Abstract
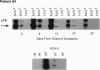
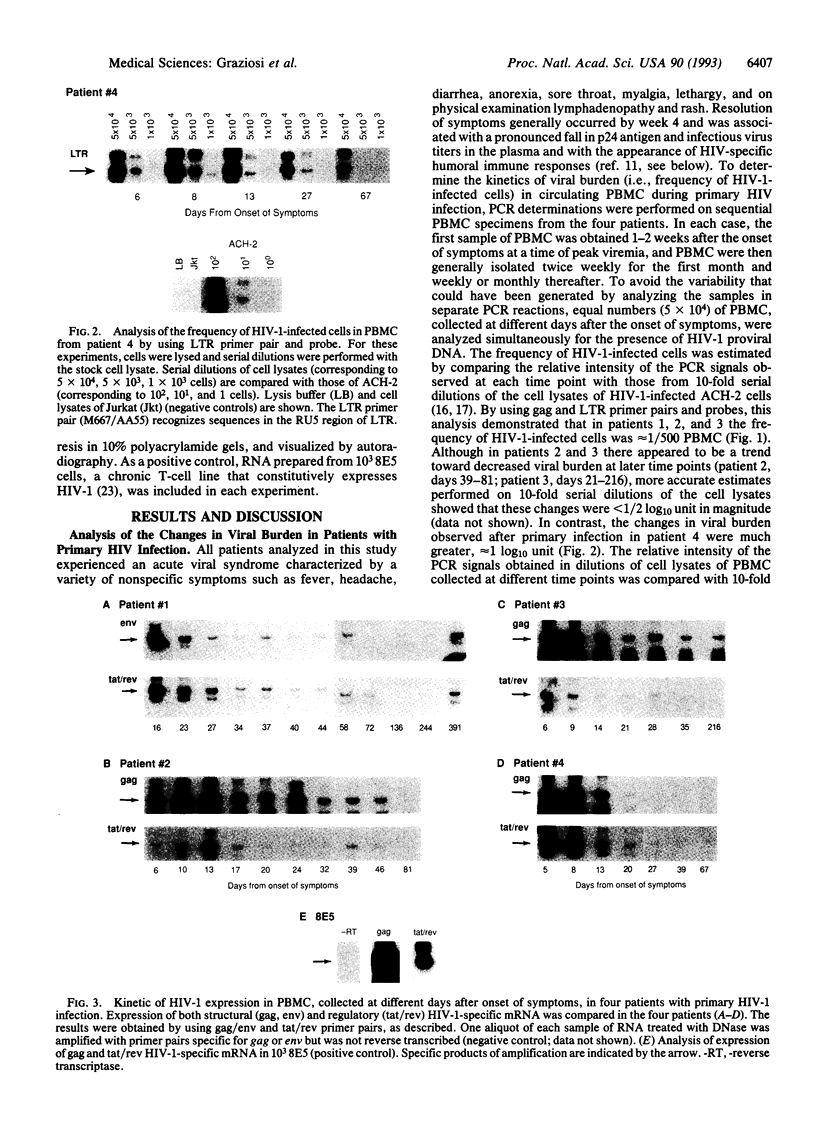
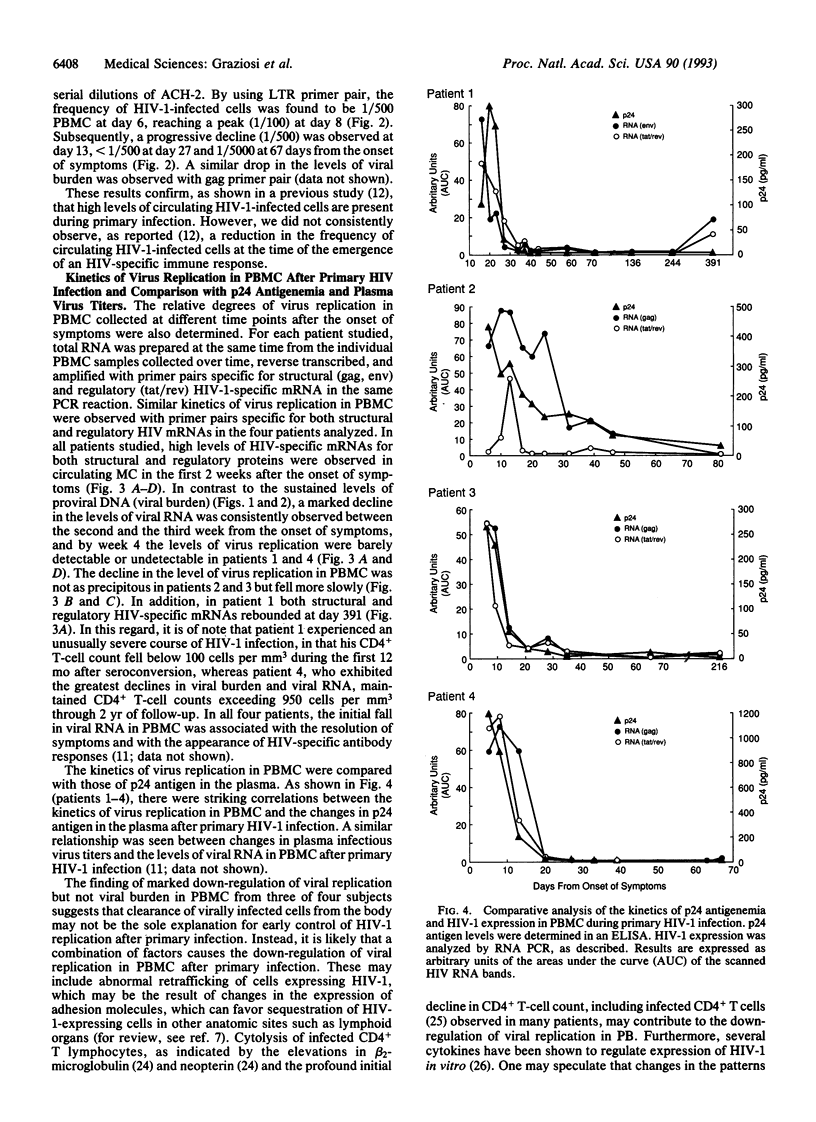
HIV-1 replication and viral burden in peripheral blood mononuclear cells (PBMC) have been reported to be high in primary infection but generally very low during the prolonged period of clinical latency. It is uncertain precisely when this transition occurs during the HIV-1 infection and what the relationship is between the changes in HIV-1 replication versus the clearance of infected cells in the overall control of viral replication. In the present study, the kinetics of viral burden (i.e., frequency of HIV-1-infected cells) and replication during primary and early-chronic infection were analyzed in PBMC of four acutely infected individuals. High frequencies of HIV-1-infected cells and high levels of virus replication were observed in PBMC after primary HIV-1 infection. Down-regulation of virus replication in PBMC was observed in all four patients coincident with the emergence of HIV-1-specific immune responses. Other parameters of virus replication, such as circulating plasma p24 antigen and plasma viremia showed similar kinetics. In contrast, a significant decline in viral burden in PBMC was observed in only one of four patients. These results indicate that the down-regulation in the levels of virus replication associated with the clinical transition from acute to chronic infection does not necessarily reflect a reduction in viral burden, thus suggesting the involvement of additional factors. Identification of these factors will be important in elucidating the host mechanisms involved in the early control of HIV-1 infection and disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besansky N. J., Butera S. T., Sinha S., Folks T. M. Unintegrated human immunodeficiency virus type 1 DNA in chronically infected cell lines is not correlated with surface CD4 expression. J Virol. 1991 May;65(5):2695–2698. doi: 10.1128/jvi.65.5.2695-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Saag M. S., Decker W. D., Campbell-Hill S., Roberson J. L., Veldkamp P. J., Kappes J. C., Hahn B. H., Shaw G. M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991 Apr 4;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Schnittman S. M., Poli G., Koenig S., Pantaleo G. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991 Apr 15;114(8):678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines H., von Sydow M. A., von Stedingk L. V., Biberfeld G., Böttiger B., Hansson L. O., Lundbergh P., Sönnerborg A. B., Wasserman J., Strannegåard O. O. Immunological changes in primary HIV-1 infection. AIDS. 1990 Oct;4(10):995–999. doi: 10.1097/00002030-199010000-00008. [DOI] [PubMed] [Google Scholar]
- Graham N. M., Zeger S. L., Park L. P., Phair J. P., Detels R., Vermund S. H., Ho M., Saah A. J. Effect of zidovudine and Pneumocystis carinii pneumonia prophylaxis on progression of HIV-1 infection to AIDS. The Multicenter AIDS Cohort Study. Lancet. 1991 Aug 3;338(8762):265–269. doi: 10.1016/0140-6736(91)90414-k. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Koenig S., Baseler M., Lane H. C., Fauci A. S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25- T cell population. J Immunol. 1990 Mar 1;144(5):1696–1704. [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- Ragni M. V., Kingsley L. A., Zhou S. J. The effect of antiviral therapy on the natural history of human immunodeficiency virus infection in a cohort of hemophiliacs. J Acquir Immune Defic Syndr. 1992;5(2):120–126. [PubMed] [Google Scholar]
- Schnittman S. M., Greenhouse J. J., Lane H. C., Pierce P. F., Fauci A. S. Frequent detection of HIV-1-specific mRNAs in infected individuals suggests ongoing active viral expression in all stages of disease. AIDS Res Hum Retroviruses. 1991 Apr;7(4):361–367. doi: 10.1089/aid.1991.7.361. [DOI] [PubMed] [Google Scholar]
- Stein D. S., Korvick J. A., Vermund S. H. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J Infect Dis. 1992 Feb;165(2):352–363. doi: 10.1093/infdis/165.2.352. [DOI] [PubMed] [Google Scholar]
- Tindall B., Cooper D. A. Primary HIV infection: host responses and intervention strategies. AIDS. 1991 Jan;5(1):1–14. [PubMed] [Google Scholar]
- Volberding P. A., Lagakos S. W., Koch M. A., Pettinelli C., Myers M. W., Booth D. K., Balfour H. H., Jr, Reichman R. C., Bartlett J. A., Hirsch M. S. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med. 1990 Apr 5;322(14):941–949. doi: 10.1056/NEJM199004053221401. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]