15). It has also been hypothesized that M. gallisepticum vlhA phase variation is stochastic, driven by anti-VlhA antibody-mediated negative selection of the initial population and resulting in random outgrowth of bacterial subpopulations expressing alternative vlhA genes (16). If that were correct, we would expect to see various patterns of vlhA expression from the individual chickens at each time point.
The function of VlhA proteins is still unknown, but it has been speculated to involve immune evasion during infection (17), and variation in vlhA gene content has been loosely associated with virulence (18). While there have been efforts to understand the antigens expressed by M. gallisepticum in vivo (19), there has not yet been a comprehensive in vivo transcriptome study of M. gallisepticum in response to the natural host over the course of early infection at the global level. Previous studies assessed selected in vivo vlhA gene expression changes using monoclonal antibodies; however, methods for comprehensive vlhA analysis were not yet available (12, 13). Now, using next-generation sequencing, which enables comprehensive gene expression assessment at the nucleotide level, we present results indicating changes in vlhA expression of M. gallisepticum sampled directly from the tracheas of experimentally infected chickens over the course of a 7-day infection.
MATERIALS AND METHODS
Animals.
Four-week-old female specific-pathogen-free White Leghorn chickens (SPAFAS, North Franklin, CT, USA) were received and divided randomly into groups, placed in HEPA-filtered isolators, and allowed to acclimate for 1 week. Nonmedicated feed and water were provided ad libitum throughout the experiment. All animal studies were performed in accordance with approved University of Connecticut (UConn) IACUC protocol number A13-001.
Chicken infection.
Stocks of M. gallisepticum strain Rlow (passage 17) were grown overnight at 37°C in complete Hayflick's medium until mid-log phase was reached as indicated by a color shift from red to orange. Bacterial concentrations were determined by the optical density at 620 nm (OD620), and 10-fold serial dilutions were conducted to confirm viable color-changing-unit titers. Bacteria were pelleted by centrifugation at 10,000 × g for 10 min and resuspended in Hayflick's complete medium. Chickens were challenged intratracheally as previously described (6) with 1 × 108 CFU/200 μl.
RNA extraction.
Five infected chickens were humanely sacrificed each day for a total of 7 days. After sacrifice, tracheas were excised and total RNA was extracted from each individual trachea by washing the lumen with 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was then purified using a Zymo Direct-zol RNA Miniprep kit (Zymo Research Corporation, Irvine, CA, USA), and standard PCR was conducted to ensure that the RNA preparations were free of any DNA. RNA was quality-checked using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and high-quality samples with RNA integrity numbers (RIN) of >8 were utilized to construct cDNA libraries.
To enrich for bacterial RNA, total RNAs were subjected to a poly(A) depletion step to remove the eukaryotic mRNA using a NEBNext poly(A) mRNA magnetic isolation module (New England BioLabs, Ipswich, MA, USA). Briefly, 5 μg of total RNA combined with an equal volume of bead binding buffer was bound to poly(T) oligonucleotide-attached magnetic beads at 65°C for 5 min. The remaining supernatant was collected, cleaned, and concentrated using a Zymo Clean & Concentrator-25 kit (Zymo Research Corporation) and eluted in 25 μl RNase-free water.
Both prokaryotic rRNA and eukaryotic rRNA were removed from 2.5 μg of poly(A)-depleted RNA using a RiboZero Magnetic Gold kit (Epidemiology) (Illumina Inc., San Diego, CA, USA) following the manufacturer's instructions. Each rRNA-depleted RNA sample obtained after cleaning and concentrating with the Zymo Clean & Concentrator-25 kit (Zymo Research Corporation) was eluted in 25 μl of RNase-free water, and the samples were used to create a cDNA library.
Illumina sequencing.
The cDNA libraries were created using an Illumina TruSeq stranded mRNA library preparation kit (Illumina Inc., San Diego, CA, USA) according to the manufacturer's instructions starting at “Make RFP” (step 14; p. 56) of the Illumina TruSeq RNA Sample Preparation v2 (HT) protocol. Briefly, 10 to 400 ng of purified mRNA was fragmented and used to synthesize first-strand cDNA using reverse transcriptase and random hexamer primers. Second-strand cDNA synthesis was performed using dUTP, DNA polymerase, and RNase. The products were amplified by PCR and purified after end repair and adaptor ligations.
The cDNA libraries were assessed for quantity using a Qubit 2.0 fluorometer (Invitrogen) and for correct fragment size (∼260 bp) using an Agilent TapeStation 2200 instrument (Agilent Technologies). Libraries were then normalized to 2 nM, pooled, denatured, and sequenced on a NextSeq500 Sequencing platform (Illumina Inc.) using a 75-bp paired-end approach.
RNA-seq analysis.
Fastq data were assembled and mapped, and differential gene expression levels were assessed, using Rockhopper, an RNA sequencing (RNA-seq) analysis program with algorithms specifically designed for the bacterial gene structures and transcriptomes (http://cs.wellesley.edu/∼btjaden/Rockhopper/) (20, 21). Bowtie2 parameters allowing 0 mismatches were used to map sequence reads to the Rlow genomic template (11). The data were normalized by the standard method of determining the ratio of reads per kilobase per million (RPKM) mapped, allowing for comparisons both within and between samples. The fold change data were determined from the log2 transformation of the RPKM data between two samples. The differential levels of gene expression were determined by pairwise comparisons between the normalized values of expression of a given vlhA gene from two different days. The program-generated P value was used to determine the significance of the differential levels of gene expression by calculating q values based on the Benjamini-Hochberg correction with a false-discovery rate of <1%. Differences in expression values were considered significant when the q value was <0.02 (21).
Sequencing of culture-passaged bacteria.
Mycoplasma cultures recovered from tracheas of the experimentally infected birds at day 7 postinfection were passaged 5 or 10 times in Hayflick's complete medium. Cultures were then grown to mid-log phase for DNA extraction using Chelex reagent (Bio-Rad, Hercules, CA, USA) or RNA extraction, enrichment, and sequencing as was described for the trachea samples above.
Targeted sequencing of vlhA 2.02 gene promoter sequences to query GAA repeat copy numbers was performed directly on specific PCR amplicons. PCR primers targeting the vlhA 2.02 promoter region designed using the M. gallisepticum R(low) genomic sequence (GenBank accession no. AE015450) (11) were as follows: forward, 5′-GATGAGATTATCAAGCTTTTAGATG-3′; reverse, 5′-CTGAACGAATCAAAGAGTTACAGC-3′. PCR was performed using AmpliTaq (Invitrogen), 20 pmol of each primer, and 200 to 300 ng DNA under the following cycling conditions: 94°C for 5 min, followed by 40 cycles at 94°C for 15 s, 54°C for 30 s, and 72°C for 1 min with a final extension at 72°C for 10 min. Amplicons were purified using a MinElute Reaction Cleanup kit (Qiagen, Valencia, CA, USA) to remove excess primers.
Sequencing was performed using 2 μl BigDye Terminator Mix (Invitrogen), 10 pmol of primer, and 300 ng of DNA (22). Excess dye terminators were removed with AutoSeq G-50 spin columns (Amersham Biosciences, Piscataway, NJ, USA), and the sequencing was performed at the University of Connecticut Biotechnology Center. Using Sequencher software (Gene Codes, Ann Arbor, MI, USA), the sequences were compared to the Rlow genome and the numbers of GAA trinucleotide repeats upstream of the gene start codon were assessed.
RESULTS
M. gallisepticum input vlhA expression.
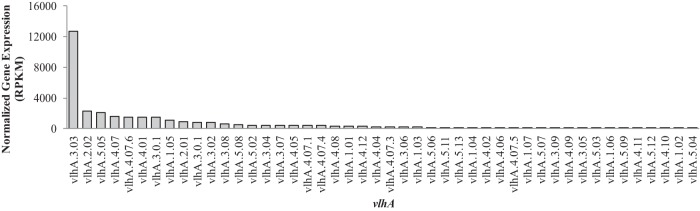
In broth-grown M. gallisepticum strain Rlow input cultures, data here indicated that 31 vlhA genes were expressed with an average normalized expression value of 719. vlhA 3.03 was the predominant vlhA expressed in this culture, with an RPKM value of >12,000 (Fig. 1). This vlhA is preceded by exactly 12 GAA repeats (11), a characteristic previously thought to be necessary for expression (12–15). vlhA 3.03 was not the only vlhA expressed, however, as several other vlhA genes, including 2.02, 5.06, and 4.07, were expressed at levels (RPKM values of 1,500 to 2,300) above the average RPKM value (1,455) for all genes in the genome that were expressed. This indicated that, while vlhA 3.03 expression predominated, additional vlhA genes that were not preceded by 12 GAA repeats were expressed at minor levels in the cultured experimental input population.
FIG 1.
The vlhA expression profile of the broth-grown M. gallisepticum input cultures as determined from RNA sequencing.
M. gallisepticum vlhA expression over the course of infection.
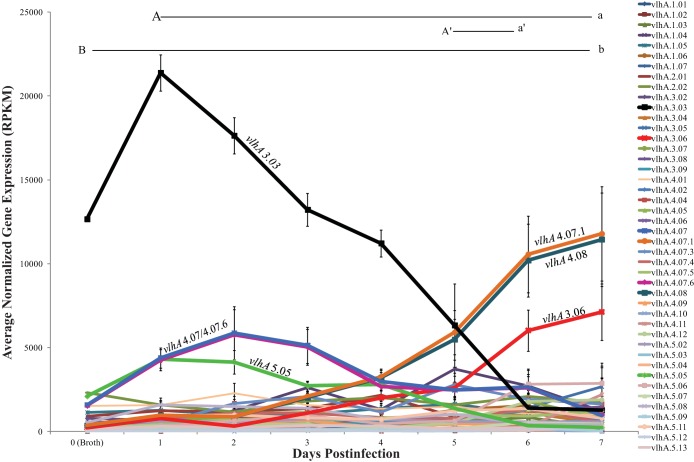
Over the course of the 7-day infection, the vlhA expression profile changed dramatically. Expression of vlhA 3.03 showed an initial increase at day 1 postinfection and then decreased daily throughout the experiment (Fig. 2). Over the course of infection, there was a 34-fold total decrease in vlhA 3.03 expression (q < 0.0001), with the largest change being a 4.5-fold decrease between days 5 and 6 postinfection (q < 0.0001).
FIG 2.
The vlhA expression profile of M. gallisepticum extracted directly from tracheas of experimentally infected birds over the course of the 7-day infection as determined through RNA sequencing. Each data point represents an average RPKM value from the results determined for five animals (with the exception of the broth sample). Error bars show standard errors of the means (SEM). Key statistically significant changes between two time points are indicated by paired upper- and lowercase letters for the following genes: vlhA 3.03 (A/a and A′/a′) and vlhA 4.07.1/4.08 (B/b).
While expression of vlhA 3.03 decreased early in infection, expression of vlhA 4.07 and its tandem repeat variant, vlhA 4.07.6, began to increase as early as 1 day postinfection. These expression levels peaked at 2 days postinfection (3.7-fold higher than input culture) before decreasing over the remainder of the 7-day infection (Fig. 2). Similarly, as vlhA 4.07 and 4.07.6 decreased in expression, vlhA 4.08 and its tandem repeat, vlhA 4.07.1, showed increased expression until peaking at 7 days postinfection with 31-fold-higher (q < 0.0001) expression than the levels of input cultures (Fig. 2). Finally, there was an initial increase in vlhA 5.05 expression at 1 day postinfection, followed by decreasing expression through the time course. The vlhA expression profile observed here at day 7 postinfection was consistent with that of a previous pilot experiment (data not shown). Taking the data together, these changes in vlhA gene expression were not completely random and appeared to be relatively coordinated, as similar vlhA profiles were seen in five independently infected birds at each time point postinfection.
M. gallisepticum vlhA expression in recovery cultures.
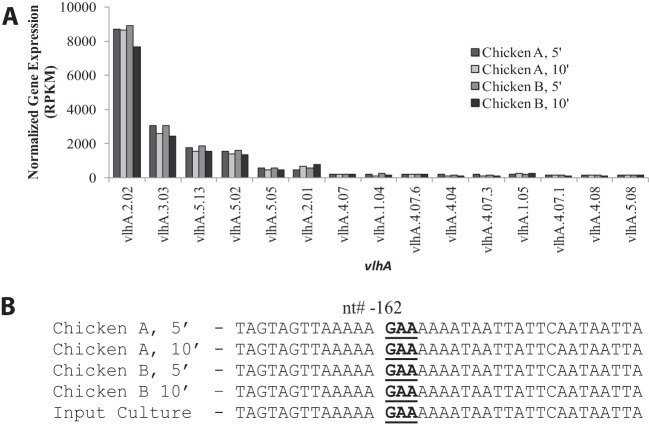
From infected birds sacrificed 7 days postinfection, M. gallisepticum was recovered directly from tracheas into Hayflick's complete medium and serially passaged in vitro. After either 5 or 10 passages, M. gallisepticum populations were predominantly expressing vlhA 2.02 with an average RPKM value of 8,463, a 4.4-fold increase relative to vlhA 2.02 expression observed directly on tracheal mucosae at 7 days postinfection (q < 0.0001) (Fig. 3A). The M. gallisepticum recovered after one passage in vitro displayed a transitional vlhA profile, with vlhA 3.03 and 2.02 increasing in expression and vlhA 4.07.1 and 4.08 decreasing in expression (data not shown). These data suggest that expression of vlhA 2.02 is favorable for the pathogen after recovery from the host trachea and passage in a nutritive medium. Several other vlhA genes, including vlhA 3.03, were also expressed in the recovery cultures but at levels much lower than vlhA 2.02. Interestingly, vlhA 3.03 was expressed at a much (4.5-fold) lower, nondominant level than in the initial input culture (Fig. 3A).
FIG 3.
(A) vlhA expression of M. gallisepticum recovered and cultured at five (5′) or 10 (10′) passages from the tracheas of the two different infected birds at 7 days postinfection. Only data from vlhA genes with an average RPKM value of >100 are displayed. (B) The sequences of the region upstream of the highly expressed vlhA 2.02 gene in the cultures recovered from the infected birds. nt, nucleotide.
When the promoter region upstream of the highly expressed vlhA 2.02 gene from the recovery cultures was PCR amplified and sequenced, data revealed only a single GAA trinucleotide upstream of the start codon, suggesting that 12 GAA repeat copies might not be essential for the expression of a given vlhA, at least in the in vitro culture following direct recovery from the trachea (Fig. 3B). Furthermore, we observed no changes in the genomic nucleotide sequence upstream of vlhA 2.02 in the cultures recovered from the infected animals and passaged in vitro compared to the broth-grown input culture.
DISCUSSION
This study has demonstrated the global population changes in M. gallisepticum vlhA gene expression assessed directly from tracheas of experimentally infected chickens over the course of a 7-day infection. It was important to recover the M. gallisepticum RNA directly from the trachea and introduce it into the TRIzol reagent to minimize the time between collection and extraction, as we observed significant changes in the gene expression profile of M. gallisepticum after just a single passage in culture medium (data not shown). This study design allowed the capture of the most accurate representation of the vlhA profile in vivo at each time point during the infection process.
These observed changes in expression are likely not antibody mediated, as the decrease in expression of vlhA 3.03 is seen as early as 2 days postinfection, well before the formation of specific antibodies (23). This finding is in agreement with a previous study that demonstrated that vlhA expression in infected chickens switches prior to the host mounting an antibody response (13). However, this study greatly expanded on those initial observations to include the expression status of all of the vlhA genes over the early course of infection.
These findings are significant as they imply that the driving force behind the shift in vlhA expression is not controlled entirely by the adaptive immune system. These data suggest that another mechanism is, at least in part, controlling changes in expression of the vlhA genes in vivo.
It is possible that early phase variation could be driven, in part, by the alterations in the cellular architecture of the tracheas during infection. As the infection progresses, the host cells experience denuding of cilia, squamous cell metaplasia, and eventual destruction of the host cell membranes. It is possible that M. gallisepticum vlhA expression is changing in response to these alterations and the pathogen is expressing a specific set of vlhA genes to best persist in the current environment. Consistent with the hypothesis that M. gallisepticum vlhA expression is nonrandom and follows the progression of pathogenesis in the host, vlhA 1.07 and 4.01, reported by Ron et al. (19) to express significant antigens at 2 to 5 weeks postinfection, were not predominantly expressed within 1 week postinfection, as reported here.
Data here indicate that, despite expression of different vlhA genes being seen in the host at day 7 postinfection, vlhA 2.02 is the predominant vlhA gene expressed in the M. gallisepticum population following recovery and passage in Hayflick's complete medium. The dominance of vlhA 2.02 and the lack of reversion to predominant vlhA 3.03 expression observed in the broth-grown input culture were unanticipated. However, in these recovery cultures, expression of vlhA 2.02 was also accompanied by expression (albeit at lower levels) of several other vlhA genes in the population, including vlhA 3.03 and 5.13, suggesting that two or more vlhA genes are expressed at a given time point in the population. These data also indicate that M. gallisepticum may favor the expression of several vlhA genes (vlhA 3.03, 2.02, and 5.13) when no longer faced with the pressures of surviving in the airway of the host.
Data here also demonstrated that 12 GAA trinucleotide repeats are not essential for the expression of a given vlhA, since the vlhA 2.02 gene that was highly expressed in the recovery samples does not contain 12 GAA repeats in upstream sequences. It had previously been hypothesized that 12 GAA repeats were necessary for expression of a given vlhA because it allowed the correct spacing between flanking sequences for necessary accessory factors to bind, as M. gallisepticum does not possess a robust consensus sequence at −10 and −35 (15). The observed expression of vlhA genes without the 12 GAA repeats upstream suggests that there may be another mechanism at play that is responsible, at least in part, for the regulation of vlhA expression in M. gallisepticum.
These results, revealing global vlhA expression changes over the course of early infection, are important in elucidating mechanisms of persistence, colonization, and overall pathogenesis of M. gallisepticum in the natural host. Full and complete understanding of these mechanisms will provide information likely to be critical for development of rationally designed M. gallisepticum therapeutics and vaccines.
ACKNOWLEDGMENTS
We thank Kathryn Korhonen and Kara Rogers for assistance with the animal studies, Kirklyn Kerr for assistance at necropsy, Gerald Kutish for assistance with data analysis, and Steven Szczepanek for helpful discussions.
We also thank the Center of Excellence for Vaccine Research for support.
REFERENCES
- 1.Razin S, Yogev D, Naot Y. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62:1094–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley DH, Berkhoff JE, McLaren JM. 1996. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis 40:480–483. doi: 10.2307/1592250. [DOI] [PubMed] [Google Scholar]
- 3.Papazisi L, Frasca S, Gladd M, Liao X, Yogev D, Geary SJ. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect Immun 70:6839–6845. doi: 10.1128/IAI.70.12.6839-6845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May M, Papazisi L, Gorton TS, Geary SJ. 2006. Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect Immun 74:1777–1785. doi: 10.1128/IAI.74.3.1777-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng CW, Kanci A, Citti C, Rosengarten R, Chiu CJ, Chen ZH, Geary SJ, Browning GF, Markham PF. 2013. MalF is essential for persistence of Mycoplasma gallisepticum in vivo. Microbiology 159:1459–1470. doi: 10.1099/mic.0.067553-0. [DOI] [PubMed] [Google Scholar]
- 6.Hudson P, Gorton TS, Papazisi L, Cecchini K, Frasca S, Geary SJ. 2006. Identification of a virulence-associated determinant, dihydrolipoamide dehydrogenase (lpd), in Mycoplasma gallisepticum through in vivo screening of transposon mutants. Infect Immun 74:931–939. doi: 10.1128/IAI.74.2.931-939.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecchini KR, Gorton TS, Geary SJ. 2007. Transcriptional responses of Mycoplasma gallisepticum strain R in association with eukaryotic cells. J Bacteriol 189:5803–5807. doi: 10.1128/JB.00667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorbachev AY, Fisunov GY, Izraelson M, Evsyutina DV, Mazin PV, Alexeev DG, Pobeguts OV, Gorshkova TN, Kovalchuk SI, Kamashev DE, Govorun VM. 2013. DNA repair in Mycoplasma gallisepticum. BMC Genomics 14:726. doi: 10.1186/1471-2164-14-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazin PV, Fisunov GY, Gorbachev AY, Kapitskaya KY, Altukhov IA, Semashko TA, Alexeev DG, Govorun VM. 2014. Transcriptome analysis reveals novel regulatory mechanisms in a genome-reduced bacterium. Nucleic Acids Res 42:13254–13268. doi: 10.1093/nar/gku976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güell M, van Noort V, Yus E, Chen W-H, Leigh-Bell J, Michalodimitrakis K, Yamada T, Arumugam M, Doerks T, Kühner S, Rode M, Suyama M, Schmidt S, Gavin A-C, Bork P, Serrano L. 2009. Transcriptome complexity in a genome-reduced bacterium. Science 326:1268–1271. doi: 10.1126/science.1176951. [DOI] [PubMed] [Google Scholar]
- 11.Papazisi L, Gorton TS, Kutish G, Markham PF, Browning GF, Nguyen DK, Swartzell S, Madan A, Mahairas G, Geary SJ. 2003. The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain Rlow. Microbiology 149:2307–2316. doi: 10.1099/mic.0.26427-0. [DOI] [PubMed] [Google Scholar]
- 12.Glew MD, Baseggio N, Markham PF, Browning GF, Walker ID. 1998. Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5′ noncoding regions. Infect Immun 66:5833–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glew MD, Browning GF, Markham PF, Walker ID. 2000. pMGA phenotypic variation in Mycoplasma gallisepticum occurs in vivo and is mediated by trinucleotide repeat length variation. Infect Immun 68:6027–6033. doi: 10.1128/IAI.68.10.6027-6033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Dybvig K, Panangala VS, van Santen VL, French CT. 2000. GAA trinucleotide repeat region regulates M9/pMGA gene expression in Mycoplasma gallisepticum. Infect Immun 68:871–876. doi: 10.1128/IAI.68.2.871-876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Panangala VS, Dybvig K. 2002. Trinucleotide GAA repeats dictate pMGA gene expression in Mycoplasma gallisepticum by affecting spacing between flanking regions. J Bacteriol 184:1335–1339. doi: 10.1128/JB.184.5.1335-1339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham PF, Glew MD, Browning GF, Whithear KG, Walker ID. 1998. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect Immun 66:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noormohammadi AH. 2007. Role of phenotypic diversity in pathogenesis of avian mycoplasmosis. Avian Pathol 36:439–444. doi: 10.1080/03079450701687078. [DOI] [PubMed] [Google Scholar]
- 18.Tulman ER, Liao X, Szczepanek SM, Ley DH, Kutish GF, Geary SJ. 2012. Extensive variation in surface lipoprotein gene content and genomic changes associated with virulence during evolution of a novel North American house finch epizootic strain of Mycoplasma gallisepticum. Microbiology 158:2073–2088. doi: 10.1099/mic.0.058560-0. [DOI] [PubMed] [Google Scholar]
- 19.Ron M, Gorelick-Ashkenazi A, Levisohn S, Nir-Paz R, Geary SJ, Tulman E, Lysnyansky I, Yogev D. 2015. Mycoplasma gallisepticum in vivo induced antigens expressed during infection in chickens. Vet Microbiol 175:265–274. doi: 10.1016/j.vetmic.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiner CR, Hunkapiller KL, Chen SM, Glass JI, Chen EY. 1998. Sequencing multimegabase-template DNA with BigDye terminator chemistry. Genome Res 8:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javed MA, Frasca S Jr, Rood D, Cecchini K, Gladd M, Geary SJ, Silbart LK. 2005. Correlates of immune protection in chickens vaccinated with Mycoplasma gallisepticum strain GT5 following challenge with pathogenic M. gallisepticum strain Rlow. Infect Immun 73:5410–5419. doi: 10.1128/IAI.73.9.5410-5419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]