Abstract
The extraintestinal pathogen termed avian pathogenic Escherichia coli (APEC) is known to cause colibacillosis in chickens. The molecular basis of APEC pathogenesis is not fully elucidated yet. In this work, we deleted a component of the Yad gene cluster (yadC) in order to understand the role of Yad in the pathogenicity of the APEC strain SCI-07. In vitro, the transcription level of yadC was upregulated at 41°C and downregulated at 22°C. The yadC expression in vivo was more pronounced in lungs than in spleen, suggesting a role in the early steps of the infection. Chicks infected with the wild-type and mutant strains presented, respectively, 80% and 50% mortality rates. The ΔyadC strain presented a slightly decreased ability to adhere to HeLa cells with or without the d-mannose analog compared with the wild type. Real-time PCR (RT-PCR) assays showed that fimH was downregulated (P < 0.05) and csgA and ecpA were slightly upregulated in the mutant strain, showing that yadC modulates expression of other fimbriae. Bacterial internalization studies showed that the ΔyadC strain had a lower number of intracellular bacteria recovered from Hep-2 cells and HD11 cells than the wild-type strain (P < 0.05). Motility assays in soft agar demonstrated that the ΔyadC strain was less motile than the wild type (P < 0.01). Curiously, flagellum-associated genes were not dramatically downregulated in the ΔyadC strain. Taken together, the results show that the fimbrial adhesin Yad contributes to the pathogenicity and modulates different biological characteristics of the APEC strain SCI-07.
INTRODUCTION
Avian pathogenic Escherichia coli (APEC) strains cause a variety of extraintestinal infections in poultry collectively known as colibacillosis (1, 2). Although the complete mechanisms of APEC pathogenicity are not fully elucidated, it is believed that colibacillosis starts with colonization of the host's upper respiratory tract, with the bacterium expressing one or more colonization factors known as adhesins (3). The infection can subsequently spread into the lungs and other inner organs, leading to a fatal septicemia (1, 4, 5). Among the known APEC adhesins, those more studied are the type 1 fimbriae, P fimbriae, and curli fimbriae, respectively (6).
Besides their roles in the adhesion and subsequent colonization of environmental surfaces, those fimbriae are assumed to be essential for the establishment of a host-parasite relationship and further disease progression (7). Both type 1 and curli fimbriae play important roles in the initial bacterial colonization of the respiratory epithelium (1, 8), while P fimbriae (9) are important for later stages of infection (1).
In addition to the fimbriae mentioned above, E. coli possesses several other fimbrial operons (i.e., Yad, Ycb, Ybg, Yfc, Yra, Sfm, Ygi, and Yeh) that display sequence and organizational homologies to type 1 fimbria and could contribute to E. coli's ability to adhere to and colonize the host epithelia (10, 11).
The adhesion operon Yad is composed of seven genes, yadN (major subunit), ecpD (usher), htrE (chaperone), yadMLK (minor subunits), and yadC (adhesive tip) (12). The fimbria Yad is more prevalent among uropathogenic E. coli (UPEC) than commensal E. coli strains and contributes to the adherence to bladder epithelial cells and biofilm formation (10). Moreover, it was demonstrated that yadK is important for adhesion of acid-stressed enterohemorrhagic Escherichia coli (EHEC) to epithelial cells, increasing bacterial colonization and virulence (12).
In APEC strain O78, the deletion of yadL has suggested that the fimbria Yad has a subtle but positive contribution to APEC virulence (13). Furthermore, in silico analyses performed by our research group (unpublished data) with 12 E. coli genomes, including five APEC genomes, revealed that yadC is under positive selection. This suggests the involvement of the fimbria Yad in the host-parasite interactions.
To better understand the role of Yad fimbria in APEC biological characteristics and pathogenicity, we deleted yadC in an APEC swollen head syndrome strain (SCI-07) and studied its effects comparing both the wild type and the isogenic mutant through comprehensive in vitro and in vivo assays.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
APEC strain SCI-07 (ONT: H31) was isolated from a laying hen diagnosed with swollen head syndrome. Its genome is available in GenBank (accession no. CP000468.1) (14). The strains were cultured in LB or Dulbecco's modified Eagle's medium (DMEM) at 37°C with appropriate antibiotics when necessary (Table 1). All of the animal experimental protocols were approved by the Ethics Committee of Animal Use-CEUA-UNICAMP (protocol no. 2669-1; Brazilian law no. 11794).
TABLE 1.
List of strains and plasmids used in this work
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| SCI-07 | APEC strain isolated from lesions of a laying hen presenting clinical signs of swollen head syndrome | Our laboratory |
| DH10β | E. coli K-12 strain | Our laboratory |
| HB101 | E. coli K-12 strain | Our laboratory |
| DH5α | E. coli K-12 strain | Our laboratory |
| EIEC strain | Enteroinvasive E. coli O164 strain | Laboratory of UEL, Londrina, Brazil |
| STM-F98 | Salmonella Typhimurium | Laboratory of Ornithopathology, FCAV-UNESP, Jaboticabal, Brazil |
| Plasmids | ||
| pKD3 | cat gene | Datsenko and Wanner (17) |
| pKD46 | Ampr-expressing λ red recombination | Datsenko and Wanner (17) |
| pACYC177 | Cloning vector | New England Biolabs |
RNA extraction.
Total RNA of strain SCI-07 was extracted from LB (in vitro) as well as from lungs and spleen of day-old infected chicks. For in vitro RNA extraction, culture was grown in LB at 22, 37, or 41°C until reaching an optical density at 600 nm (OD600) of 0.5 to 0.6. Afterwards, RNA was extracted using the “RNAeasy minikit” (Qiagen). For in vivo assays, an inoculum of 0.1 ml was injected into the right air sac of five 1-day-old chicks. After 24 h postinfection (hpi), the left lung and spleen were removed and homogenized in 1 ml TRIzol solution (Ambion). RNA was extracted using the Purelink RNA minikit (Ambion, Life Technologies).
Relative expression of yadC in SCI-07 by qRT-PCR.
Real-time PCR (RT-PCR) was performed to quantify the transcription of yadC in LB at different temperatures (22, 37, and 41°C). Primers for quantitative RT-PCR (qRT-PCR) were constructed with Primer Express software version 3.0 (Applied Biosystems) (see Table S1 in the supplemental material), using the SCI-07 genome as the template. The qRT-PCR experiments were performed as described previously (15). RNA polymerase subunit A (rpoA) was used as the endogenous control (see Table S1). Data were normalized based on the transcription level of rpoA in the wild type and then analyzed using the comparative critical threshold cycle (CT) method described by Walters and Sperandio (16).
Deletion of yadC.
The ΔyadC strain was constructed following the methodology described by Datsenko and Wanner (17). To replace gene yadC by homologous recombination, a chloramphenicol (Cm) cassette from pKD3 was amplified, and strain SCI-07, harboring the λ red plasmid pKD46, was transformed by electroporation as described by de Paiva et al. (15). The ΔyadC deletion was confirmed by PCR using primers F-yadC 1, R-yadC 2 (internal), F-yadC 3, and R-yadC 4 (external) (see Table S1 in the supplemental material).
Construction of the yadC complemented strain.
For construction of a complemented strain, yadC (1,263 bp), along with its promoter, was cloned into the cloning vector pACYC177. Using primers F-C.ΔyadC and R-C.ΔyadC (see Table S1 in the supplemental material), a fragment containing gene yadC and the enzyme DraIII and HindIII restriction sites was amplified and purified. The fragment was then ligated to plasmid pACYC177 DraIII and HindIII restriction sites. The recombinant plasmid was electroporated into competent cells of strain DH10β (15). Positive colonies were tested for the presence of yadC by PCR using the same complementation primers. The resulting plasmid, pACYC177-yadC, was isolated from strain DH10β and then transformed into competent cells of the ΔyadC strain to make the C.ΔyadC strain.
qRT-PCR assays for adhesion- and motility-related genes.
Bacterial cultures of wild-type, mutant, and complemented strains were aerobically grown until they reached an OD600 of 0.5 to 0.6. RNA from three biological samples was extracted using the RNAeasy minikit (Qiagen). Real-time PCR assays were performed to quantify the transcription of bacterial genes related to adhesion (fimH, csgA, and ecpA) and motility (fliC, flhD, and flgE) and the motility modulator ompR in each strain. Primers were designed (see Table S1 in the supplemental material) and qRT-PCR experiments were performed as described previously (6).
Bacterial adhesion to HeLa cells.
The SCI-07, ΔyadC, and C.ΔyadC strains were evaluated for their ability to adhere to HeLa cells in the presence (1%) and absence of the d-mannose analog. E. coli strains O164 (enteroinvasive E. coli [EIEC]) and HB101 were used as positive and negative controls, respectively. Monolayers of HeLa cells were grown in DMEM containing 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator until they reached confluent growth. These incubation conditions were used for all eukaryotic cell lines. HeLa cells were infected with 10 μl of bacterial inocula at a concentration of 107 CFU/ml in triplicate in 24-well plates as described previously (6). The obtained suspension was diluted 1- to 10-fold and plated onto MacConkey agar (MAC) containing 100 μg ml−1 streptomycin (Sm) for colony-forming unit determination.
Bacterial invasion of human epithelial Hep-2 cells.
The invasion abilities of the three studied strains were assessed in vitro with cultured Hep-2 cells following the methodology described by Scaletsky et al. (18). E. coli strains O164 (EIEC) and DH5α were used as positive and negative controls, respectively. A volume of 10 μl of a bacterium–phosphate-buffered saline (PBS) suspension (107 CFU/ml) was inoculated onto monolayers of Hep-2 cells in triplicate. Each well was filled with DMEM and 10% FBS up to 1 ml and incubated for 3 h. The extracellular bacteria were removed by washing the cells twice with PBS. Cells were replenished with fresh DMEM containing 20 mg ml−1 of ampicillin (Amp) for the wild-type and mutant strains and 25 mg ml−1 of kanamycin (Km) for the complementation strain and then incubated for another 3 h. Afterwards, cells were washed with PBS and lysed with subsequent colony-forming unit determination.
Bacterial survival in HD11 macrophage cells.
For the macrophage bacterial survival assay, HD11 chicken macrophage cells (19) were infected with the wild-type, ΔyadC, and C.ΔyadC strains. Salmonella enterica serovar Typhimurium STM-F98 and E. coli HB101 were used as positive and negative controls, respectively. A volume of 10 μl of bacterium-PBS suspension (107 CFU/ml) was inoculated onto monolayers of HD11 cells in triplicate. Each well was filled with RPMI 1640, supplemented with 2 mM glutamine and 10% FBS up to 1 ml, and incubated for 1 h. The extracellular bacteria were removed by washing the plate with PBS. After this, 1 ml of the glutamine-FBS-RPMI medium containing 20 mg ml−1 Amp for wild-type and mutant strains and 25 mg ml−1 of Km for the complemented strain was added to each well and incubated for 3 h or 18 h. Then the plate was washed 3 times with PBS, and cells were lysed with 1% Triton X-100. The lysed cell suspension was used for colony-forming unit determination.
Motility assay.
Cultures of each strain grown overnight in LB broth were stabbed into the center of LB–0.3% agar plates. The agar plates were incubated at 37°C and motility (in centimeters) was measured after 6, 8, and 10 h.
Bacterial growth assay.
Overnight-incubated cultures (LB broth) of the SCI-07, ΔyadC, and C.ΔyadC strains were diluted in LB as well as in DMEM (1:100) and grown at 37°C with agitation of 150 rpm. Optical densities of each bacterial culture were monitored spectrophotometrically at 600 nm every 30 min for 6 h.
In vivo mortality test.
Cultures from the SCI-07, ΔyadC, and C.ΔyadC strains were grown (LB medium) at 37°C for 24 h. Briefly, groups of 20 birds for each strain were inoculated in the right thoracic air sac with 0.1 ml of a suspension containing 109 CFU or with 0.1 ml of PBS as negative control (15). Birds were observed every 12 h up to 7 days after inoculation, and the numbers of deaths were recorded as described by Antão et al. (20).
Systemic infection.
The group of three 1-day-old chicks for each strain (the SCI-07, ΔyadC, and C.ΔyadC strains) was infected with 0.1 ml of a suspension containing 108 CFU, in the right thoracic air sac. At 24 and 48 hpi, chickens were euthanized. Afterwards, samples of the lung, liver, and heart were homogenized, and colony forming unit determination was performed as described previously (15).
Statistical analysis.
For statistical analysis, qRT-PCR, motility assays, and agar plate counting experiments were performed in triplicate. Statistical analysis was performed by analysis of variance (ANOVA), with Tukey's test, using ASSITAT software version 7.7.
RESULTS
Transcription level of gene yadC changed according to different temperatures.
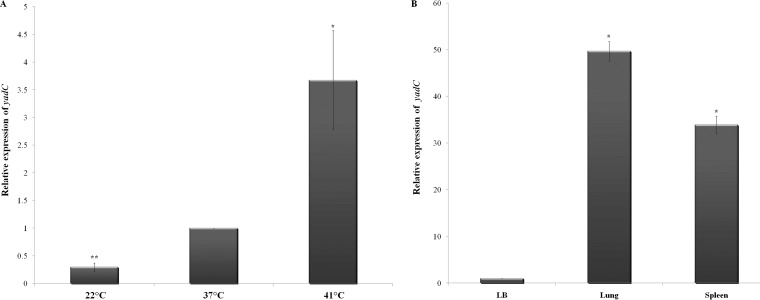
The transcription of gene yadC in strain SCI-07 was verified in vitro and in vivo by qRT-PCR. The transcription level of gene yadC was found to be upregulated, by almost 4-fold, when the strain was cultivated at the highest temperature (41°C), which is the usual temperature of chicken bodies, comparing to the incubation at 37°C. On the other hand, it was downregulated, by 0.2-fold, when the strain was cultivated at room temperature (22°C) (Fig. 1A). In vivo, the transcription of gene yadC was upregulated in lungs and spleen compared to its transcription in LB culture (37°C) (Fig. 1B). When comparing lungs and spleen, yadC expression was more pronounced in lungs.
FIG 1.
Relative fold expression of yadC by strain SCI-07, as determined by qRT-PCR. All levels were based on the comparison with the transcriptions in LB at 37°C. (A) In vitro (LB) at 22, 37, and 41°C. (B) In vivo (lung and spleen). One asterisk indicates statistical significance at P < 0.05, and two asterisks indicate significance at P < 0.01.
The bacterial growth assay performed with the wild-type, mutant, and complemented strains showed the same trend, suggesting that YadC does not play a role in this APEC strain growth (see Fig. S1 in the supplemental material).
Deletion of yadC effects in SCI-07 pathogenicity and intrabody survival.
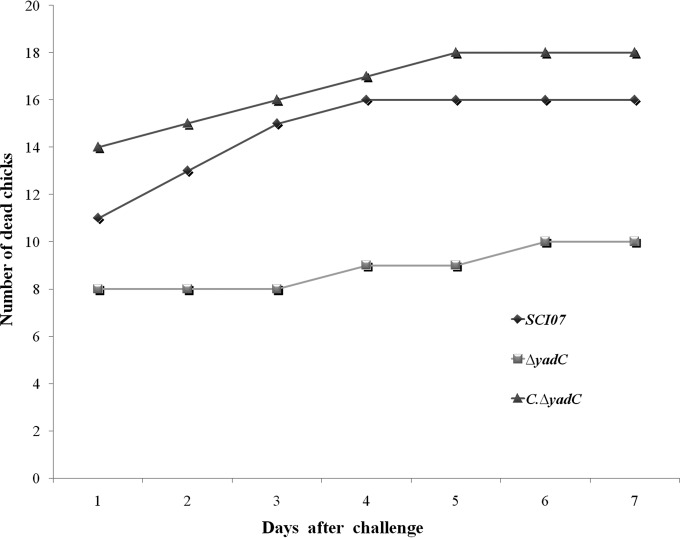
To verify if gene yadC could play a role in the virulence of strain SCI-07, as suggested by the previous transcription assays, in vivo mortality assays were performed. Chicks infected with the wild-type strain (SCI-07) presented an 80% mortality rate (n = 16), with 11 animals being killed at the first day of observation. The mutant (ΔyadC) strain presented overall a 50% mortality rate (n = 10) during the 7 days of experimentation, with only 8 of the chicks being killed at the first day of observation. The complemented (C.ΔyadC) strain presented a complete restoration and increased virulence levels with a global mortality rate of 90% (n = 18) and 14 animals dead on the first day. No mortality was observed for the negative control (data not shown) (Fig. 2).
FIG 2.
Mortality test in day-old chicks infected with 109 CFU of wild-type (SCI-07), mutant (ΔyadC), and complemented (C.ΔyadC) strains.
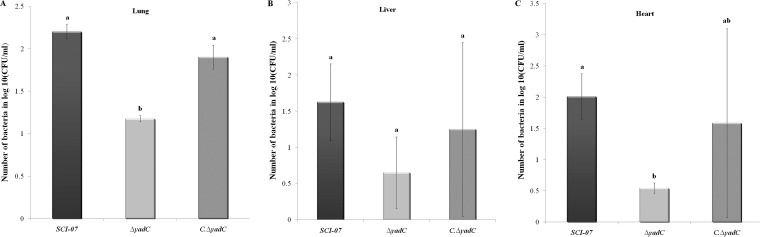
Moreover, the systemic in vivo infection assays demonstrated that less of the ΔyadC strain was recovered from lungs and heart than the wild-type strain 24 hpi (P < 0.05) (Fig. 3A, B, and C). No statistical difference was observed in the number of colony-forming units for any of the analyzed organs 48 hpi.
FIG 3.
Bacterial recovery of wild-type (SCI-07), mutant (ΔyadC), and complemented (C.ΔyadC) strains from the lungs (A), liver (B), and heart (C) after infection with 108 CFU of bacteria. Different letters indicate statistical difference (P < 0.05).
Role of yadC in bacterial adhesion.
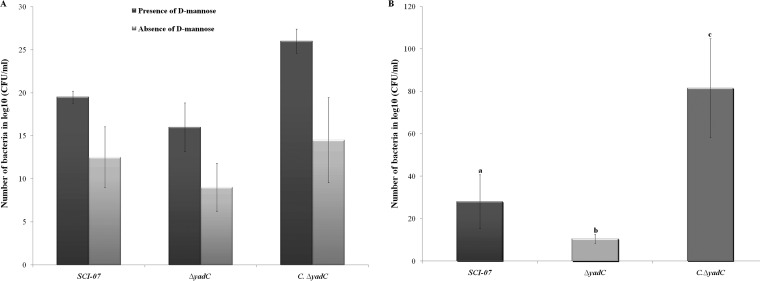
In order to better understand if yadC absence affects the initial infection processes, we performed biological tests, including an adhesion assay with HeLa cells in the presence and absence of the d-mannose analog. The adhesion studies showed that the adhesion levels of the wild-type strain decreased in the presence of the d-mannose analog, indicating that strain SCI-07 expresses type 1 fimbriae as previously described (15). Moreover, strain SCI-07, under these conditions, was still able to express a d-mannose-resistant adhesin, suggesting the expression of another type of fimbria. The ΔyadC strain, with the same experimental assays, presented a slightly decreased ability to adhere to HeLa cells under both conditions (with and without d-mannose analog) compared with the wild type. This suggests that yadC plays a modest direct role in APEC adhesion and may be involved in the regulation of type 1 fimbriae (Fig. 4A). The remaining adhesion capacity of the SCI-07 and ΔyadC strains either in the presence or in the absence of the d-mannose analog could be due to the possible expression of other adhesins that might exist in strain SCI-07.
FIG 4.
(A) Adhesion of wild-type (SCI-07), mutant (ΔyadC), and complemented (C.ΔyadC) strains to HeLa cells. Although adhesion levels were decreased, no statistical differences were found among the wild-type, mutant, and complemented strains. (B) Invasion of wild-type, mutant, and complemented strains into Hep-2 cells. Different letters indicate statistical difference (P < 0.05).
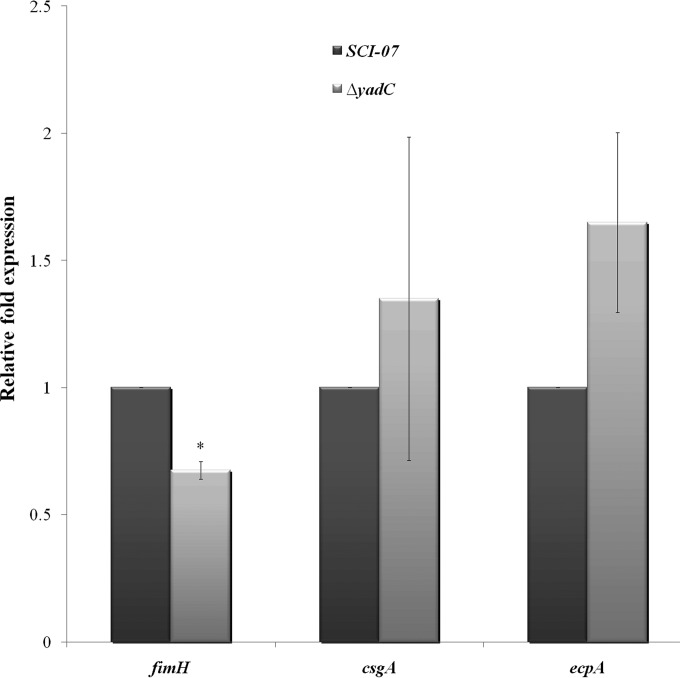
To evaluate if the transcription of other fimbriae known to exist in strain SCI-07 could be regulated by gene yadC, the qRT-PCR assays for type 1, curli, and E. coli common pilus (ECP) were performed in vitro. These tests showed that csgA and ecpA were slightly increased without statistical significance, but fimH was downregulated in the ΔyadC strain (Fig. 5).
FIG 5.
Relative fold expression of fimbrial fimH, csgA, and ecpA genes as determined by qRT-PCR in wild type (SCI-07) and mutant (ΔyadC) strains. An asterisk indicates statistical significance at P < 0.05.
Deletion of yadC also affects the APEC strain ability to invade Hep-2 cells.
The invasion ability of the mutant strain in Hep-2 cells was tested to help us better understand the key role gene yadC plays in the ΔyadC strain's virulence attenuation. This assay showed that the ΔyadC strain had a lower number of intracellular bacteria recovered from Hep-2 cells than the wild-type strain (P < 0.05) (Fig. 4B).
Replication and survival of strain SCI-07 inside macrophage cells was compromised by yadC deletion.
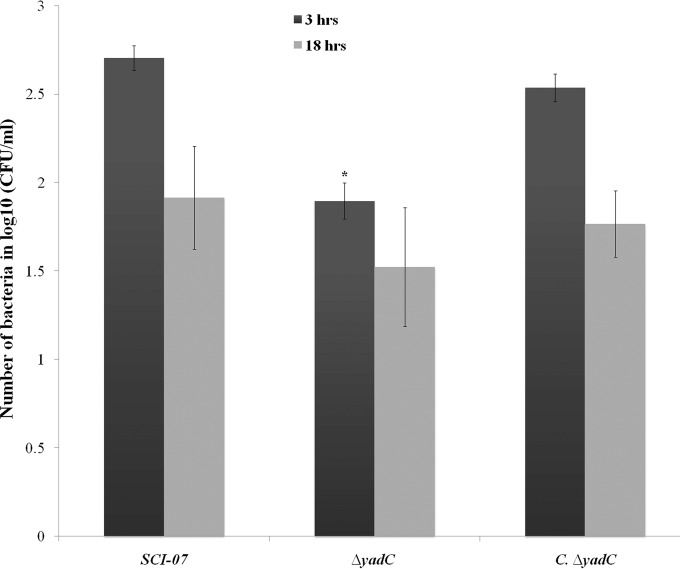
The capacity of some pathogenic bacteria to survive and replicate inside macrophages indicates an increased ability to evade host defenses. To test if yadC contributes to this process, the ability of the ΔyadC strain to replicate and survive within the HD11 macrophage cells was tested. The assay showed that the Δyad mutant had lower survivability in this macrophage cell line than the wild-type strain 3 hpi (P < 0.05) (Fig. 6).
FIG 6.
Bacterial survival in avian macrophage cells (HD11) infected with the wild-type (SCI-07), mutant (ΔyadC), and complemented (C.ΔyadC) strains. Infected macrophages were lysed at 3 or 18 h postinfection, and the numbers of bacteria were determined in triplicate. An asterisk indicates statistical significance at P < 0.05.
Deletion of gene yadC affects motility.
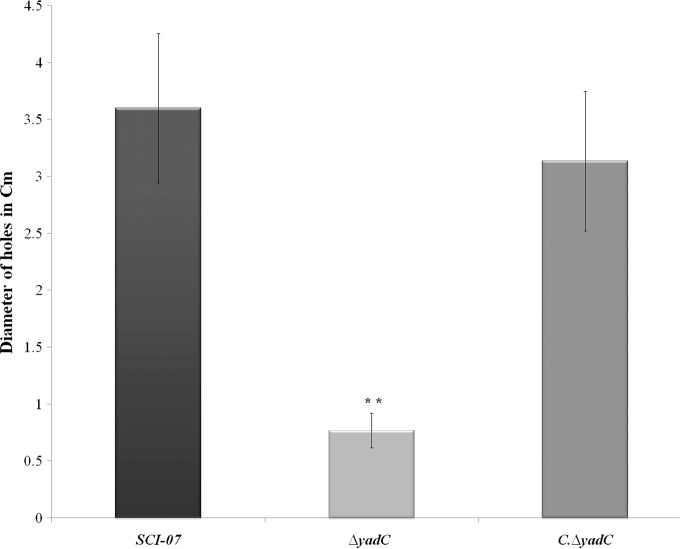
The motility assays showed that the ΔyadC mutant strain has a decreased motility degree compared with the wild-type strain (P < 0.01) (Fig. 7). To better understand how an alteration in expression of fimbria Yad could influence motility or flagellar protein expression in the mutant strain, qRT-PCR analysis was performed to quantify the relative transcription of structural and regulator flagellar genes in all studied strains.
FIG 7.
Motility assay in soft agar with the wild type (SCI-07), mutant (ΔyadC), and (C.ΔyadC) complementation strains. The presence of two asterisks indicates statistical significance at P < 0.01.
The obtained results showed that the expression levels of flgE and fliC (structural genes; class II and III, respectively) were slightly decreased in the ΔyadC strain compared to the wild-type strain. On the other hand, the expression of flhD (global regulator; class I) and ompR (motility regulator) was slightly increased in the mutant strain (see Fig. S2 in the supplemental material).
DISCUSSION
Escherichia coli's ability to establish colonization in several different niches and to infect the host's surfaces depends first on its ability to adhere, which prevents physical clearance mechanisms and engages the bacteria in a further colonization process. Many putative adhesins have been reported and their roles studied in distinct E. coli pathotypes. The Yad-encoding gene cluster has been associated with adhesion in an O157:H7 E. coli strain (12). In the present work, we showed the role of this gene in an avian pathogenic E. coli (APEC) strain.
Because yadC was upregulated both at the avian host regular temperature and in in vivo assays, it could play an important role in SCI-07 virulence. The high expression of yadC in lungs indicates that the fimbria Yad plays a role in APEC during the initial steps of the infection. Moreover, this effect would not be a consequence of an increased number of bacteria once the mutant presented the same bacterial growth as the wild-type and complemented strains.
In a previous work, the biofilm formation capacity associated with the fimbria Yad was more pronounced at lower (30°C) than higher (37°C) temperatures, with an assumed constant level of Yad expression (11). This would indicate that the fimbria Yad works better at lower than higher temperatures, similarly to the temperature-sensitive hemagglutinin (Tsh) (21). The in vitro upregulation at higher temperatures of a yad gene cluster component (yadC) found in the present work was also detected in other yad components (ecpD-htrE) in another work (22). Admitting that biofilm formation is a pathogenicity-related process, the higher transcription of an assumed less effective product could be a way of balancing the ratio of efficiency to quantity in order to keep a constant final function.
In the current proposed model of avian colibacillosis pathogenesis, the respiratory tract is colonized first, with subsequent spread of the pathogenic bacteria to the inner organs (23). The higher yadC expression in lungs than in spleen suggests that this adhesin would play a more intense role in the earlier steps of the infection mainly influencing the adhesion process. Moreover, the decreased ability of the ΔyadC mutant strain to survive in macrophages at 3 hpi indicates that the presence of these fimbriae in the wild-type strain would hamper the initial macrophage recognition, facilitating bacterial spreading inside the host body and further septicemic development of the infection.
Although several adhesins are associated with APEC (e.g., type 1 fimbria, curli, and ECP) (23), the complete mechanisms involved in APEC pathogenesis are poorly understood. The roles of Yad-mediated adhesion seem to be distinct between pathogenic and nonpathogenic E. coli. In the laboratory strain K-12, Yad does not promote adherence to A549, T24, HeLa, and Henle cells but increases biofilm formation (11). On the other hand, Yad is required for adherence to bladder epithelial cells (UM-UC-3) in uropathogenic E. coli (UPEC) (10) and to Caco-2 cells in EHEC under stress conditions (12). Many APEC and UPEC strains share genetic determinants (24) that led to a specialization in causing extraintestinal diseases. The Yad fimbria of the APEC strain tested in this work followed the same trend found with UPEC, contributing to adhesion. Due to the crucial role of adhesion for bacterial colonization and disease progression, it is possible that pathogenic E. coli presents further traits absent in commensal strains that mediate Yad-associated adhesion.
The role of E. coli adhesins in the internalization into eukaryote cell lines is under debate. Expression of curli fimbriae at high levels was able to mediate the internalization of a uropathogenic E. coli strain into bladder cell lines (25). Also, the expression of adhesin AfaD/DraD was suggested to partially mediate the internalization of bacteria into HeLa cells (26).This work showed that the mutant ΔyadC strain was less invasive in human epithelial cells (Hep-2). It is not possible to confirm if Yad has a crucial role in internalization or if the increased invasion is secondary to increased adhesion.
After physical barriers, bacterial pathogens must overcome the innate host defense in order to produce disease. In this context, the ability to survive in macrophages is an advantage for pathogenic E. coli. It was previously demonstrated that the deletion of a gene encoding a DNA-binding protein of APEC (RstA) had a role in diminishing the ability to survive in avian macrophage cell lines (HD11) (27). The present work showed that Yad increased the survival in macrophages 3 hpi but not 18 hpi. This fact was expected since the battle between APEC and avian macrophages is supposed to take place at the beginning of the infection.
Flagellar motility can be modulated by fimbrial expression. In UPEC, it was shown that a mutant lacking genes encoding type 1 (fim) and P (pap) fimbriae presented slight decreases in motility compared to the wild-type strain (28). This was similar to the present work, where the ΔyadC strain was less motile than the wild type. However, the decreased motility of the mutant strain was not accompanied by a significant downregulation of flagellar genes. A similar paradox was observed with an enterotoxigenic E. coli (ETEC) strain. In that case, an isogenic ΔfaeG strain (faeG encodes the major subunit of F4 fimbriae) did not present a different degree of motility but presented fliC (encoding the major flagellin protein) upregulated compared to the wild type (29). This indicates that the level of flagellar gene transcription does not always correspond to the degree of bacterial motility.
Taken together, these results suggest that the fimbrial adhesin YadC plays a role in adhesion, internalization, and motility (all of them basic biological bacterial characteristics) of the APEC strain here studied and contributes to its pathogenicity, particularly in the beginning of the infection process.
Supplementary Material
ACKNOWLEDGMENTS
R.V. (2012/23289-3), T.C.G.R. (2013/09167-5), J.L.L.G. (2014/04860-7), R.P.M. (2012/05073-3), and L.P.M.S. (2012/04391-1) were supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). W.D.S. has a scientific fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01138-15.
REFERENCES
- 1.Dho-Moulin M, Fairbrother JM. 1999. Avian pathogenic Escherichia coli (APEC). Vet Res 30:299–316. [PubMed] [Google Scholar]
- 2.Mellata M, Dho-Moulin M, Dozois CM, Curtiss R III, Brown PK, Arné P, Brée A, Desautels C, Fairbrother JM. 2003. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect Immun 71:536–540. doi: 10.1128/IAI.71.1.536-540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes HJ, Vaillancourt JP, Gross W. 2003. Colibacillosis, p 631–656. In Saif Y. (ed), Diseases of poultry, 11th ed Iowa State University Press, Ames, IA. [Google Scholar]
- 4.Gross WB. 1991. Colibacillosis, p 138–144. In Calnek BW. (ed), Diseases of poultry, 9th ed Iowa State University Press, Ames, IA. [Google Scholar]
- 5.La Ragione RM, Woodward MJ. 2002. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res Vet Sci 73:27–35. doi: 10.1016/S0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 6.de Pace F, Nakazato G, Pacheco A, Boldrin de Paiva J, Sperandio V, Dias da Silveira W. 2010. The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect Immun 78:4990–4998. doi: 10.1128/IAI.00531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaper JB, Nataro JP, Mobley HLT. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 8.Klemm P, Jorgensen BJ, van Die I, de Ree H, Bergmans H. 1985. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol Gen Genet 199:410–414. doi: 10.1007/BF00330751. [DOI] [PubMed] [Google Scholar]
- 9.Latham RH, Stamm WE. 1984. Role of fimbriated Escherichia coli in urinary tract infections in adult women: correlation with localization studies. J Infect Dis 149:835–840. doi: 10.1093/infdis/149.6.835. [DOI] [PubMed] [Google Scholar]
- 10.Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. 2011. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun 79:4753–4763. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korea CG, Badouraly R, Prevost MC, Ghigo JM, Beloin C. 2010. Escherichia coli K-12 possesses multiple cryptic but functional chaperone-usher fimbriae with distinct surface specificities. Environ Microbiol 12:1957–1977. doi: 10.1111/j.1462-2920.2010.02202.x. [DOI] [PubMed] [Google Scholar]
- 12.Chingcuanco F, Yu Y, Kus JV, Que L, Lackraj T, Lévesque CM, Barnett Foster D. 2012. Identification of a novel adhesin involved in acid-induced adhesion of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 158:2399–2407. doi: 10.1099/mic.0.056374-0. [DOI] [PubMed] [Google Scholar]
- 13.Dziva F, Hauser H, Connor TR, van Diemen PM, Prescott G, Langridge GC, Eckert S, Chaudhuri RR, Ewers C, Mellata M, Mukhopadhyay S, Curtiss R, Dougan G, Wieler LH, Thomson NR, Pickard DJ, Stevens MP. 2013. Sequencing and functional annotation of avian pathogenic Escherichia coli serogroup O78 strains reveal the evolution of E. coli lineages pathogenic for poultry via distinct mechanisms. Infect Immun 81:838–849. doi: 10.1128/IAI.00585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas TC, Parizzi LP, Tiba MR, Chen L, Pereira GA, Sangal V, Yang J, Yu J, Dias da Silveira W. 2012. Draft genome of a Brazilian avian-pathogenic Escherichia coli strain and in silico characterization of virulence-related genes. J Bacteriol 194:3023. doi: 10.1128/JB.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Paiva JB, Leite JL, Silva LP, Rojas TC, de Pace F, Conceicao RA, Sperandio V, Silveira WD. 2015. Influence of the major nitrite transporter NirC on the virulence of a swollen head syndrome avian pathogenic E. coli (APEC) strain. Vet Microbiol 175:123–131. doi: 10.1016/j.vetmic.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Walters M, Sperandio V. 2006. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun 74:5445–5455. doi: 10.1128/IAI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaletsky IC, Silva ML, Trabulsi LR. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun 45:534–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T. 1979. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- 20.Antao EM, Glodde S, Li G, Sharifi R, Homeier T, Laturnus C, Diehl I, Bethe A, Philipp HC, Preisinger R, Wieler LH, Ewers C. 2008. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC). Microb Pathog 45:361–369. doi: 10.1016/j.micpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Provence DL, Curtiss R. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun 62:1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raina S, Missiakas D, Baird L, Kumar S, Georgopoulos C. 1993. Identification and transcriptional analysis of the Escherichia coli htrE operon which is homologous to pap and related pilin operons. J Bacteriol 175:5009–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guabiraba R, Schouler C. 2015. Avian colibacillosis: still many black holes. FEMS Microbiol Lett 362:fnv118. doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 24.Maluta RP, Logue CM, Casas MR, Meng T, Guastalli EA, Rojas TC, Montelli AC, Sadatsune T, de Carvalho Ramos M, Nolan LK, da Silveira WD. 2014. Overlapped sequence types (STs) and serogroups of avian pathogenic (APEC) and human extra-intestinal pathogenic (ExPEC) Escherichia coli isolated in Brazil. PLoS One 9:e105016. doi: 10.1371/journal.pone.0105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gophna U, Barlev M, Seijffers R, Oelschlager TA, Hacker J, Ron EZ. 2001. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun 69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jouve M, Garcia MI, Courcoux P, Labigne A, Gounon P, Le Bouguénec C. 1997. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect Immun 65:4082–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Q, Ye Z, Wang X, Mu X, Gao S, Liu X. 2015. RstA is required for the virulence of an avian pathogenic Escherichia coli O2 strain E058. Infect Genet Evol 29:180–188. doi: 10.1016/j.meegid.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Lane MC, Simms AN, Mobley HL. 2007. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J Bacteriol 189:5523–5533. doi: 10.1128/JB.00434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M, Duan Q, Zhu X, Guo Z, Li Y, Hardwidge PR, Zhu G. 2013. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet Res 44:30. doi: 10.1186/1297-9716-44-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.