Abstract
The intracellular parasite Toxoplasma gondii has unique dense granule antigens (GRAs) that are crucial for host infection. Emerging evidence suggests that GRA7 of T. gondii is a promising serodiagnostic marker and an effective toxoplasmosis vaccine candidate; however, little is known about the intracellular regulatory mechanisms involved in the GRA7-induced host responses. Here we show that GRA7-induced MyD88 signaling through the activation of TRAF6 and production of reactive oxygen species (ROS) is required for the induction of NF-κB-mediated proinflammatory responses by macrophages. GRA7 stimulation resulted in the rapid activation of mitogen-activated protein kinases and an early burst of ROS in macrophages in a MyD88-dependent manner. GRA7 induced a physical association between GRA7 and TRAF6 via MyD88. Remarkably, the C terminus of GRA7 (GRA7-V) was sufficient for interaction with and ubiquitination of the RING domain of TRAF6, which is capable of inflammatory cytokine production. Interestingly, the generation of ROS and TRAF6 activation are mutually dependent on GRA7/MyD88-mediated signaling in macrophages. Furthermore, mice immunized with GRA7-V showed markedly increased Th1 immune responses and protective efficacy against T. gondii infection. Collectively, these results provide novel insight into the crucial role of GRA7-TRAF6 signaling in innate immune responses.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular apicomplexan parasite in a broad range of warm-blooded vertebrates and is the causative agent of the anthropozoonotic disease called toxoplasmosis (1–3). T. gondii infection during pregnancy is of significant concern because it can lead to abortion or congenital toxoplasmosis (4). Currently, there is no vaccine available to prevent toxoplasmosis in humans. Therefore, understanding how protective immune responses to these parasites are mounted is crucial for the development of effective vaccination strategies or better therapeutics for human and veterinary medicine.
Previous studies have shown that promising vaccine candidate antigens include surface antigen, dense granule proteins, rhoptry proteins, and micronemal proteins (5). Dense granule antigen (GRA) is a secretory vesicular organelle present in all infectious forms of T. gondii. Among the GRA proteins, GRA7 induces a strong antibody (Ab) response during acute infection (6) and is a target antigen in the intracerebral immune response during the chronic phase of infection (7). Importantly, GRA7 has the ability to stimulate significant humoral and cellular immune responses to T. gondii (1, 6, 7). Transmembrane GRA7 proteins are released as soluble proteins and then trafficked to the parasitophorous vacuole membrane (PVM) and/or the intravacuolar network, a structure that forms at the posterior end of the parasite and unfolds throughout the lumen of the vacuole (1, 4), and play important roles in interaction with the host. However, the roles of GRA7-induced host innate immune responses and their regulatory mechanisms have not been fully elucidated.
Toll-like receptors (TLRs) are a family of innate immune recognition receptors that recognize molecular patterns associated with intracellular parasites and contribute to the host defense during T. gondii infection (3, 8, 9). MyD88 is an adaptor molecule involved in most TLR signaling cascades, and it is believed that the TLR recognition of T. gondii is crucial for host resistance. Among them, TLR11 and TLR12 have a key role in interleukin-12 (IL-12) production by macrophages after recognition of the T. gondii-derived protein profilin (3, 10). The recognition of glycosylphosphatidylinositol-anchored proteins or heat shock protein 70 by TLR2 and TLR4 is required for tumor necrosis factor alpha (TNF-α) production by macrophages (10–13), and TLR7 and TLR9 have been implicated in the sensing of T. gondii RNA and genomic DNA, respectively (8, 14, 15). In addition, plasmacytoid dendritic cells produce beta interferon (IFN-β) through the activation of TLR12 (3). However, although multiple TLRs have been suggested to recognize T. gondii antigens, mice deficient in TLR2, TLR4, or TLR11 survive T. gondii infection, while death of TLR12- or MyD88-deficient mice has been observed (3, 10, 11, 16). TLR-dependent signaling leads to NF-κB activation and the production of inflammatory cytokines through the recruitment of MyD88, IRAK, TRAF6, and TAK-1 (17). The adaptor molecule TRAF6 is involved in TLR signaling pathways and associates with serine/threonine kinases involved in the activation of mitogen-activated protein kinase (MAPK) pathways that are crucial for the macrophage signaling induced by T. gondii (8, 18).
There is a growing body of evidence suggesting that reactive oxygen species (ROS) contribute to diverse signaling processes, including TLR-induced innate immune responses, antimicrobial activity, and inflammation (8, 19, 20). It was demonstrated that NADPH oxidase (NOX)-derived ROS plays an essential role in inflammatory responses and has antiparasite activity against T. gondii (19, 20). Additionally, ROS generation with T. gondii is essential for mammalian innate immunity and acts through the phosphatidylinositol 3-kinase (PI3K)/AKT (19), endoplasmic reticulum (ER) stress, apoptosis, or JNK pathway (21). However, the roles of TRAF6 and ROS in the regulation of GRA7-induced intracellular signaling and innate immune responses are largely uncharacterized.
In this study, we investigated the roles of GRA7-induced NF-κB signaling in innate immune responses and the mechanisms by which GRA7 can trigger TRAF6 activation in macrophages. We found that GRA7/MyD88-dependent NF-κB activation is essential for the activation of TRAF6 and ROS generation and enhances the release of inflammatory mediators. GRA7 stimulation led to a physical and functional association between GRA7 and TRAF6. The generation of GRA7-dependent ROS and the association of GRA7 with TRAF6 were dependent on MyD88. Furthermore, GRA7-induced Th1 immune responses and protective efficacy was crucial for T. gondii infection in vivo. These data provide novel insight into the role of GRA7-induced MyD88–NF-κB signaling in innate immune responses through the activation of TRAF6 and ROS generation.
MATERIALS AND METHODS
Mice and cells.
Primary bone marrow-derived macrophages (BMDMs) were isolated from gp91phox, p47phox, TLR2, TLR4, and MyD88 knockout (KO) mice (on the C57BL/6 background) as described previously (22). All animals were maintained in a pathogen-free environment. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University. The mouse macrophage cell line RAW264.7 (American Type Culture Collection [ATCC] TIB-71) and HEK293T (ATCC 11268) cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen), sodium pyruvate, nonessential amino acids, penicillin G (100 IU/ml), and streptomycin (100 μg/ml). THP-1 (ATCC TIB-202) human monocytic cells were grown in RPMI 1640-GlutaMAX supplemented with 10% FBS and treated with 20 nM phorbol myristate acetate (Sigma-Aldrich) for 24 h to induce their differentiation into macrophage-like cells and then washed three times with phosphate-buffered saline.
Reagents, DNA, and Abs.
Caffeic acid phenethyl ester (CAPE), N-acetyl-l-cysteine (NAC), and diphenyleneiodonium (DPI) were purchased from Calbiochem. Dimethyl sulfoxide (DMSO; Sigma-Aldrich) was added to the cultures at a concentration of 0.1% (vol/vol) as a solvent control. The NF-κB–luciferase reporter and hemagglutinin (HA)-ubiquitin (Ub) plasmids were generous gifts from J. U. Jung (University of Southern California, Los Angeles, CA). Glutathione S-transferase (GST)-tagged GRA7 and truncated mutant genes were cloned into a pEBG derivative encoding an N-terminal GST epitope tag between the BamHI and NotI sites. Flag-tagged TRAF6 and truncated mutant genes were cloned into the XbaI and BamHI sites in pcDNA3.0. All constructs were sequenced with an ABI PRISM 377 automatic DNA sequencer to verify 100% correspondence to the original sequence. Specific Abs against phospho-(Thr202/Tyr204)-p42/44, phospho-(Thr180/Tyr182)-p38, phospho-(Thr183/Tyr185)-SAPK/JNK, phospho-(Ser32/36)-IkB-α, and phospho-(Ser176/180)-IKKα/β were purchased from Cell Signaling Technology (Danvers, MA, USA). Abs specific for actin (I-19), IκB-α (C-21), IRAK4 (H-100), TRAF6 (H-274), TRAF2 (C-20), gp91phox (h-60), p47phox (H-195), Ub (P4D1), His (His17), HA (12CA5), Flag (D-8), and GST (B-14) were purchased from Santa Cruz Biotechnology.
Lentiviral shRNA production and transduction.
Lentiviral short hairpin RNA (shRNA) produced by transient transfection with packaging plasmids (psPAX2, pMD2.VSV-G; purchased from Addgene) and the target shRNA plasmid DNA (human TLR1, TLR2, TLR4, TLR5, TLR6, TLR10, MyD88, and TRAF6 and mouse TLR11 and TLR12; purchased from Open Biosystems) were cotransfected into 293T cells with Lipofectamine 2000 (Invitrogen catalog no. 11668) as described previously (22). Virus-containing medium was concentrated by ultracentrifugation, titration was determined in 293T cells, and transduction into THP-1 or RAW264.7 cells was carried out as described previously (22). A parallel experiment with a green fluorescent protein-encoding lentivirus (the pGIPZ lentiviral vector; Open Biosystems) indicated that 80% of the cells were successfully transduced by the virus.
Western blotting and coimmunoprecipitation.
RAW264.7, THP-1, and 293T cells and BMDMs were treated as indicated and processed for analysis by Western immunoblotting (IB) and immunoprecipitation as previously described (22, 23). For Western blot analysis, primary Abs were used at a 1/1,000 dilution. For the immunoprecipitation assays, cells were harvested and lysed as described previously (22). The lysates were mixed and precipitated with Abs and protein A-Sepharose by incubation at 4°C for 18 h on a rotator. The samples were subsequently solubilized in SDS sample buffer and separated by SDS-PAGE for Western blot analysis. Ab binding was visualized by chemiluminescence (ECL; Millipore) and detected with a Vilber chemiluminescence analyzer (Fusion SL 3; Vilber Lourmat).
RNA extraction and reverse transcription (RT)-PCR.
Total RNA was extracted with TRIzol reagent (Invitrogen) as described previously (22). Total RNA (2 μg) was used for first-strand cDNA synthesis with Moloney murine leukemia virus reverse transcriptase (Promega) according to the manufacturer's instructions. The PCR conditions were as described previously (23). The primer pairs used for PCR are listed in Table S1 in the supplemental material. PCR products were resolved on 1.5% agarose gels and stained with ethidium bromide.
ELISA.
Murine BMDMs and THP-1 cells were treated as indicated and processed for analysis by sandwich enzyme-linked immunosorbent assay (ELISA). Cell culture supernatants and mouse sera were analyzed for cytokine content with a BD OptEIA ELISA set (BD Pharmingen) for the detection of TNF-α, IL-6, IL-12p40, IFN-γ, IL-4, and IL-10. T. gondii-specific serum IgG, IgG1, and IgG2a Ab levels were determined by ELISA as described previously (6). All assays were performed as recommended by the manufacturers.
Measurement of intracellular ROS levels and determination of NOX activity.
Intracellular ROS levels were measured with an assay involving the oxidative fluorescent dye dihydroethidium (DHE for superoxide; Calbiochem) and 5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA for H2O2) as previously described (22, 24). The cells were examined with an LSM510 laser scanning confocal microscope (Zeiss), and the mean relative fluorescence intensity of each group of cells was measured with a Zeiss LSM510 vision system (version 2.3). Lucigenin (bis-N-methylacridinium nitrate, 5 × 10−6 M) chemiluminescence assays were used to measure NOX activity (22). The values are expressed in relative light units per 1 × 105 cells.
Recombinant GRA7 (rGRA7) protein.
To obtain purified GRA7 (GenBank accession no. DQ459443.2) protein, GRA7 amino acid residues 1 to 236 and 201 to 236 were cloned with an N-terminal 6×His tag into the pRSFDuet-1 Vector (Novagen) and induced, harvested, and purified from Escherichia coli expression strain BL21(DE3)pLysS as described previously (1, 7) in accordance with the standard protocols recommended by Novagen. rGRA7 was dialyzed with permeable cellulose membrane and tested for lipopolysaccharide contamination with a Limulus amebocyte lysate assay (BioWhittaker) and contained <20 pg/ml at the concentrations of rGRA7 protein used in the experiments described here.
T. gondii infection and parasite burden in the brain in vivo.
Cysts of T. gondii strain Me49 were obtained from the brains of infected mice and purified as described previously (6). BALB/c mice (six per group) were immunized intraperitoneally with rGRA7 (100 μg/kg of body weight) once a week for 4 weeks. Blood and spleens were collected to assess serum IgG levels, in vitro T cell proliferation, and cytokine levels at 1 week after final immunization. One week after the final immunization, 10 mice in each group were orally challenged with 1,500 cysts of strain Me49 as previously reported (2, 6) and monitored for death daily for 4 weeks. Immunized BALB/c mice were orally challenged with 20 cysts of strain Me49 at 1 week after the last booster. Their brain parasite burdens were evaluated 4 weeks after infection. The mean number of cysts per 25 μl of brain sample was determined by observation under an optical microscope. The results are shown as means ± standard deviations (SD) of the results from the experiments performed with groups of six mice.
Statistical analysis.
For statistical analysis, data obtained from independent experiments (means ± SD) were analyzed with a two-tailed Student t test. Differences were considered significant at a P value of <0.05. For survival, data were graphed and analyzed by the product limit method of Kaplan and Meier by using the log-rank (Mantel-Cox) test for comparisons (Prism, version 5.0; GraphPad Software) (2, 22, 23).
RESULTS
T. gondii GRA7 protein leads to MAPK and NF-κB activation and proinflammatory cytokine expression in macrophages.
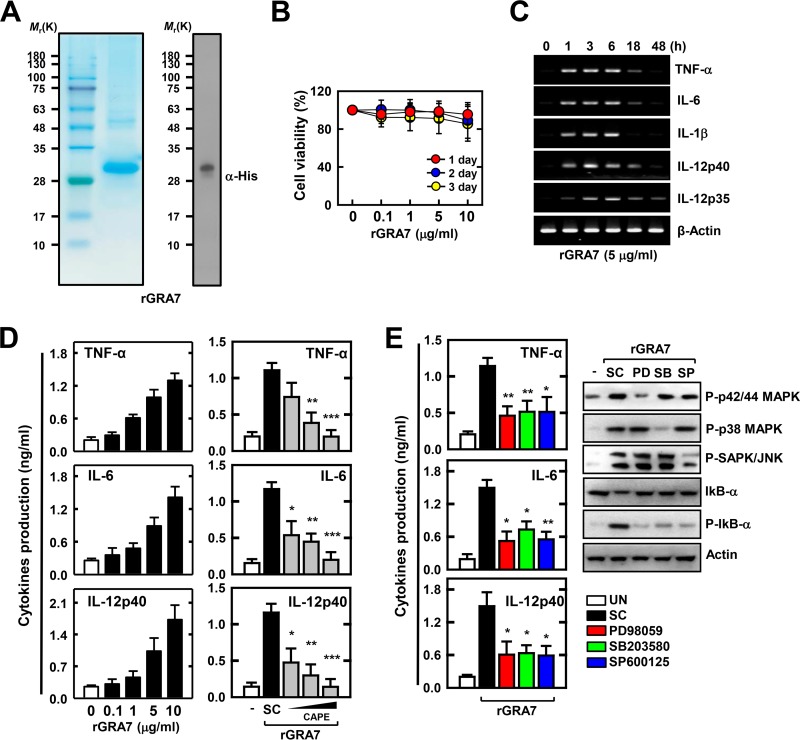
To examine the role of T. gondii GRA7 in innate immune responses by macrophages, we generated bacterially purified His-tagged GRA7 protein as described previously (1, 7). Purified rGRA7 (29 kDa) was confirmed through SDS-PAGE and IB analysis (Fig. 1A). In addition, we tested whether rGRA7 induces cytotoxicity in murine macrophages. As shown in Fig. 1B, no significant difference in rGRA7-induced cell viability was observed at the times indicated up to 3 days (P ≥ 0.05). We first determined the expression of proinflammatory cytokine genes and proteins in the macrophages following stimulation with rGRA7 at various concentrations (0.1 to 10 μg/ml) and time courses (1 to 96 h). After stimulation with rGRA7, the peak levels of TNF-α, IL-6, IL-1β, IL-12p40, and IL-12p35 mRNA expression were detected at 3 to 6 h, and TNF-α, IL-6, and IL-12p40 protein synthesis was detected at 48 to 96 h (Fig. 1C; see Fig. S1A in the supplemental material). Additionally, the expression levels of cytokines in the rGRA7-treated BMDMs were increased in a dose-dependent manner (Fig. 1D).
FIG 1.
rGRA7 rapidly induces MAPK and NF-κB activation and proinflammatory cytokine production in macrophages. (A) Bacterially purified 6×His-GRA7 was analyzed by Coomassie blue staining (left) or IB with an anti-His Ab (right). (B) 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay for cell viability. Cells were incubated with rGRA7 for various times and at various concentrations. Quantitative cell viability data are presented as the mean ± SD of three experiments. (C) BMDMs from mice were stimulated with rGRA7 for the times indicated. The cells were then harvested and subjected to semiquantitative RT-PCR for TNF-α, IL-6, IL-1β, IL-12p40, IL-12p35, and β-actin. (D) BMDMs from mice were stimulated with rGRA7 for 18 h (left). After preincubation for 45 min with CAPE (5, 10, or 25 μM), BMDMs were stimulated with rGRA7 (5 μg/ml) for 18 h (right). Culture supernatants were harvested, and the levels of TNF-α, IL-6, and IL-12p40 were measured by ELISA. (E) After pretreatment for 45 min with an inhibitor of MEK (PD98059, 10 μM), p38 (SB203580, 5 μM), or JNK/SAPK (SP600125, 20 μM), BMDMs were stimulated with rGRA7 (5 μg/ml) for 18 h. Culture supernatants were harvested and analyzed by cytokine ELISA (left), and BMDMs were stimulated with rGRA7 (5 μg/ml) for 1 h. Cells were harvested and then subjected to IB with the phosphorylated and total forms of MAPKs (p42/p44, p38, and SAPK/JNK), IkB-α, or actin (right). The data are representative of three independent experiments with similar results (C and E, right side). Data shown are the means ± SD of three experiments. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) from control cultures treated with solvent alone are shown (D and E, left side). UN, untreated; SC, solvent control (0.1% DMSO).
NF-κB transcription factors and MAPKs (p42/44, p38, and JNK/SAPK) have an essential role in inflammation and innate immunity against T. gondii infection (3, 25). Therefore, we investigated the role of NF-κB in rGRA7-induced immune responses in BMDMs. The expression levels of cytokines in the rGRA7-treated BMDMs were significantly reduced by the NF-κB inhibitor CAPE (Fig. 1D) or Bay 11-7085 (data not shown). As shown in Fig. S1B in the supplemental material, rGRA7 stimulated the transient phosphorylation of MAPKs in BMDMs. The maximal response was obtained within 2 h of rGRA7 stimulation and then declined to basal levels within 8 h. Notably, pretreatment with MAPK inhibitors markedly attenuated rGRA7-stimulated NF-κB activation and cytokine production (Fig. 1E). These results indicate that T. gondii GRA7-induced immune responses are involved in MAPKs, NF-κB, and proinflammatory signal activation in macrophages.
Intracellular ROS formation is involved in GRA7-induced proinflammatory signaling in macrophages.
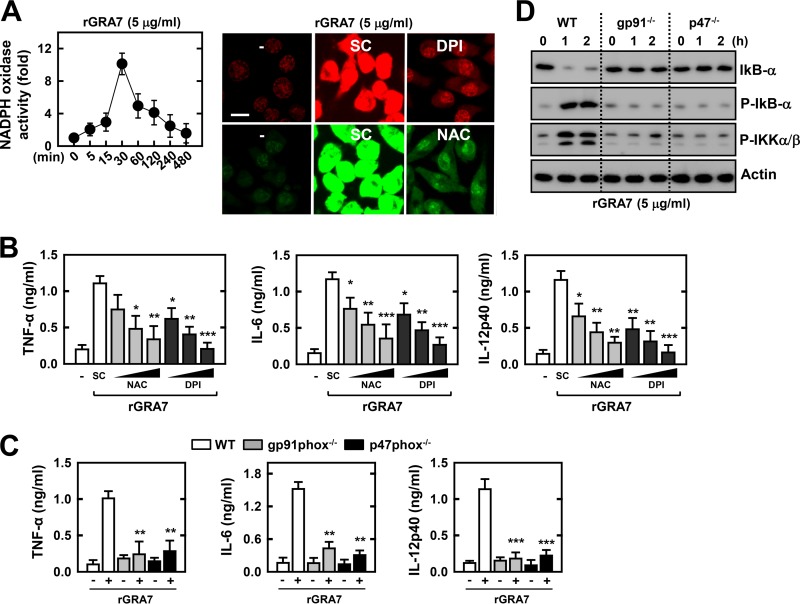
Although intracellular ROS as a secondary messenger can regulate infection signaling against T. gondii (19, 26), ROS formation in response to toxoplasma antigens is poorly characterized. We first determined whether stimulation with rGRA7 leads to ROS generation in murine BMDMs. As shown in Fig. 2A, rGRA7 induced a robust burst of ROS production in macrophages within 30 min of stimulation. The NOX inhibitor DPI and the antioxidant NAC significantly attenuated rGRA7-induced superoxide and H2O2 production, respectively (Fig. 2A). We next examined whether ROS are involved in rGRA7-mediated proinflammatory responses and NF-κB activation. The rGRA7-mediated production of TNF-α, IL-6, and IL-12p40 was significantly attenuated by pretreatment of macrophages with the antioxidant and NOX inhibitor (Fig. 2B). In addition, the rGRA7-induced activation of cytokine production from the BMDMs of gp91phox or p47phox KO mice were completely abrogated compared with that of BMDMs from wild-type (WT) mice (Fig. 2C). Furthermore, ROS play an indispensable role in rGRA7-induced NF-κB activation; the rGRA7-induced attenuation of IκB-α expression levels and the phosphorylation of IκB-α and IKKα/β were completely abrogated in BMDMs from gp91phox or p47phox KO mice (Fig. 2D). Taken together, these results indicate that ROS play an indispensable role in T. gondii GRA7-induced proinflammatory cytokine expression and NF-κB activation in macrophages.
FIG 2.
Intracellular ROS is essential for GRA7-induced proinflammatory cytokine production. (A) NOX activity of BMDMs was stimulated with rGRA7 (5 μg/ml) for the times indicated (left). The DHE and CM-H2DCFDA fluorescence of BMDMs was stimulated with rGRA7 (5 μg/ml) for 30 min in the presence of 20 μM DPI (upper) and 30 mM NAC (lower) to detect O2− and H2O2 production, respectively. The images shown are representative of three independent experiments with similar results. Bar, 10 μm. (B and C) Culture supernatants were harvested and analyzed for cytokine ELISA. (B) After pretreatment for 45 min with an inhibitor of either NAC (10, 30, or 50 mM) or DPI (10, 20, or 50 μM), BMDMs were stimulated with rGRA7 (5 μg/ml) for 18 h. (C) rGRA7 (5 μg/ml)-induced cytokine production in BMDMs from WT, gp91phox−/−, and p47phox−/− mice for 18 h. (D) rGRA7-induced NF-κB activation in BMDMs from WT, gp91phox−/−, and p47phox−/− mice. Cells were harvested and then subjected to IB with the phosphorylated and total forms of IkB-α, IKK-α/β, or actin. The data are representative of three independent experiments with similar results. Data shown are the means ± SD of three experiments. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) from treatment with solvent alone are shown (B and C). SC, solvent control (0.1% DMSO).
T. gondii GRA7 induces proinflammatory signaling in a MyD88-dependent manner.
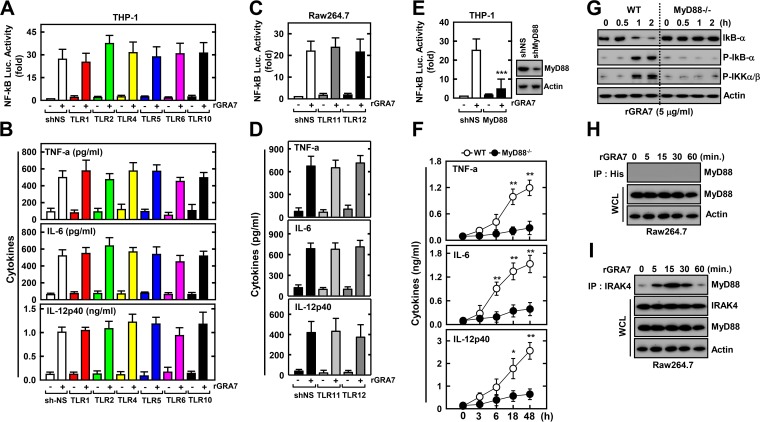
It has been demonstrated that the recognition of T. gondii by the innate immune system occurs via the TLR-MyD88 pathway (3, 8). We first determined whether rGRA7 leads to innate immune responses in macrophages in a TLR-dependent manner. To achieve this, we performed a shRNA screen against TLRs involved in the recognition of T. gondii in human and murine macrophages. As shown in Fig. 3A to D, human TLRs (hTLRs) were knocked down by transduction with lentiviral particles carrying shRNAs against hTLR1, -2, -4, -5, -6, and -10 (shTLR1, -2, -4, -5, -6, and -10) (Fig. 3A and B), and RAW264.7 cells were knocked down by transduction with lentiviral shRNA specific to mouse TLRs, including TLR11 or TLR12 (Fig. 3C and D). NF-κB–luciferase promoter assays and ELISA analysis were performed with THP-1 and RAW 264.7 cells (Fig. 3A to D). Treatment of macrophages with rGRA7 significantly increased NF-κB–luciferase promoter activity and cytokine production; however, no significant differences in rGRA7-induced NF-κB-activation and/or mediated enhanced inflammation were detected between THP-1 or RAW264.7 cells knocked down with nonspecific control shRNA (shNS) and cells knocked down with shRNA specific for TLRs (Fig. 3A to D; see Fig. S2A and B in the supplemental material). Consistent with the findings shown in Fig. 3A and B, BMDMs from TLR2 and TLR4 KO mice also confirmed the data from knockdown experiments with hTLR2 and hTLR4 shRNA (see Fig. S2C and D). Thus, TLR signaling appears not to be required for GRA7-induced NF-κB signaling in macrophages.
FIG 3.
MyD88 plays an essential role in rGRA7-mediated NF-κB activation and cytokine expression. (A to E) THP-1 cells (A, B, and E) or RAW264.7 cells (C and D) were transduced with lentivirus expressing nonspecific shRNA (shNS) or shRNAs specific for TLR1, -2, -4, -5, -6, and -10 (A and B); TLR11 and -12 (C and D); or MyD88 (E) with Polybrene (8 μg/ml). THP-1 (A and E) or RAW264.7 (C) cells were transfected with an NF-κB reporter plasmid, and a pRL-TK Renilla plasmid was cotransfected for normalization. After 2 days, THP-1 (A, B, and E) or RAW264.7 (C and D) cells were treated with rGRA7 (5 μg/ml) for 6 h and luciferase assays were performed (A, C, and E) or for 18 h and culture supernatants were analyzed by cytokine ELISA (B and D). (F and G) rGRA7 (5 μg/ml)-induced cytokine production (F) or NF-κB activation (G) in BMDMs from WT and MyD88−/− mice at the times indicated. (G) Cells were harvested and then subjected to IB with the phosphorylated and total forms of IkB-α, IKK-α/β, or actin. (H and I) RAW264.7 cells were stimulated with rGRA7 (5 μg/ml) for the times indicated and then subjected to IP with anti-His (H) or anti-IRAK4 (I) Ab and IB with anti-MyD88 or anti-IRAK4 Ab. Whole-cell lysates (WCLs) were used for IB with anti-MyD88, anti-IRAK4, or anti-actin Ab. The data are representative of three independent experiments with similar results (G to I). Data shown are the means ± SD of three experiments. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) from the treated control cultures are shown (E and F). IP, immunoprecipitation.
We next investigated whether MyD88 plays a role in T. gondii GRA7-induced proinflammatory signaling in macrophages. MyD88 plays an indispensable role in the rGRA7-induced activation of NF-κB and cytokine production. NF-κB–luciferase gene activity in THP-1 cells, the production of cytokines, IκB-α expression levels, and the phosphorylation of IκB-α and IKKα/β were completely abrogated in BMDMs from MyD88 KO mice (Fig. 3E to G). Previous studies have shown that MyD88- and IRAK4-dependent pathways are essential for the intracellular signaling triggered by the intracellular parasite (17, 27). We further investigated the potential physical association of GRA7 with MyD88 in macrophages stimulated with rGRA7 in a coimmunoprecipitation assay. As shown in Fig. 3H, no association was detected during prolonged incubation with rGRA7; however, rGRA7-induced endogenous MyD88 rapidly and strongly associated with IRAK4 (from 5 to 30 min) (Fig. 3I). Taken together, these results indicate that GRA7-induced inflammatory responses are mediated by the MyD88-IRAK4 complex but not by TLRs.
GRA7 associates with TRAF6 and controls its ubiquitination in macrophages.
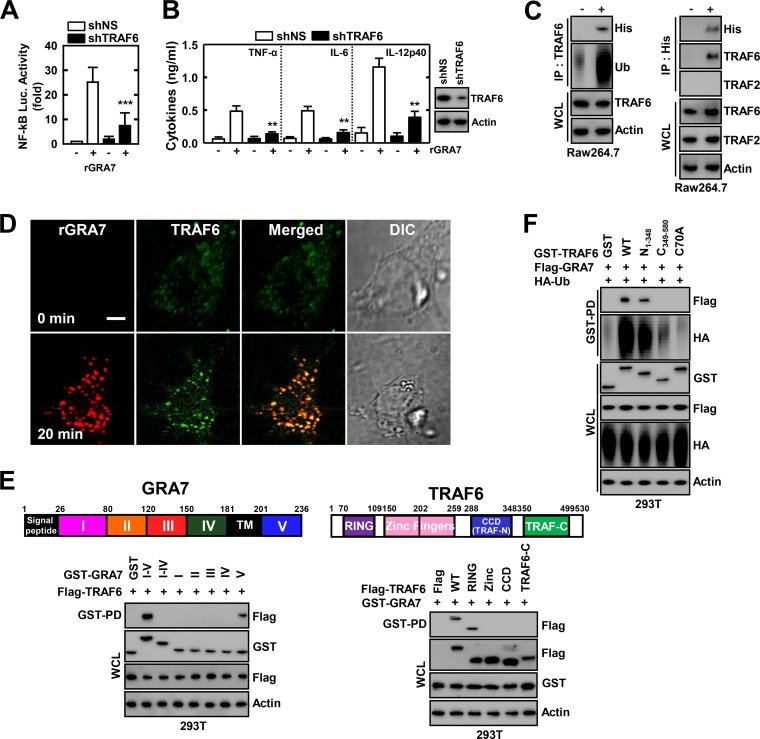
MyD88 and IRAK-4 play crucial roles as adaptor molecules in signal transduction of the TLR/IL-1R superfamily, and it is known that the expression of these proteins leads to the activation of NF-κB in a TNF receptor-associated factor 6 (TRAF6)-dependent manner (3, 8, 17, 27, 28). To explore the involvement of TRAF6 in MyD88- and IRAK4-mediated activation of NF-κB signaling, we examined the effect of knocking down the gene with shRNA specific for TRAF6 in THP-1 cells. rGRA7-induced NF-κB-dependent reporter activity and proinflammatory cytokine production were significantly lower in shTRAF6-transduced THP-1 cells than in those from shNS (Fig. 4A and B). We further investigated whether GRA7 associates with TRAF6 in macrophages. Coimmunoprecipitation showed that GRA7 interacted with endogenous TRAF6 but not with TRAF2 after stimulation with rGRA7 in RAW264.7 and vice versa (Fig. 4C). The intracellular interaction of GRA7 and TRAF6 was confirmed by their colocalization after stimulation with rGRA7, as documented by immunostaining and image overlay (Fig. 4D). GRA7 localized with TRAF6 in the cytoplasm, appearing as small speckles and punctate spots (Fig. 4D). A detailed mapping study with various mammalian GST-GRA7 fusions indicates that while the C-terminal V (amino acids 201 to 236) motif of GRA7 exhibited only minimal binding affinity for TRAF6, TRAF6 carrying the N-terminal RINIG domain bound GRA7 as strongly as WT TRAF6 (Fig. 4E).
FIG 4.
GRA7 interacts with TRAF6 to modulate its ubiquitination. (A and B) THP-1 cells were transduced with lentivirus expressing nonspecific shRNA (shNS) or shRNA specific for TRAF6 with Polybrene (8 μg/ml). THP-1 (A) was transfected with an NF-κB reporter plasmid, and a pRL-TK Renilla plasmid was cotransfected for normalization. After 2 days, THP-1 cells were treated with rGRA7 (5 μg/ml) for 6 h and luciferase assays were performed (A) or for 18 h and culture supernatants were analyzed by cytokine ELISA (B). (C) RAW264.7 cells were stimulated with rGRA7 (5 μg/ml) for 20 min, and then immunoprecipitation (IP) with anti-TRAF6 (left) or anti-His (right) Ab and IB with anti-His, anti-Ub, or anti-TRAF6 were performed. Whole-cell lysates (WCLs)s were used for IB with anti-TRAF6 or anti-actin Ab. (D) Microscopy of BMDMs stimulated for 20 min with rGRA7 and then immunolabeled with Ab to TRAF6 (conjugated to the green fluorescent dye Alexa Fluor 488) or Ab to 6×His-GRA7 (conjugated to the red fluorescent dye tetramethyl rhodamine isocyanate). Scale bar, 5 μm. (E) Binding mapping. Schematic diagram of the structures of GRA7 and TRAF6. At 48 h posttransfection with mammalian GST or GST-GRA7 and truncated mutant constructs together with Flag-TRAF6 (left) or with GST or GST-GRA7 constructs together with Flag-TRAF6 and truncated mutant constructs (right), 293T cells were used for GST pulldown, followed by IB with anti-Flag Ab. WCLs were used for IB with anti-GST, anti-Flag, or anti-actin Ab. TM, transmembrane. (F) 293T cells were cotransfected with GST, GST-GRA7, or Flag-GRA7 together with HA-Ub and subjected to GST pulldown, followed by IB with anti-Flag or anti-HA Ab. WCLs were used for IB with anti-GST, anti-Flag, anti-HA, or anti-actin Ab. Data shown are the means ± SD of three experiments. Significant differences (**, P < 0.01; ***, P < 0.001) from control treated cultures are shown (A and B). The data are representative of three independent experiments with similar results (C to F).
E3 Ub protein ligase TRAF6 catalyzes Lys63 (K63)-linked polyubiquitin chains of target proteins, including IRAK1, NF-κB modulator IKKγ (NEMO), and TRAF6 itself (17, 28, 29). Having found that GRA7 interacts with TRAF6, we next assessed whether GAR7 modulates TRAF6 ubiquitination. As shown in Fig. 4C, GRA7 induces the ubiquitination of TRAF6 but not the Ub ligase TRAF2 in a binding-dependent manner (data not shown). The RING domains of TRAF6 are required for binding to an E2 Ub-conjugating enzyme and promote the oligomerization of TARF6, which is critical for the E3 Ub ligase activity that is essential for NF-κB activation (17, 29). Remarkably, the physical interaction through the RING domain of TRAF6 was functionally relevant because K63-linked polyubiquitination of TRAF6 was much lower in cells transfected with plasmids encoding mutant TRAF6 with deletion of the RING domain (C349–580) or with a loss of ligase activity and autoubiquitination (C70A) (Fig. 4C and F). Furthermore, rGRA7-induced endogenous TRAF6 rapidly and strongly associates with IRAK4 (from 15 to 60 min) (see Fig. S3 in the supplemental material). These data suggest that GRA7 induced NF-κB signaling by interacting with TRAF6 and modulating the K63-linked polyubiquitination of TRAF6 and that an intact RING domain of TRAF6 is functionally required for its interaction with C-terminal GRA7.
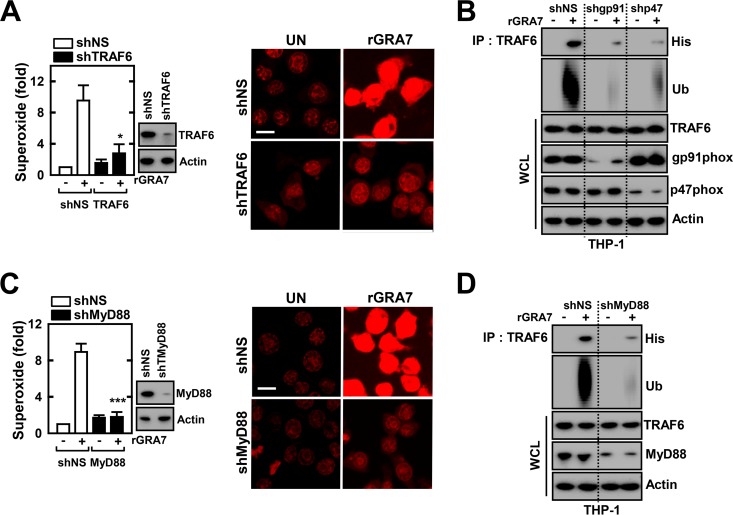
Production of ROS and TRAF6 activation is mutually dependent on GRA7/MyD88-mediated signaling in macrophages.
The specific roles of TRAF6 in ROS generation and the priming of TRAF6 activation remain to be determined. To examine whether ROS generation is dependent on TRAF6 activation, TRAF6 was first knocked down in THP-1 cells by the transduction of lentiviral shRNA specific for TRAF6. The results showed that ROS generation in THP-1 cells transduced with lentiviral shTRAF6 was significantly decreased when they were stimulated with rGRA7 (Fig. 5A).
FIG 5.
ROS generation and TRAF6 activation are mutually dependent on GRA7/MyD88-mediated signaling in macrophages. (A to D) THP-1 cells were transduced with lentivirus expressing nonspecific shRNA (shNS) or shRNA specific for TRAF6 (shTRAF6; A), gp91phox, p47phox (shgp91phox, p47phox; B), or MyD88 (shMyD88; C and D) with Polybrene (8 μg/ml). After 2 days, rGRA7 (5 μg/ml)-induced superoxide production was assessed by confocal microscopy for 30 min (A and C). Data shown are the means ± SD of three experiments. Significant differences (*, P < 0.05; ***, P < 0.001) from treated control cultures are shown. The images shown are representative of three independent experiments with similar results. Bars, 10 μm. (B and D) THP-1 cells were stimulated with rGRA7 for 30 min; this was followed by immunoprecipitation (IP) with anti-TRAF6 and IB with anti-His or anti-Ub Ab. Whole-cell lysates (WCLs) were used for IB with anti-TRAF6, anti-gp91phox, anti-p47phox, anti-MyD88, or anti-actin Ab. The data are representative of three independent experiments with similar results. UN, untreated.
Given that GRA7-induced ROS production and TRAF6 activation showed similar kinetics (data not shown), we examined whether these two events are dependent. To this end, we examined whether knockdown of the NOX component affected rGRA7-induced TRAF6 binding and its ubiquitination. Human THP-1 cells were transduced with lentiviral shgp91phox or shp47phox, and the cells were treated with or without rGRA7. As shown in Fig. 5B, rGRA7-induced binding and ubiquitination of TRAF6 decreased considerably in cells with gp91phox or p47phox knocked down. We further examined the role of MyD88 in the GRA7-induced production of ROS and TRAF6 activation in macrophages. Human THP-1 cells transduced with lentiviral shRNA against hMyD88 showed a blockade of rGRA7-induced ROS production and the interaction with TRAF6 and its ubiquitination (Fig. 5C and D). Taken together, these findings indicate that ROS production and TRAF6 activation are mutually dependent on GRA7/MyD88-induced inflammatory signaling pathways.
GRA7-V-dependent host protective immune responses against T. gondii infection.
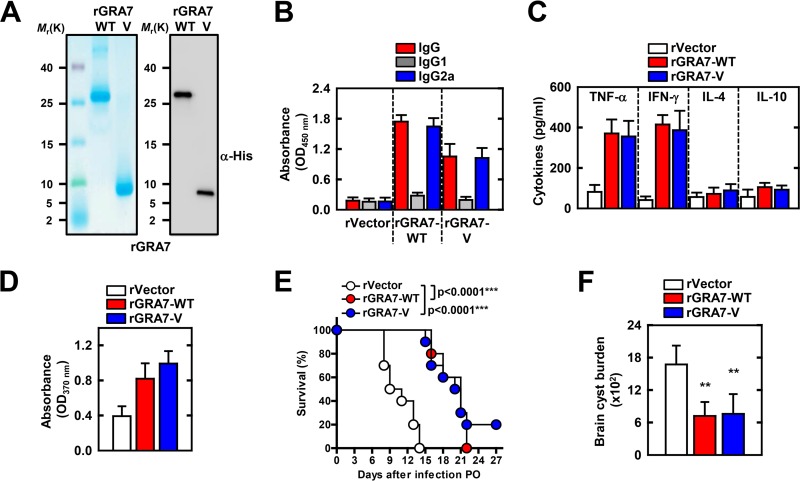
T. gondii with GRA7 deleted was attenuated for virulence in mice (1), and mice that were immunized with pGRA7 and rGRA7 showed enhanced protective host responses to and survival of T. gondii infection (4, 6). Drawing on the observation that GRA7-V associated with TRAF6, we investigated whether GRA7-V has a vaccine effect and protects against T. gondii. To evaluate the protective efficacy of a recombinant protein vaccine expressing GRA7-V of T. gondii, we generated bacterially purified His-tagged GRA7-V protein (Fig. 6A; see Fig. S4 in the supplemental material). We examined whether TH1 or TH2 responses were elicited in immunized mice by analyzing the T. gondii-specific IgG1 and IgG2a titers in their serum with GRA7-WT or -V antigen. As shown in Fig. 6B, T. gondii-specific IgG2a was present at high levels in GRA7-WT- and -V-immunized mice (IgG2a optical density [OD], >1.0; IgG1 OD, <0.3). In addition, purified rGRA7-treated culture supernatants of splenocytes were obtained from immunized mice, which augmented the TH1 or TH2 cytokine response. The TNF-α and IFN-γ levels of mice immunized with GRA7-WT or -V were markedly higher than the IL-4 and IL-10 levels (Fig. 6C). Furthermore, in vitro splenocyte proliferation was significantly higher in GRA7-WT- or GRA7-V-immunized mice (Fig. 6D). These results indicate that GRA7-V-immunized mice produced specific Abs against T. gondii GRA7 and showed a predominant Th1-type immune response.
FIG 6.
GRA7-V-immunized mice show enhanced protection from a T. gondii challenge. (A) Bacterially purified 6×His-GRA7 was analyzed by Coomassie blue staining (left) or IB with anti-His Ab (right). (B to D) BALB/c mice (six per group) were immunized with rGRA7 (100 μg/kg body weight; intraperitoneally) or empty vector protein as a negative-control once a week for 4 weeks. Blood and spleens were collected to assess serum IgG, cytokine levels, and in vitro T cell proliferation at 1 week after the final immunization. (B) Detection of anti-T. gondii IgG, lgG1, and lgG2a levels in the vaccinated mouse sera (diluted 1:100). (C) Cytokine concentrations of culture supernatants of the rGRA7-stimulated splenocytes determined by ELISA. The levels of IL-4, IL-10, IFN-γ, and TNF-α were assessed at 24, 72, 96, and 48 h, respectively. (D) In vitro proliferation of splenocytes from mice after stimulation with the soluble tachyzoite antigen of T. gondii (STAg). Splenocytes were cultured with 10 μg/ml STAg for 72 h, and proliferation was measured with a chemiluminescent bromodeoxyuridine ELISA kit. Absorbance was evaluated with an ELISA reader at 370 nm with a 492-nm reference. (E) One week after the final immunization, 10 mice in each group were orally challenged with 1,500 cysts of strain Me49 and monitored for death daily for 2 weeks. Significant differences from the control mice are indicated (log-rank test). (F) The brain parasite burden was evaluated 4 weeks after immunized BALB/c mice were orally challenged with 20 cysts of strain Me49. Results are expressed as means ± SD (six mice per group). For panels B to D, three independent experiments were performed and data from one representative experiment are shown. For panels E and F, two independent experiments were performed and data from one representative experiment are shown. **, P < 0.01.
To further investigate GRA7-V's effect on the in vivo host responses to T. gondii infection, mice were immunized with GRA7-WT or -V protein for 4 weeks. Mice were orally challenged with strain Me49 (1,500 cysts per mouse) (Fig. 6E). Mice immunized with empty vector protein had a median survival of 10 days, while mice immunized with GRA7-WT and -V survived significantly longer (median survival time, 20.5 to 21 days) and had an increased survival rate (Fig. 6E). To determine whether these effects were due to enhanced parasite clearance, we measured their brain cyst burdens (Fig. 6F). Immunized mice were orally challenged with strain Me49 (20 cysts per mouse) for 4 weeks. In correlation with the survival rate, GRA7-WT and -V showed a significantly 2.1- to 2.3-fold lower brain cyst burden compared with treatment with empty vector protein. These results unambiguously show that host defenses against T. gondii infection are substantially affected by GRA7-V and GRAT-WT.
DISCUSSION
The necessity of pattern recognition receptors, such those in the TLR pathway, for the innate recognition of T. gondii has been well established (8). Previously, it was suggested that a range of TLRs, including two cell surface TLRs (TLR2 and TLR4) and four endosomal TLRs (TLR7, TLR9, TLR11, and TLR12), are involved in the innate sensing of T. gondii infection in vivo and T. gondii-derived proteins and nucleic acids in vitro (3, 8–11, 13–15). Furthermore, recent studies have shown that the UNC93 homologue B1, an ER-resident protein, has a central role in the function of endosomal TLRs and is essential for resistance to T. gondii (30, 31). Most notably, the TLR-induced adaptor MyD88-mediated signaling pathway, which is dependent on NF-κB or IRF-8 activation, is essential for host resistance to T. gondii (8). Our findings indicate a broader role for GRA7 in the expression of MyD88-dependent inflammatory mediators in macrophages. Our data partially correlate with those of previous studies showing that GRA7-induced innate immune responses were MyD88 dependent but not TLR dependent, suggesting that other members of the TLR family or pattern recognition receptors are involved. A recent study showed that the recognition of T. gondii through the activation of the intracellular sensor NLRP1 and NLRP3 inflammasomes induced cell death and the production of IL-1β in in vitro and in vivo experiments with mouse and rat cells (32–34). Furthermore, T. gondii has been reported to be a novel activator of the NLRP1 and NLRP3 inflammasomes in vivo and a role for these sensors in host resistance to toxoplasmosis has been established (34). In this study, we focused on the role of GRA7-MyD88-dependent innate immunity; however, future studies will clarify the role of NLRP1 and NLRP3 inflammasome activation in GRA7-induced inflammatory responses.
Innate immune responses triggered by TLR signaling are mediated through the generation of intracellular ROS, which are now being recognized as important secondary messengers (21, 22, 24). Zhou et al. showed that T. gondii infection or excretory/secretory protein treatment induces NOX4-dependent ROS generation via activation of the PI3K/AKT signaling pathway in human retinal epithelium cells (19). Additionally, a recent study showed that T. gondii induced the NOX1-dependent production of excessive ROS, which contributed to mitochondrial structural damage and mitochondrial dysfunction in placentas, as well as the cleavage of caspase-9 and caspase-3, which resulted in the apoptosis of trophoblasts (35). T. gondii also induced pyroptosis-like features with ROS production, as well as the activation of caspase-1 and IL-1β secretion, in macrophages (36). Our results correlate with those of previous studies that showed that NOX is an important source of GRA7-induced ROS that initiate intracellular signaling cascades. In addition to its effect on ROS, nitric oxide (NO) restricts parasite replication by depleting arginine, another amino acid essential for T. gondii growth, by IFN-γ-mediated innate protective mechanisms in a cell type- and species-specific manner (37). More interestingly, GRA15 carried by the type II strain of T. gondii directly activates NF-κB and drives host macrophages toward the polarization of classically activated macrophages, which generates the ROS and NO responsible for cell apoptosis (37, 38). Future studies should reveal the precise molecular mechanisms by which NOX regulates the fine control of GRA7-induced innate signaling in the context of ROS generation and will clarify the role of NO production in host defense mechanisms.
The modulation of host innate immunity by the intracellular parasite T. gondii in the early phases of infection is critical for the establishment of both the initial invasion and the subsequent maintenance of latent infection. Growing evidence suggests that host-pathogen interactions have led to the coevolution of toxoplasmosis-causing T. gondii with its host (1, 8, 39, 40). Previously, it was suggested that GRA proteins interact with a number of host cell proteins, including enzymes and structural and functional subcellular organelles from a broad spectrum, by yeast two-hybrid methods, with some pattern specific to each GRA protein. Ahn et al. showed that a search using GRA7 in the HeLa cDNA library resulted in two Homo sapiens genes, those for poly(rC) binding protein 1/PCBP1 and thymosin beta-10/TMSB10. GRA7 binds to PCBP1 along with PCBP2 and hnRNPK, corresponding to the principal cellular poly(rC) binding proteins. PCBP1 plays a part in the formation of a sequence-specific α-globin mRNP complex that is associated with the stability of α-globin mRNA (39). Additionally, GRA16 binds two host enzymes, the deubiquitinase HAUSP and PP2A phosphatase. GRA16 alters p53 levels in a HAUSP-dependent manner and induces the nuclear translocation of the PP2A holoenzyme for virulence in T. gondii (40). Furthermore, different polymorphic rhoptry proteins ROP5 and ROP18 have been shown to inhibit the immunity-related GTPase (IRG) resistance system by phosphorylation of highly conserved threonine residues in the switch I region of the nucleotide binding site for virulence of T. gondii (41–45). This mechanism is facilitated by direct binding of the pseudokinase ROP5 to IRG proteins (44, 45) and to ROP18, which control T. gondii virulence by upregulation of ROP18 activity (43). In mice, this two-component ROP5/ROP18 parasite virulence system is counteracted by polymorphic tandem IRG proteins (46), most likely because of active decoying of ROP5 and ROP18, leaving IRG a6/Irga6 unphosphorylated. Another active rhoptry kinase, ROP17, was demonstrated only recently to phosphorylate Irga6 and Irgb6 in vitro. ROP17 shows a preference for Irgb6, and unlike that of ROP18, its kinase activity is independent of ROP5 (47). GRA7 associates with ROP2 and ROP4 in infected host cells (48). In a recent study, GRA7 directly bound to the active dimer of Irga6 in a GTP-dependent manner. The binding of GRA7 to Irga6 led to enhanced polymerization, rapid turnover, and eventual disassembly, which contributed to acute virulence in the mouse (1). Furthermore, GRA7 is an additional component of the T. gondii ROP5/ROP18 kinase complex (1, 49). Hermanns et al. showed that GRA7 is directly associated with ROP5 and required for efficient phosphorylation of Irga6. GRA7 contributes to inhibition of vacuolar IRG protein accumulation at the PVM and is Irga6 specific. It has been demonstrated that T. gondii GRA7 as a regulator of ROP18-specific inactivation of Irga6 in vivo (49). The structural diversity of the IRG proteins implies that certain family members constitute additional specific targets for other, as-yet-unknown, T. gondii virulence effectors. Our results have further expanded the role of GRA7 to include the modulation of K63-linked TRAF6 polyubiquitination by direct binding to the TRAF6 RING domain, which is required for NF-κB activation and cytokine expression in the host defense strategy. It is critically involved in the autoubiquitination of TRAF6 and downstream signaling activation (17, 29). The role of GRA7 in TRAF6 ubiquitination does not seem to involve the deubiquitinases A20 and CYLD (data not shown), which are known inhibitors of NF-κB signaling (50). GRA7 does not contain a deubiquitination enzyme domain. It is possible that GRA7 controls additional E3 or E2 Ub ligases that also participate in TLR or non-TLR signaling. Given these findings, secreted T. gondii effector protein GRA7 represents a potentially emerging subfamily of exported dense granule proteins that modulate host function. Taken together, these data reveal a new facet of the role of GRA7 in TRAF6 targeting, which affects the regulation of innate host immune responses.
We have shown here that the C terminus of the GRA7-V domain specifically binds to the C70 residue with ligase activity containing the RING domain of TRAF6. rGRA7-V-immunized mice showed significantly enhanced Th1 immune responses and protective efficacy against T. gondii infection, and similar results were observed with rGRA7-WT in a binding-dependent manner (1). Taken together, these data strongly suggest that the GRA7-V domain is essential for the initiation of innate signaling pathways through NOX-dependent ROS generation and TRAF6 activation in a MyD88-dependent manner and provides protective mechanisms in vivo. These events may be potential targets for pharmacological modulation of GRA7 and associated signaling pathways in the inflammatory reaction during toxoplasmosis. Furthermore, the TRAF6 and NOX activation pathways are mutually dependent on GRA7-induced inflammatory signaling by macrophages.
In conclusion, we provide evidence of a critical role for MyD88-dependent TRAF6 activation and ROS production in the regulation of GRA7-mediated innate immune responses in macrophages. GRA7 interacts with TRAF6 to activate inflammatory responses; this is regulated by the association of GRA7-V with the RING domain of TRAF6, which modulates its ubiquitination. These observations reveal a new role for GRA7 in the regulation of innate immune responses in host protective immunity. Further studies are needed to develop more effective GRA7-based vaccines and to understand how GRA7 regulates host defense strategies against toxoplasmosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. S. Bae (Ewha University, Seoul, South Korea) for the kind provision of gp91phox KO mice and S. Akira (Osaka University, Osaka, Japan) for the kind provision of TLR2, TLR4, and MyD88 KO mice.
We have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00734-15.
REFERENCES
- 1.Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD. 2014. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc Natl Acad Sci U S A 111:1126–1131. doi: 10.1073/pnas.1313501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Echeverria PC, de Miguel N, Costas M, Angel SO. 2006. Potent antigen-specific immunity to Toxoplasma gondii in adjuvant-free vaccination system using Rop2-Leishmania infantum Hsp83 fusion protein. Vaccine 24:4102–4110. doi: 10.1016/j.vaccine.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. 2013. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min J, Qu D, Li C, Song X, Zhao Q, Li XA, Yang Y, Liu Q, He S, Zhou H. 2012. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine 30:5631–5636. doi: 10.1016/j.vaccine.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 5.Jung C, Lee CY, Grigg ME. 2004. The SRS superfamily of Toxoplasma surface proteins. Int J Parasitol 34:285–296. doi: 10.1016/j.ijpara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Quan JH, Chu JQ, Ismail HA, Zhou W, Jo EK, Cha GH, Lee YH. 2012. Induction of protective immune responses by a multiantigenic DNA vaccine encoding GRA7 and ROP1 of Toxoplasma gondii. Clin Vaccine Immunol 19:666–674. doi: 10.1128/CVI.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selseleh M, Keshavarz H, Mohebali M, Shojaee S, Selseleh M, Eshragian MR, Mansouri F, Modarressi MH. 2012. Production and evaluation of Toxoplasma gondii recombinant GRA7 for serodiagnosis of human infection. Korean J Parasitol 50:233–238. doi: 10.3347/kjp.2012.50.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarovinsky F. 2014. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol 14:109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 9.Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. 2013. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13:42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 11.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol 179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 12.Debierre-Grockiego F, Azzouz N, Schmidt J, Dubremetz JF, Geyer H, Geyer R, Weingart R, Schmidt RR, Schwarz RT. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J Biol Chem 278:32987–32993. [DOI] [PubMed] [Google Scholar]
- 13.Mun HS, Aosai F, Norose K, Piao LX, Fang H, Akira S, Yano A. 2005. Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70. Infect Immun 73:4634–4642. doi: 10.1128/IAI.73.8.4634-4642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. 2006. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 15.Haas T, Metzger J, Schmitz F, Heit A, Müller T, Latz E, Wagner H. 2008. The DNA sugar backbone 2′ deoxyribose determines Toll-like receptor 9 activation. Immunity 28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 17.Lin SC, Lo YC, Wu H. 2010. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason NJ, Fiore J, Kobayashi T, Masek KS, Choi Y, Hunter CA. 2004. TRAF6-dependent mitogen-activated protein kinase activation differentially regulates the production of interleukin-12 by macrophages in response to Toxoplasma gondii. Infect Immun 72:5662–5667. doi: 10.1128/IAI.72.10.5662-5667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Quan JH, Lee YH, Shin DW, Cha GH. 2013. Toxoplasma gondii proliferation require [sic] down-regulation of host Nox4 expression via activation of PI3 kinase/Akt signaling pathway. PLoS One 8:e66306. doi: 10.1371/journal.pone.0066306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aline F, Bout D, Dimier-Poisson I. 2002. Dendritic cells as effector cells: gamma interferon activation of murine dendritic cells triggers oxygen-dependent inhibition of Toxoplasma gondii replication. Infect Immun 70:2368–2374. doi: 10.1128/IAI.70.5.2368-2374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Liu T, Zhang A, Huo X, Luo Q, Chen Z, Yu L, Li Q, Liu L, Lun ZR, Shen J. 2012. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect Immun 80:2121–2132. doi: 10.1128/IAI.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Yang CS, Lee JS, Rodgers M, Min CK, Lee JY, Kim HJ, Lee KH, Kim CJ, Oh B, Zandi E, Yue Z, Kramnik I, Liang C, Jung JU. 2012. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 11:264–276. doi: 10.1016/j.chom.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuk JM, Shin DM, Lee HM, Kim JJ, Kim SW, Jin HS, Yang CS, Park KA, Chanda D, Kim DK, Huang SM, Lee SK, Lee CH, Kim JM, Song CH, Lee SY, Hur GM, Moore DD, Choi HS, Jo EK. 2011. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat Immunol 12:742–751. doi: 10.1038/ni.2064. [DOI] [PubMed] [Google Scholar]
- 24.Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. 2008. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signaling. Cell Microbiol 10:741–754. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Ahn MH, Song HO, Choi JH, Ryu JS, Min DY, Cho MH. 2006. Involvement of MAPK activation in chemokine or COX-2 productions by Toxoplasma gondii. Korean J Parasitol 44:197–207. doi: 10.3347/kjp.2006.44.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves CM, Silva DA, Azzolini AE, Marzocchi-Machado CM, Lucisano-Valim YM, Roque-Barreira MC, Mineo JR. 2013. Galectin-3 is essential for reactive oxygen species production by peritoneal neutrophils from mice infected with a virulent strain of Toxoplasma gondii. Parasitology 140:210–219. doi: 10.1017/S0031182012001473. [DOI] [PubMed] [Google Scholar]
- 27.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, Garty BZ, Camcioglu Y, Doffinger R, Kumararatne D, Davies G, Gallin JI, Haraguchi S, Day NK, Casanova JL, Meffre E. 2008. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity 29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muroi M, Tanamoto K. 2008. TRAF6 distinctively mediates MyD88- and IRAK-1-induced activation of NF-kappaB. J Leukoc Biol 83:702–707. [DOI] [PubMed] [Google Scholar]
- 29.Ferrao R, Li J, Bergamin E, Wu H. 2012. Structural insights into the assembly of large oligomeric signalosomes in the Toll-like receptor–interleukin-1 receptor superfamily. Sci Signal 5:re3. doi: 10.1126/scisignal.2003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pifer R, Benson A, Sturge CR, Yarovinsky F. 2011. UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J Biol Chem 286:3307–3314. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melo MB, Kasperkovitz P, Cerny A, Könen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. 2010. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog 6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gov L, Karimzadeh A, Ueno N, Lodoen MB. 2013. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4:e00255–13. doi: 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, Grigg ME, Saeij JP, Moayeri M. 2014. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog 10:e1003927. doi: 10.1371/journal.ppat.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M, Saeij JP, Grigg ME. 2014. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio 5:e01117–13. doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, He L, Zhang A, Li Q, Hu W, Chen H, Du J, Shen J. 2015. Toxoplasma gondii isolate with genotype Chinese 1 triggers trophoblast apoptosis through oxidative stress and mitochondrial dysfunction in mice. Exp Parasitol 154:51–61. doi: 10.1016/j.exppara.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Cavailles P, Flori P, Papapietro O, Bisanz C, Lagrange D, Pilloux L, Massera C, Cristinelli S, Jublot D, Bastien O, Loeuillet C, Aldebert D, Touquet B, Fournié GJ, Cesbron-Delauw MF. 2014. A highly conserved Toxo1 haplotype directs resistance to toxoplasmosis and its associated caspase-1 dependent killing of parasite and host macrophage. PLoS Pathog 10:e1004005. doi: 10.1371/journal.ppat.1004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo MB, Jensen KD, Saeij JP. 2011. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol 27:487–495. doi: 10.1016/j.pt.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn HJ, Kim S, Kim HE, Nam HW. 2006. Interactions between secreted GRA proteins and host cell proteins across the parasitophorous vacuolar membrane in the parasitism of Toxoplasma gondii. Korean J Parasitol 44:303–312. doi: 10.3347/kjp.2006.44.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, Pelloux H, Hakimi MA. 2013. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13:489–500. doi: 10.1016/j.chom.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD. 2010. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinfeldt T, Könen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, Hunn JP, Howard JC. 2010. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol 8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, Sibley LD. 2012. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog 8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleckenstein MC, Reese ML, Konen-Waisman S, Boothroyd JC, Howard JC, Steinfeldt T. 2012. A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol 10:e1001358. doi: 10.1371/journal.pbio.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Farid Arenas A, Melo MB, Spooner E, Yaffe MB, Saeij JP. 2012. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog 8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lilue J, Muller UB, Steinfeldt T, Howard JC. 2013. Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife 2:e01298. doi: 10.7554/eLife.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. 2014. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn JD, Ravindran S, Kim SK, Boothroyd JC. 2008. The Toxoplasma gondii dense granule protein GRA7 is phosphorylated upon invasion and forms an unexpected association with the rhoptry proteins ROP2 and ROP4. Infect Immun 76:5853–5861. doi: 10.1128/IAI.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermanns T, Müller UB, Könen-Waisman S, Howard JC, Steinfeldt T. 6 August 2015. The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell Microbiol doi: 10.1111/cmi.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun SC. 2008. Deubiquitylation and regulation of the immune response. Nat Rev Immunol 8:501–511. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.