Abstract
Schistosomiasis is a tropical disease affecting over 230 million people worldwide. Although effective drug treatment is available, reinfections are common, and development of immunity is slow. Most antibodies raised during schistosome infection are directed against glycans, some of which are thought to be protective. Developing schistosomula are considered most vulnerable to immune attack, and better understanding of local antibody responses raised against glycans expressed by this life stage might reveal possible glycan vaccine candidates for future vaccine research. We used antibody-secreting cell (ASC) probes to characterize local antiglycan antibody responses against migrating Schistosoma japonicum schistosomula in different tissues of rats. Analysis by shotgun Schistosoma glycan microarray resulted in the identification of antiglycan antibody response patterns that reflected the migratory pathway of schistosomula. Antibodies raised by skin lymph node (LN) ASC probes mainly targeted N-glycans with terminal mannose residues, Galβ1-4GlcNAc (LacNAc) and Galβ1-4(Fucα1-3)GlcNAc (LeX). Also, responses to antigenic and schistosome-specific glycosphingolipid (GSL) glycans containing highly fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches that are believed to be present at the parasite's surface constitutively upon transformation were found. Antibody targets recognized by lung LN ASC probes were mainly N-glycans presenting GalNAcβ1-4GlcNAc (LDN) and GlcNAc motifs. Surprisingly, antibodies against highly antigenic multifucosylated motifs of GSL glycans were not observed in lung LN ASC probes, indicating that these antigens are not expressed in lung stage schistosomula or are not appropriately exposed to induce immune responses locally. The local antiglycan responses observed in this study highlight the stage- and tissue-specific expression of antigenic parasite glycans and provide insights into glycan targets possibly involved in resistance to S. japonicum infection.
INTRODUCTION
Schistosomiasis is one of the neglected tropical diseases with the highest impacts on human health. Over 230 million people are infected worldwide, and over 500 million are at risk of infection (1–3). Infection leads to chronic disease characterized by pronounced immunological reactions against eggs deposited into host tissues by the adult schistosome worms, which eventually lead to fibrosis and organ failure (2). Although effective treatment is available, reinfection occurs rapidly and immunity develops only slowly, stressing the need for a prophylactic vaccine as part of a sustainable control strategy (4–6).
Schistosomes have a complex life cycle with different life stages that interact with the human host and that each play a role in immunology, immunopathology, and maintenance of infection. Schistosoma infection occurs after direct contact with water containing the larval form of the parasite (cercariae). Cercariae penetrate the host skin and transform into schistosomula, which enter the vasculature and mature while migrating via the lungs to the portal veins. When fully developed, male and female worms pair and, in the cases of Schistosoma japonicum and Schistosoma mansoni, migrate to the mesenteric venous plexus, where the female worms start producing eggs. Adult worm pairs can survive for several years, producing hundreds of eggs per day, unless the infection is treated by chemotherapy. The specific locations of the different life stages of the schistosome lead to unique interactions with different parts of the host immune system (2, 7). At the molecular level, the expression of proteins and glycans is developmentally regulated and subject to constant change during schistosome development (8, 9). This specific antigen expression by different developmental stages could be a strategy to escape the host's immune system, but it may also lead to specific local protective immune responses of the host (10).
Schistosomula, the migratory larvae, are probably the most important targets of immunity. Effective elimination of these life stages would prevent the establishment of infection, thereby preventing egg-induced pathology and transmission of the disease. It has been shown in vitro that schistosomula can be targets of effective antibody-mediated immune responses and that secondary infection in the laboratory rat is a good model of this humoral immunity (11, 12). IgG1, IgG2, and IgG3 have been associated with killing of schistosomula through an antibody-dependent cellular cytotoxic process mediated by activated, as well as nonactivated, eosinophils (13). IgG2 has a dual function, as it has cytotoxic activity in the presence of activated eosinophils, while in the presence of nonactivated eosinophils it was able to block protective responses. IgG4 has no cytotoxic activity and can block the effects of IgG1, IgG2, and IgG3 (13). Furthermore, other studies have indicated a role for IgE and IgA in the elimination of schistosomula through similar mechanisms (14, 15).
Like all other schistosome life stages, schistosomula are abundantly glycosylated. Since schistosome glycans have repeatedly been described as major targets of antibody responses (16–18), they could play a role in the induction of immunity. Over the past several decades, glycans expressed during schistosome development have been characterized (9, 19–27). Overall, the glycans of schistosomula up to 3 days old were similar to those present in cercariae (9, 20–22, 24, 26, 27). N-glycans feature oligomannosyl structures, Galβ1-4GlcNAc (LacNAc) and Galβ1-4(Fucα1-3)GlcNAc (Lewis X, or LeX) antennae, and core xylosylation, while glycosphingolipid (GSL) glycans are mainly presented with terminal LeX, Fucα1-3Galβ1-4(Fucα1-3)GlcNAc (pseudo-Lewis Y, or pseudo-LeY), and multifucosylated GalNAcβ1-4GlcNAc (LacDiNAc, or LDN) motifs. O-glycans displaying partly similar motifs were abundantly present in cercariae, but they gradually disappeared in the developing schistosomula (9). In contrast, N-glycan expression remained during further development of schistosomula, although the N-glycan profile showed a gradual change toward oligomannosyl glycans and complex-type structures, which predominantly expressed LDN antennae without core xyloses, similar to the adult worm (9, 20–22, 24, 26, 27). The overall GSL glycan expression remained largely unchanged after transformation and early schistosomula development (9). Nonetheless, regardless of these overall expression patterns, clear changes in the surface-exposed glycan antigens of early schistosome larvae were observed in studies using antiglycan monoclonal antibodies and immunofluorescence microscopy. Mono- and multifucosylated LDN motifs, except LDN-F, present on O- or GSL glycans, were present at the surface before and after transformation, whereas glycans carrying LeX and LDN-F motifs appeared at the surface for the first time shortly after transformation (28). Such glycans exposed on the surfaces of the vulnerable schistosomula may form the targets of antibody-mediated immunity against infection with schistosomes.
To study local antibody responses to glycans of migrating schistosomula, we used the antibody-secreting cell (ASC) probe method in rats upon secondary infection with S. japonicum (29, 30). Rats are semipermissive hosts for schistosomes, as they become infected by cercariae but the parasite does not complete its life cycle. The initial course of infection is normal, but rats are capable of a self-cure mechanism resulting in a drop in parasite burden between 3 and 4 weeks after initial infection. Also, immunity to reinfection is developed, and reinfections are rapidly eliminated, mainly before or during passage through the lung vasculature. These protective responses are antibody mediated and resemble the type 2 response seen in humans (31, 32). Therefore, the rat is a good model to study protective immune responses. Using this method, it was shown previously that migrating S. japonicum schistosomula in rats induced antibodies in the lung to several high-molecular-weight protein antigens of larvae and worms, as well as antibodies to synthetic glycan elements, including GlcNAc, LeX, and LacNAc and fucosylated N-glycan core structures (10, 33). While the use of these nonspecific synthetic glycans provided insights into the potential importance of antiglycan antibodies, the naturally occurring glycans on the surfaces and in secretions of schistosomula are far more complex than the glycans previously studied and often carry glycan motifs unique to the schistosome. In the current study, therefore, we analyzed ASC probe samples derived from lungs of S. japonicum-infected rats using shotgun glycan microarrays of naturally occurring glycans isolated from several schistosome life stages. In addition, we included skin ASC probes to study antiglycan responses that are induced early upon transformation into schistosomula. We show that N-glycans expressing terminal GlcNAc, LDN, LeX, and LacNAc motifs and oligomannosyl glycans and GSL glycans expressing unique highly fucosylated GalNAcβ1-4(GlcNAc β1)n stretches were the major targets of antibodies raised in the skin of infected rats, whereas antibodies raised in the lung tissue were predominantly directed against N-glycans expressing nonfucosylated LDN and terminal GlcNAc motifs. These observations indicate which glycan structures might be involved in antibody-mediated protection against S. japonicum in rats.
MATERIALS AND METHODS
ASC probes.
ASC probes were produced as described previously (10). In short, a Chinese strain of Oncomelania hupensis snails (Anhui Province, People's Republic of China) was used to obtain S. japonicum cercariae, which were subsequently used for the infection of female Wistar rats. The rats were infected percutaneously with 125 (primary infection) and 350 (secondary infection, 6 weeks after primary infection) cercariae. Axillary and inguinal lymph nodes (LN) (skin LN) and mediastinal LN (lung LN) were isolated from an uninfected control group and from two groups of rats at 5 and 9 days after secondary infection, respectively. The timing of the isolation of LN corresponded to the expected peak in antibody production in the skin (5 days postinfection) and lung (9 days postinfection) LN (10). The LN were dissected, and cell suspensions were made and cultured at 37°C and 5% CO2 for 5 days. Supernatants containing antibodies secreted by in vivo-induced ASCs (ASC probes) were then collected and used for incubation on glycan microarrays.
Glycan microarray development.
In previous studies, the development of glycan arrays has been described extensively (34, 35). Briefly, N-glycans, O-glycans, and GSL glycans were obtained from cercariae, adult worms, and eggs from a Puerto Rican strain of S. mansoni. 2-Aminobenzoic acid (2-AA)-derivatized glycans were fractionated in two dimensions by hydrophilic interaction liquid chromatography (HILIC)- and reversed-phase liquid chromatography (RP-LC), and the collected glycan-containing fractions were subsequently printed in triplicate to epoxysilane-coated glass slides, together with synthetic glycoconjugates. Three individual arrays were printed to each glass slide, each array containing a total of 888 LC fractions (127 from cercarial N-glycans, 115 from adult worm N-glycans, 110 from egg N-glycans, 37 from cercarial GSL glycans, 66 from egg GSL glycans, 137 from cercarial O-glycans, 79 from adult worm O-glycans, 146 from egg O-glycans, and 71 from synthetic glycoconjugates and proteins).
Glycan microarray analysis.
Glycan microarray slides were covered with hand-cut silicone gaskets to create barriers between individual arrays. Prior to use, any remaining reactive epoxysilane groups were blocked for 60 min at room temperature with phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA) and 50 mM ethanolamine. Then, the slides were rinsed with PBS and incubated with ASC probe sample diluted 1:5 in PBS-0.01% Tween 20 with 1% BSA for 60 min at room temperature. The slides were subsequently rinsed with PBS-0.05% Tween 20 and PBS, followed by incubation for 30 min at room temperature with Alexa Fluor 555-labeled goat anti-rat IgG and Alexa Fluor 647-labeled goat anti-rat IgM, both diluted 1:1,000 in PBS-0.01% Tween 20 with 1% BSA. Then, the slides were flushed with PBS-0.05% Tween 20, PBS, and Milli-Q. After drying by centrifugation, the slides were stored in the dark before scanning. Scanning was performed using a G2565BA scanner (Agilent Technologies, Santa Clara, CA) at 10-μm resolution with 2 lasers (532 nm and 633 nm). The scanned images were analyzed with Genepix Pro 7.0 software. Spots were aligned and resized using round features and no composite pixel intensity (CPI) threshold. The median fluorescence intensities of each of the spots with background subtracted were then averaged for each glycan sample. The data sets were log2 transformed to remove the basic trends of variance.
Response patterns and statistics.
LC fractions showing significant differences in signal intensities for the different ASC probes were identified using analysis of variance (ANOVA) (P < 0.05) and were grouped based on response patterns using hierarchical-clustering analysis (HCA) (complete linkage clustering using Euclidean distance). Branches with approximately the same horizontal distribution and strongest/largest distance between clusters were used to classify the most discriminative clusters.
Mass spectrometry and structural assignments.
LC fractions were analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using Ultraflex II and Ultraflextreme mass spectrometers (Bruker Daltonics, Bremen, Germany) in negative-ion reflectron mode with 2,5-dihydroxybenzoic acid (DHB) (Bruker Daltonics) as the matrix. The detected masses were translated into putative glycan structures using Glycopeakfinder (http://glyco-peakfinder.org), the literature (9, 19–27, 36–41), and our own unpublished tandem-MS (MS-MS) data. The three most abundant signals in each mass spectrum were translated into glycan structures, unless the individual signals made up less than 10% of the total intensity of all glycan masses in the spectrum of a glycan sample.
RESULTS
To study the local antibody responses to schistosome glycans, we used ASC probes isolated from rats with a secondary S. japonicum infection and compared the binding of antibodies generated in skin and lung LN using shotgun glycan microarrays. These LN and the timing of their isolation at 5 (I5) and 9 (I9) days after secondary infection reflected the migratory pathway of invading schistosome larvae and corresponded to the expected peak in antibody production in the skin and lungs, respectively. Glycan LC fractions showing significant differences in antibody binding between controls (C), I5, and I9 were clustered according to the antibody response patterns raised against the antigenic glycan motifs present in these LC fractions in a specific LN over time. Glycan-binding patterns were defined for IgG, as well as IgM, in the skin and lung LN ASC probes.
Skin LN ASC probe IgG.
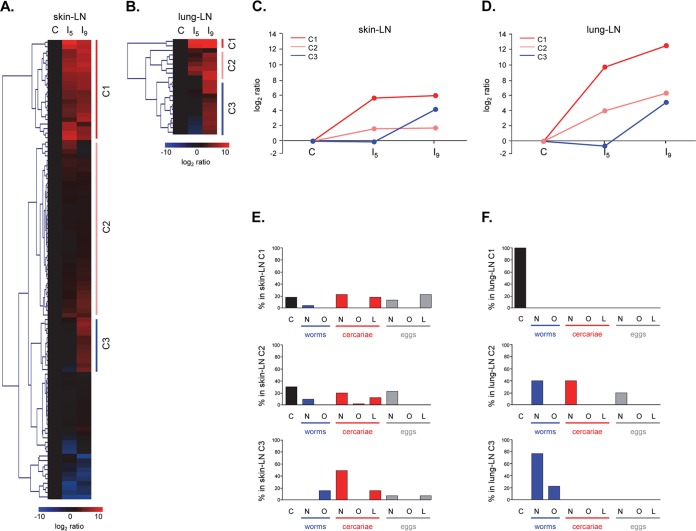
For the skin LN ASC probes, a total of 101 LC fractions, synthetic glycoconjugates, and glycoprotein mixtures/isolates showed differential IgG binding that could be grouped in three major glycan clusters (Fig. 1A) with different response patterns (Fig. 1C). Glycan cluster skin-IgG-C1 showed a rise in signal intensity for group I5 compared to the control rats, which remained equally high or slightly increased for group I9. A total of 18 LC fractions were found within this cluster and contained mainly N- and GSL glycans derived from cercariae and eggs (Fig. 1E). N-glycans present within these LC fractions showed numerous different terminal glycan motifs, including GlcNAc (egg N-glycan fractions 28 and 30), LDN (worm N-glycan fraction 16.4 and egg N-glycan fraction 28), LacNAc (cercarial N-glycan fraction 22, worm N-glycan fraction 16.4, and egg N-glycan fractions 28 and 30), and LeX (cercarial N-glycan fractions 26, 27, and 34 and worm N-glycan fraction 16.4). Also, mannosyl glycans were targeted (cercarial N-glycan fraction 22 and egg N-glycan fractions 14 and 30) (Table 1). Other N-glycan motifs recognized by antibodies in the skin LN ASC probes within this cluster were GalNAcβ1-4(Fucα1-3)GlcNAcβ1 (LDN-F) (worm N-glycan fraction 16.4) and F-GlcNAc (cercarial N-glycan fraction 34) (Table 1). Furthermore, numerous GSL glycan fractions present within glycan cluster skin-IgG-C1 were characterized by the presence of highly fucosylated GalNAcβ1-4(GlcNAc β1)n stretches (cercarial GSL glycan fractions 19, 21, and 25 and egg GSL glycan fractions 22.1, 23.1, 24.1, 25.2, and 26.1) (Table 1).
FIG 1.
(A and B) Hierarchical-clustering analysis of log2-transformed median fluorescence intensities with classification of the antiglycan IgG responses in skin LN (A) and lung LN (B) into three major clusters each. (C and D) Fluorescence intensity signals were averaged for each cluster and showed different response profiles for skin LN IgG (C) and for lung LN IgG (D). (E and F) Distribution of glycan origins within each skin LN (E) and lung LN (F) glycan cluster. C, (synthetic) glycoconjugates; N, N-glycan; O, O-glycan; L, GSL glycan.
TABLE 1.
Glycan fractions identified in the different clusters for IgG responses in the skin LN ASC probesa
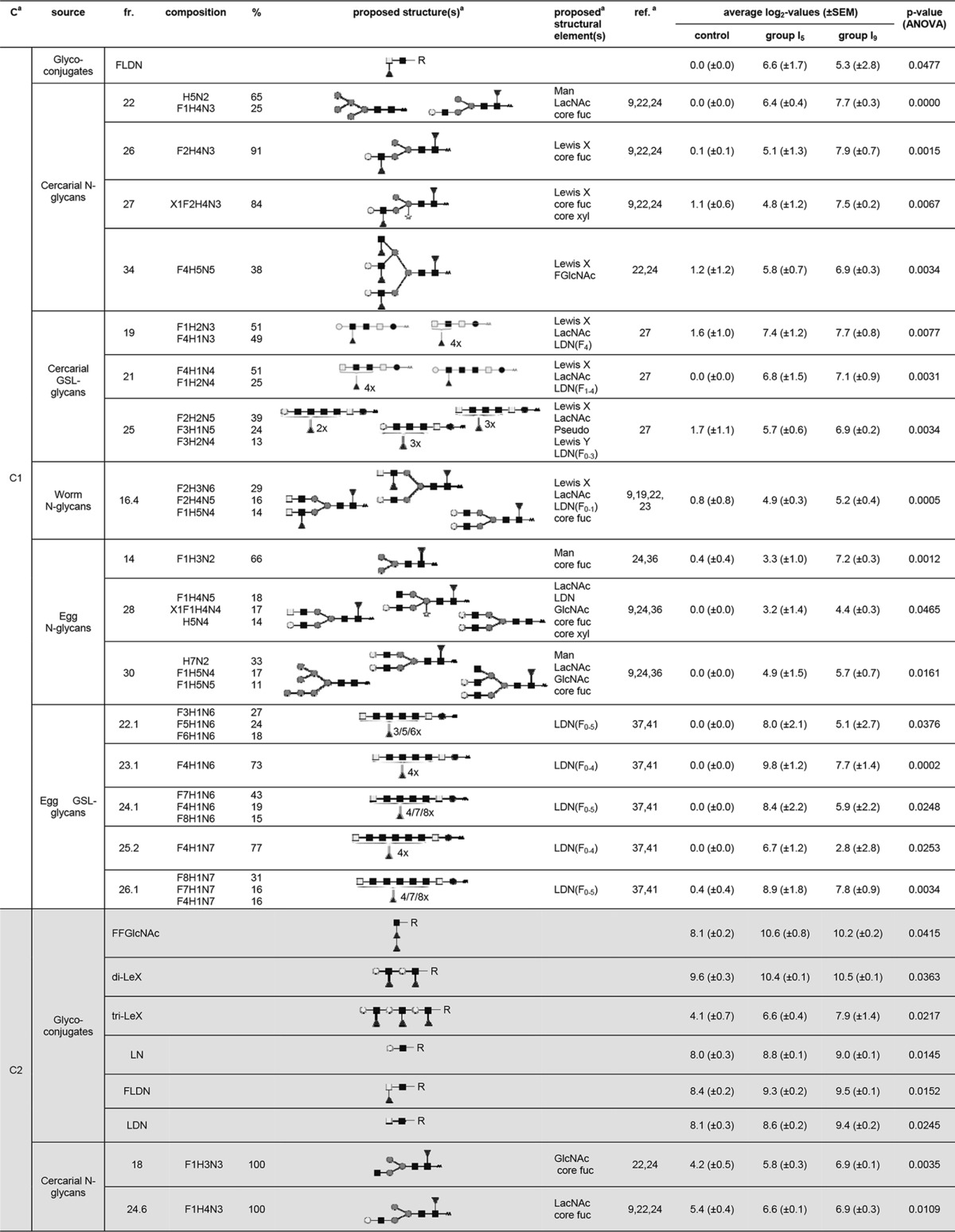
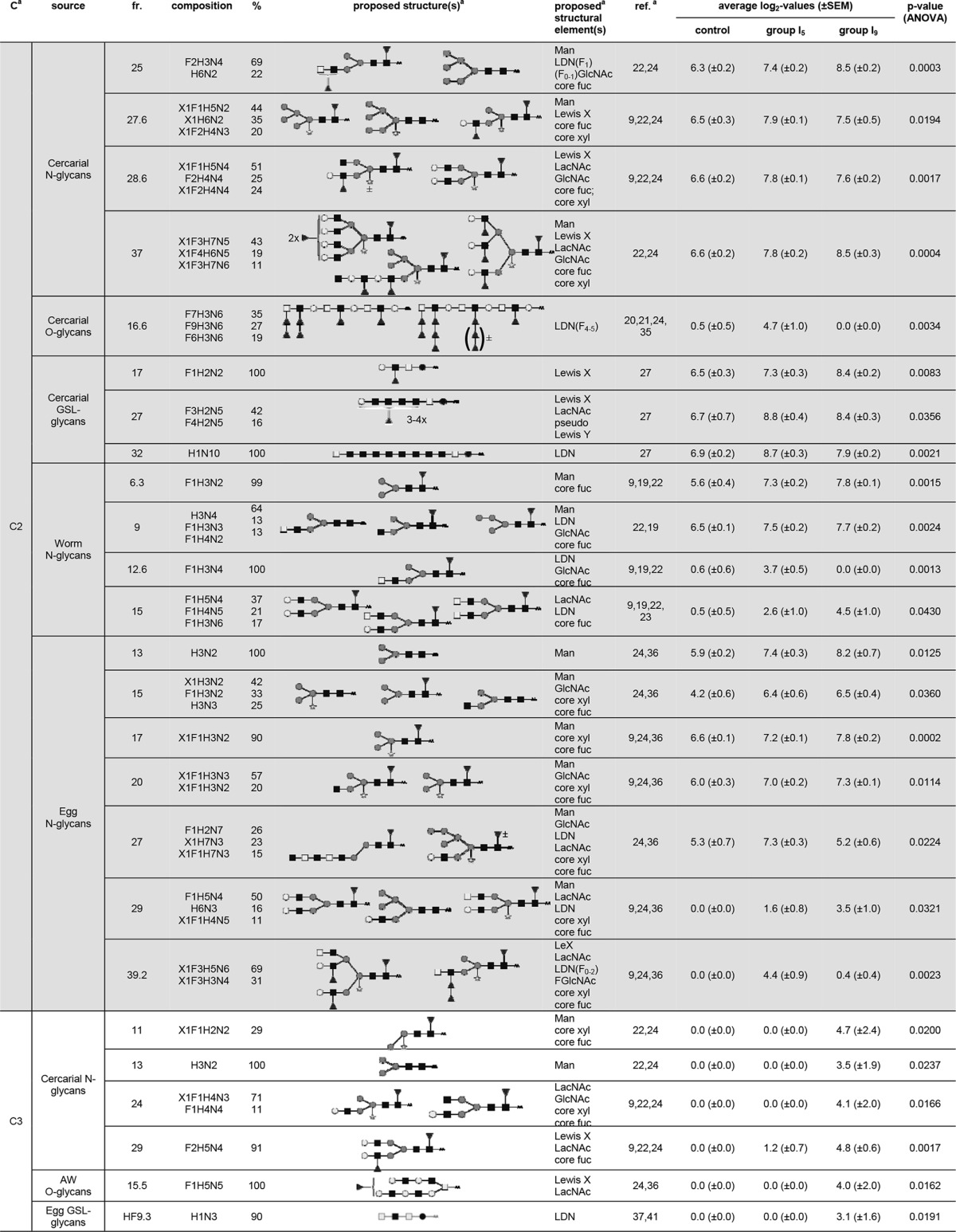
Putative structures and structural elements are proposed using literature-guided interpretations of the glycan compositions obtained by MALDI-TOF MS on the basis of the references listed. C, cluster; fr., fraction; ref., references; Man, terminal mannose(s); core fuc, core fucosylation; core xyl, core xylosylation; X, xylose; F, fucose; H, hexose; N, N-acetylhexosamine; dark square, N-acetylglucosamine (GlcNAc); light square, N-acetylgalactosamine; medium dark circle, mannose; dark circle, glucose; light circle, galactose; dark triangle, fucose; white star, xylose; AA, AA label; LacNAc, Galβ1-4GlcNAcβ1; LewisX, Galβ1-4(Fucα1-3)GlcNAcβ1; LDN, GalNAcβ1-4GlcNAcβ1.
Glycan cluster skin-IgG-C2 also showed a rise in antibody signal intensities for group I5 compared to the control rats, which remained equally high or slightly increased for group I9; however, signal intensities were lower than for cluster skin-IgG-C1 (Fig. 1C). Cluster skin-IgG-C2 contained 39 LC fractions, synthetic glycoconjugates, and glycoprotein mixtures/isolates, the majority of which (around 50%) were N-glycans derived from different life stages, including cercariae, worms, and eggs (Fig. 1E). This specific set of N-glycans was characterized by the presence of a wide variety of terminal motifs, which we observed for cluster skin-IgG-C1, as well. The most abundant terminal motifs within cluster skin-IgG-C2 were GlcNAc (cercarial N-glycan fractions 18, 28.6, and 37; worm N-glycan fraction 9; and egg N-glycan fractions 15, 20, and 27), LDN (worm N-glycan fractions 9, 12.6, and 15 and egg N-glycan fractions 29 and 39.2), LacNAc (cercarial N-glycan fractions 24.6, 28.6, and 37 and egg N-glycan fractions 27 and 29), LeX (cercarial N-glycan fractions 27.6, 28.6, and 37 and egg N-glycan fraction 39.2), and mannosyl (cercarial N-glycan fractions 25, 27.6, and 37; worm N-glycan fractions 6.3 and 9; and egg N-glycan fractions 13, 15, 17, 20, 27, and 29) residues (Table 1). Terminal motifs less frequently found within these N-glycans than in cluster skin-IgG-C1 included LDN-F (cercarial N-glycan fraction 25) and GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAβ1- (LDN-DF) (egg N-glycan fraction 39.2) (Table 1). In contrast to skin IgG cluster C1, GSL glycans bearing highly fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches were absent in cluster skin-IgG-C2. Other LC fractions found within skin IgG cluster C2 contained GSL glycans derived from cercariae, displaying either LDN (cercarial GSL glycan fraction 32), LeX (cercarial GSL glycan fraction 17), or pseudo-LeY (cercarial GSL glycan fraction 27) motifs (Table 1). One LC fraction with cercarial O-glycans presenting Fucα1-2Fucα1-3 (DF) motifs attached to terminal GlcNAc, as well as internal LDN motifs (cercarial O-glycan fraction 16.6), was also found within this cluster (Table 1). Binding of the ASC probe antibodies to synthetic glycoconjugates related to these motifs was in line with these observations (Table 1). Since the antibody responses within skin IgG clusters C1 and C2 were induced in I5 and sustained in I9, it is likely that the glycan structures identified within these clusters are associated with the migrating schistosomula or with schistosomulum- or cercaria-derived material that is left behind in the skin, e.g., secreted glycoproteins, fragments of the glycocalyx, or dying schistosomula.
Glycan cluster skin-IgG-C3 showed no change in signal intensity for group I5 compared to the control group, but signal intensities increased in group I9 (Fig. 1C). This cluster contained 12 LC fractions, half of which were cercaria-derived N-glycans (Fig. 1E). These N-glycans again contained terminal GlcNAc (cercarial N-glycan fraction 24), LacNAc (cercarial N-glycan fractions 24 and 29), and LeX (cercarial N-glycan fraction 29) motifs, as well as mannosyl termini (cercarial N-glycan fractions 11 and 13) (Table 1). Worm-derived O-glycans found within this cluster also contained LeX and LacNAc motifs (worm O-glycan fraction 15.5). Also, a specific GSL glycan sample (egg GSL glycan fraction HF 9.3) with terminal LDN was observed in this response pattern. Antibody responses to these glycan motifs in this specific context thus seem to be induced after the schistosomula have already left the skin tissue and migrated toward the lungs, suggesting that these antibodies are triggered by schistosomulum- or cercaria-derived material trapped in the skin.
Most notably, these different response patterns show that highly fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches on GSL glycans were identified only in cluster skin-IgG-C1, which already displayed the largest increases in IgG signal intensities in group I5. Furthermore, the IgG response patterns of the skin LN ASC probes recognized mostly N- and GSL glycans, whereas O-glycans were hardly recognized. Besides the highly fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches on GSL glycans, the N- and GSL glycans recognized in the different response patterns carry several other terminal motifs, including terminal mannose and terminal GlcNAc, LeX, LacNAc, and LDN motifs. These motifs could be found in all identified response patterns.
Lung LN ASC probe IgG.
For the lung LN ASC probes, a total of 20 LC fractions and glycoprotein isolates/mixtures showed differential IgG binding, for which we observed three clusters (Fig. 1B) with different response patterns (Fig. 1D). Cluster lung-IgG-C1, which showed a rise in antibody signal intensities for group I5 compared to the control rats and even higher signal intensities for group I9 (Fig. 1D), contained only two glycoprotein isolates/mixtures from Schistosoma, making it impossible to define which motifs are bound (Fig. 1F). Cluster lung-IgG-C2 showed a response pattern very similar to that of cluster lung IgG-C1, albeit with much lower antibody signal intensities for all groups (Fig. 1D). This cluster included 5 LC fractions that were all N-glycans derived from cercariae, adult worms, and eggs (Fig. 1F). Structures with terminal mannose residues were detected in most of the LC fractions of this cluster (worm N-glycan fractions 2.1 and 8.5 and egg N-glycan fraction 32.5) (Table 2). In addition, structures with LeX (cercarial N-glycan fractions 27 and 32.7), LDN-DF (egg N-glycan fraction 32.5), and terminal GlcNAc (cercarial N-glycan fraction 32.7) were observed, as well (Table 2). It is worth noting that these structures thus already give rise to antibodies in the lung LN ASC probes very early upon contact with migrating schistosomula, possibly because the time the migrating schistosomula reside in the skin varies and some schistosomula might have already migrated toward the lung tissue, where they could induce immunological reactions (42, 43).
TABLE 2.
Glycan fractions identified in the different clusters for the IgG responses in the lung LN ASC probesa
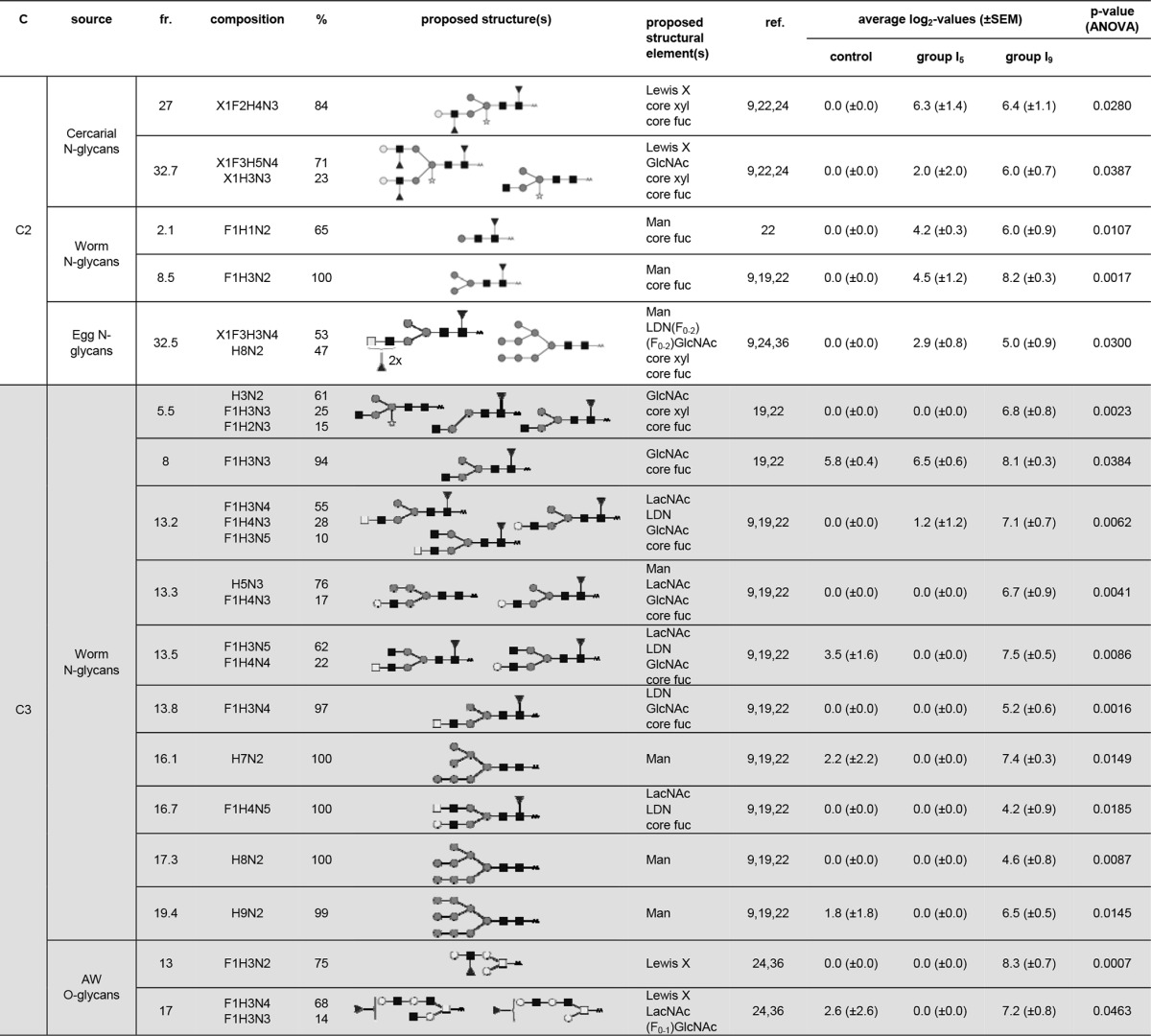
Putative structures and structural elements are proposed using literature-guided interpretations of the glycan compositions obtained by MALDI-TOF MS on the basis of the references listed. C, cluster; fr., fraction; ref., references; Man, terminal mannose(s); core fuc, core fucosylation; core xyl, core xylosylation; X, xylose; F, fucose; H, hexose; N, N-acetylhexosamine; dark square, N-acetylglucosamine (GlcNAc); light square, N-acetylgalactosamine; medium dark circle, mannose; dark circle, glucose; light circle, galactose; dark triangle, fucose; white star, xylose; AA, AA label; LacNAc, Galβ1-4GlcNAcβ1; LewisX, Galβ1-4(Fucα1-3)GlcNAcβ1; LDN, GalNAcβ1-4GlcNAcβ1.
Cluster lung-IgG-C3 showed no change in signal intensity for group I5 compared to the control group, but signal intensities increased in group I9 (Fig. 1D), corresponding to the expected peak in antibody production after the schistosomula reached the lungs. This cluster contains 13 LC fractions, containing exclusively N- and O-glycans derived from adult worms (Fig. 1F). Most of the LC fractions detected in cluster lung-IgG-C3 contained N-glycan structures with GlcNAc and LDN motifs (worm N-glycan fractions 5.5, 8, 13.2, 13.3, 13.5, 13.8, and 16.7) (Table 2). A single O-glycan structure presented terminal GlcNAc, as well (worm O-glycan fraction 17) (Table 2). In contrast to the responses raised in the skin, fucosylated variants of these motifs were not seen in the response pattern. In addition to the LDN and GlcNAc motifs, structures with terminal mannose residues were detected in multiple LC fractions (worm N-glycan fractions 13.3, 16.1, 17.3, and 19.4), while other structures displayed terminal LacNAc (worm N-glycan fractions 13.2, 13.3, 13.5, and 16.7) and LeX (worm O-glycan fractions 13 and 17) motifs.
Strikingly, multifucosylated GSL glycan structures, abundantly expressed at the surfaces of lung stage schistosomula (28), were not associated with any of the lung IgG response patterns. The clearest targets of the IgG in the lung LN ASC probes are N-glycans with nonfucosylated LDN and GlcNAc motifs. In addition, terminal mannose residues and LacNAc and LeX motifs on N- and O-glycans are targets for IgG in the lung, as well.
Skin LN ASC probe IgM.
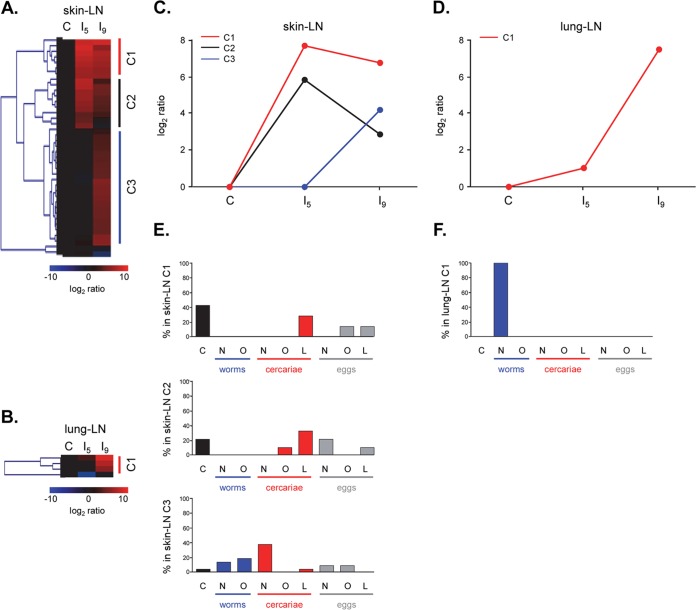
A total of 39 LC fractions, synthetic glycoconjugates, and glycoprotein mixtures/isolates showed differential IgM binding for the skin LN ASC probes, for which we observed three clusters (Fig. 2A) with different response patterns (Fig. 2C). Cluster skin-IgM-C1 showed an increase in antibody signal intensities in group I5 compared to the control rats, which only slightly decreased in group I9 (Fig. 2C). The 4 LC fractions that were included in the cluster consisted of mostly GSL glycans from eggs and cercariae (3 LC fractions), whereas 3 synthetic glycoconjugates were included in the cluster, as well (Fig. 2E). The GSL glycans all carried fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches (cercarial GSL glycan fractions 18 and 19 and egg GSL glycan fraction 22.1) (Table 3). Furthermore, one of the synthetic glycoconjugates found in the cluster was the LDN-F conjugate (Table 3). Another terminal motif frequently observed in LC fractions within cluster skin-IgM-C1 was the LeX motif, as part of cercaria-derived GSL glycans and egg-derived O-glycans (cercarial GSL glycan fraction 19 and egg O-glycan fraction 36.2) and as a synthetic glycoconjugate (Table 3). Finally, GlcNAc- and F-GlcNAc motifs were seen as part of egg-derived O-glycans (egg O-glycan fraction 36.2) (Table 3).
FIG 2.
(A and B) Hierarchical-clustering analysis of log2-transformed median fluorescence intensities with background subtracted, classifying the antiglycan IgM responses in skin LN (A) and lung LN (B) into three and one major cluster(s), respectively. (C and D) Fluorescence intensity signals were averaged for each cluster and showed different response profiles for skin LN IgM (C) and for lung LN IgM (D). (E and F) Distribution of glycan origins within each skin LN (E) and lung LN (F) glycan cluster. C, (synthetic) glycoconjugates; N, N-glycan; O, O-glycan; L, GSL glycan.
TABLE 3.
Glycan fractions identified in the different clusters for IgM responses in the skin LN ASC probesa
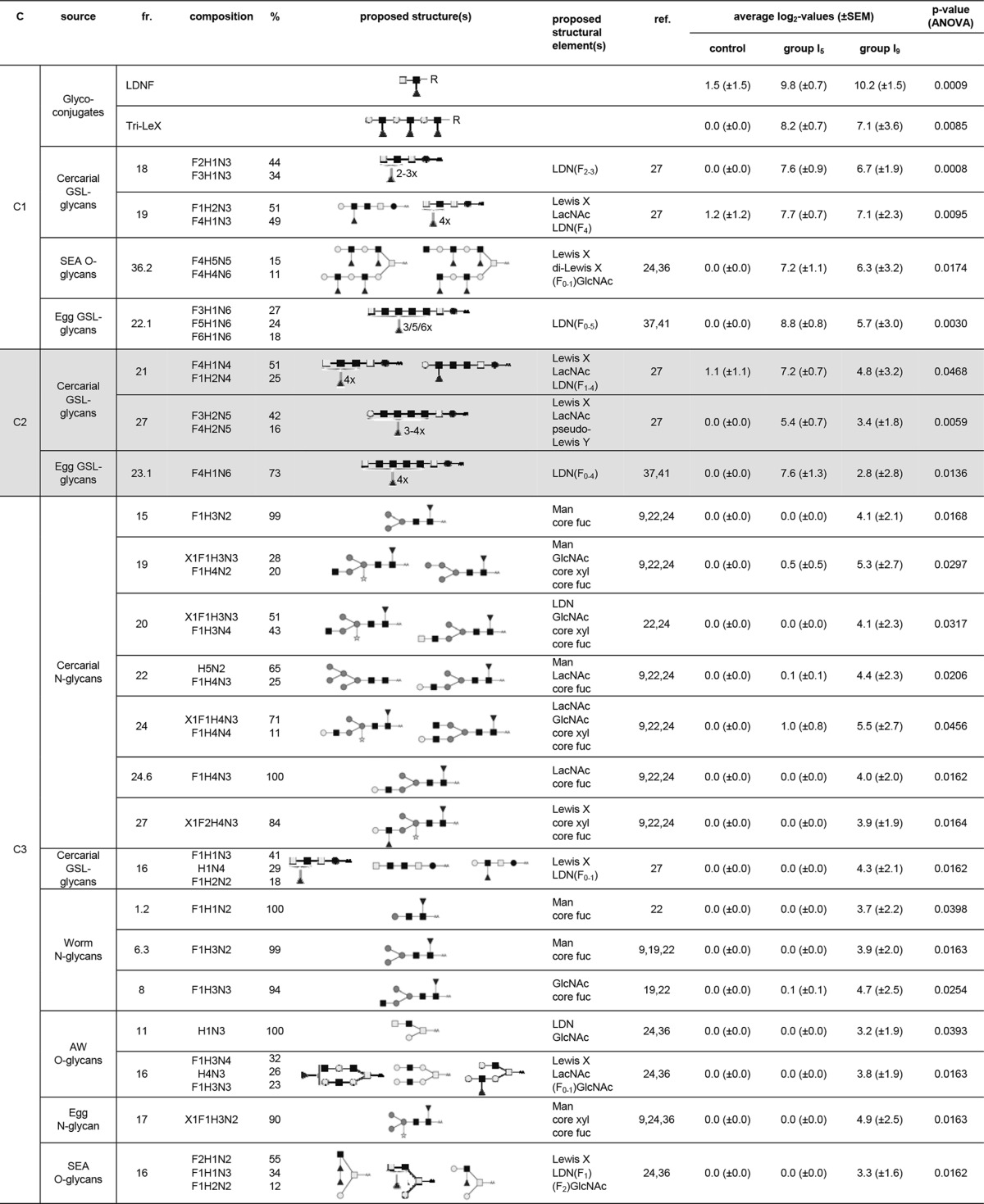
Putative structures and structural elements are proposed using literature-guided interpretations of the glycan compositions obtained by MALDI-TOF MS on the basis of the references listed. C, cluster; fr., fraction; ref., references; Man, terminal mannose(s); core fuc, core fucosylation; core xyl, core xylosylation; X, xylose; F, fucose; H, hexose; N, N-acetylhexosamine; dark square, N-acetylglucosamine (GlcNAc); light square, N-acetylgalactosamine; medium dark circle, mannose; dark circle, glucose; light circle, galactose; dark triangle, fucose; white star, xylose; AA, AA label; LacNAc, Galβ1-4GlcNAcβ1; LewisX, Galβ1-4(Fucα1-3)GlcNAcβ1; LDN, GalNAcβ1-4GlcNAcβ1.
A total of 9 LC fractions and glycoprotein mixtures/isolates were found in cluster skin-IgM-C2, which showed enhanced antibody signal intensities in group I5 compared to the control rats. However, in group I9, signal intensities decreased to intermediate levels compared to group I5, although they were still enhanced compared to the control rats (Fig. 2C). GSL glycans derived from eggs and cercariae predominated in this cluster (Fig. 2E) and contained structures with DF-LDN-DF (cercarial GSL glycan fraction 21 and egg GSL glycan fraction 23.1), LeX (cercarial GSL glycan fraction 21), or pseudo-LeY (cercarial GSL glycan fraction 27) motifs. Because the responses observed in skin IgM clusters C1 and C2 showed the highest intensities in group I5 and again decreased in group I9, these responses nicely reflect the migratory pathway of the schistosomula.
Cluster skin-IgM-C3 did not show changes in signal intensity for group I5 compared to the control rats, but signal intensities increased in group I9 (Fig. 2C). This cluster contained 20 LC fractions, approximately 38% of which were cercarial N-glycans. N- and O-glycans of both adult worms and eggs were also found within the cluster (Fig. 2E). The glycan structures present in cluster skin-IgM-C3 showed numerous different terminal glycan motifs on different types of glycans (Table 3). Among the most prominently represented glycan motifs were terminal mannose residues (cercarial N-glycan fractions 15, 19, and 22; worm N-glycan fractions 1.2 and 6.3; and egg N-glycan fraction 17) and LacNAc (cercarial N-glycan fractions 22, 24, and 24.6 and worm O-glycan fraction 16) and LeX (cercarial N-glycan fraction 27, cercarial GSL glycan fraction 16, worm O-glycan fraction 16, and egg O-glycan fraction 16) termini (Table 3). Also terminal GlcNAc (cercarial N-glycan fractions 19, 20, and 24; worm N-glycan fraction 8; and worm O-glycan fraction 16) and LDN (cercarial N-glycan fraction 20, cercarial GSL glycan fraction 16, and worm O-glycan fraction 16) motifs were observed, some of which were more abundantly fucosylated (cercarial GSL glycan fraction 16 and egg O-glycan fraction 16) (Table 3). These glycan structures thus show great similarity (but are not completely identical) to the terminal motifs observed in skin IgG response clusters C1 and C2 (Table 1); however, the IgM antibodies recognizing these motifs are induced at a later stage (Fig. 1C and 2C), when the schistosomula have already migrated out of the skin.
Taken together, the IgM responses of the skin LN ASC probes thus target fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches on GSL glycans 5 days after secondary infection, at the expected peak in antibody production, possibly together with specific O- and GSL glycans expressing LeX and (fucosylated) GlcNAc motifs. IgM responses targeting terminal mannose residues and LeX and LacNAc motifs were observed only after the schistosomula had already migrated out of the skin.
Lung LN ASC probe IgM.
Only 4 LC fractions were differentially recognized by the IgM responses of the lung LN ASC probes. One cluster of 3 LC fractions was observed (Fig. 2B). The IgM antibodies within this lung-IgM-C1 cluster gave only slightly increased signal intensities in group I5 compared to the control group, whereas for group I9, signal intensities were far higher than in the control group (Fig. 2D). All LC fractions found in the cluster were composed of N-glycans derived from adult worms (Fig. 2F). Most of the glycan structures within these LC fractions presented terminal LDN motifs (worm N-glycan fractions 9.2 and 10), but LacNAc motifs (worm N-glycan fraction 9.2) and terminal mannose residues (worm N-glycan fraction 8.5) were detected, as well (Table 4). These observations further underline the fact that N-glycans with LDN and GN motifs that are expressed by the schistosomula can be targets for antibody responses when the schistosomula pass through the lungs.
TABLE 4.
Glycan fractions identified in cluster C1 for IgM responses in the lung LN ASC probesa
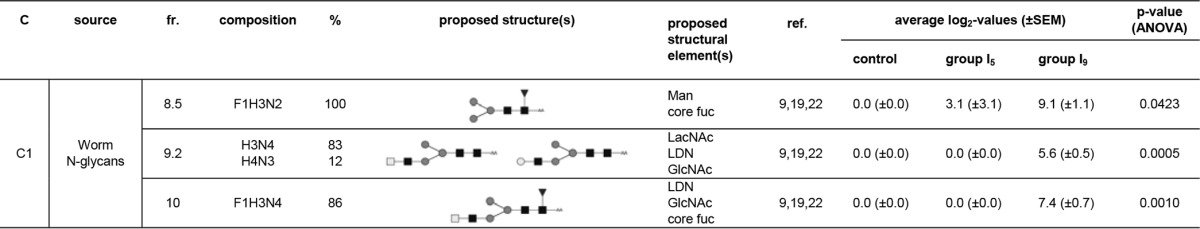
Putative structures and structural elements are proposed using literature-guided interpretations of the glycan compositions obtained by MALDI-TOF MS on the basis of the references listed. C, cluster; fr., fraction; ref., references; Man, terminal mannose(s); core fuc, core fucosylation; core xyl, core xylosylation; X, xylose; F, fucose; H, hexose; N, N-acetylhexosamine; dark square, N-acetylglucosamine (GlcNAc); light square, N-acetylgalactosamine; medium dark circle, mannose; dark circle, glucose; light circle, galactose; dark triangle, fucose; white star, xylose; AA, AA label; LacNAc, Galβ1-4GlcNAcβ1; LewisX, Galβ1-4(Fucα1-3)GlcNAcβ1; LDN, GalNAcβ14GlcNAcβ1.
DISCUSSION
Our data show that antiglycan antibody responses in skin and lung LN ASC probes of S. japonicum-infected rats peaked at the time points (5 days and 9 days, respectively) reflecting the migration of the invading larvae. Using an elaborate microarray constructed of naturally occurring glycans isolated from schistosomes, we were able to confirm that in the lung LN ASC probes, antibody responses were mainly directed against N-glycans with terminal GlcNAc, LeX, and LacNAc motifs and terminal mannose residues, as was shown by McWilliam et al. using a glycan array with synthetic glycan structures (10). In addition, we found that LDN motifs present on N-glycans were also recognized by IgG and IgM produced by lung LN ASC probes. Furthermore, by defining the different response patterns, we revealed that antibodies to N-glycans with LDN motifs and terminal GlcNAc and mannose residues were detected in the lung LN ASC probes in significant amounts only at 9 days postinfection, corresponding to the passage of schistosomula through the lung vasculature around that time point. In addition to the antiglycan responses in the lung, in this study, we also addressed the glycan structures recognized by responses in the skin LN ASC probes. For these ASC probes, we showed that antibody responses were mostly targeted at N-glycans with terminal mannose residues and LacNAc, LeX, GlcNAc, and LDN motifs and at GSL glycans with fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches. Antibody levels either peaked at 5 days postinfection and were sustained or were induced in later infection stages. Responses to the fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches present on GSL glycans were exclusively found among those clusters showing sustained high antibody binding levels up to 9 days postinfection. The sustained binding of antibodies to all of these different glycans in the skin LN ASC probes might indicate that at least some of the glycans can still be found in the skin after the schistosomula have already migrated toward the lungs.
Interestingly, antibodies produced by the skin LN ASC probes bound only to the surfaces of cercariae and not to 24-h schistosomula (10). A possible explanation would be the relatively fast migration through the skin and subsequently to the lungs by S. japonicum, in combination with a change in surface-exposed antigens after transformation, thereby preventing the development of antibodies to schistosomula (43). Cercarial secretions and parts of the cercarial glycocalyx remain in the skin for a longer time, allowing the immune system to develop local antibody responses to antigenic motifs present in these schistosome-derived products. Cercarial secretions contain many LacNAc and LeX elements in both the N- and O-glycans (24). In the glycan microarray analysis, we observed a reaction against these types of glycans by skin LN ASC probes. In particular, the clusters skin-IgG-C1, skin-IgG-C2, and skin-IgM-C1 showed prolonged antibody response profiles that might be associated with the presence of secretions or material shed into the skin by penetrating cercariae or developing schistosomula, rather than with surface-exposed structures. For S. mansoni, it was shown that skin stage schistosomula still express glycocalyx-related antigens and leave traces in the skin upon further migration to the lungs (43–46) and that O-glycans expressed by the glycocalyx are quite abundant and antigenic (20, 35). However, we observed no significant binding of IgG or IgM from skin ASC probes to the glycocalyx-associated O-glycans that were on the glycan microarray. This might suggest that the expression level of these O-glycan types is absent or too low to mount an antibody response or that antibody responses are induced at a later time point. Alternatively, differences in the O-glycan structures between S. japonicum and S. mansoni might be a reason for the absence of responses to O-glycans on the microarray, since we studied the antiglycan responses raised to S. japonicum by making use of a shotgun glycan microarray containing glycans derived from S. mansoni. Glycosylations in both species are highly similar based on the limited literature available (22, 36, 37). The few differences that have been identified are related to glycan structures that are lacking in S. japonicum compared to S. mansoni (36, 37). Given this high resemblance and in view of the big investment needed to make an S. japonicum-specific array to add perhaps just a few (if any) S. japonicum-specific glycans, we decided to use the previously generated S. mansoni glycan microarray to study antiglycan antibodies induced in S. japonicum-infected rats. However, some differences that have to be considered when interpreting our array data have been described, as well. First, core xylosylation is absent from the N-glycans of S. japonicum cercariae, making it likely that the same will be true for schistosomula (22). Antibodies to the xylosylated N-glycans, as observed in sera of schistosome-infected humans and animals, would logically be absent in the ASC probes studied. Furthermore, multifucosylated terminal structures expressed in all life stages of S. mansoni (9, 20, 21, 24, 25, 36, 41) and highly antigenic in S. mansoni infection (47–49) were not detected on protein- or lipid-linked glycans from eggs of S. japonicum (36, 37). Whether such multifucosylated terminal motifs, including DF motifs, are present or absent in other life stages of S. japonicum needs further research. If DF elements are not expressed by S. japonicum larvae, this would be a possible explanation for the relatively low number of multifucosylated O-glycan structures bound by the skin LN ASC probes. However, some of the GSL glycans that contained many fucoses were bound by IgM and IgG in skin LN ASC probes, suggesting that S. japonicum cercariae and/or schistosomula do express this type of GSL glycan structure or similar terminal glycan motifs. Besides differences in expression profiles, the localization of these glycans might also be different across different species, indicating that it would be worthwhile to investigate the structures and localization of glycans expressed by S. japonicum schistosomula and other life stages.
In the lung LN ASC probes, we observed intense antibody binding to LDN and GlcNAc motifs. LDN motifs appear to be surface expressed throughout worm development (19, 49), but LDN expression becomes more abundant on N-glycans when the worms mature, while LDN expression by other types of glycans, especially in later stages of development, including lung stage schistosomula, is hardly present (9). Together with the previously observed binding of lung LN ASC probes to the surface of the developing schistosomula, this indicates that these antibodies were raised against surface-exposed antigens, including N-glycans with LDN motifs (10). Responses to N-glycans displaying LacNAc and LeX motifs were also observed for the lung LN ASC probes. For S. mansoni, it has been shown that these motifs are still present in schistosomula up to several days after transformation, especially in the N-glycan pool (9), and LeX motifs were also shown to be surface exposed on schistosomula (28). Given the high resemblance of cercarial N-glycans (22) and the expression and antigenicity of LeX (50) between S. mansoni and S. japonicum, it is likely that schistosomula of S. japonicum also surface expose these motifs.
Strikingly, in the lung LN ASC probes, we observed no significant IgG or IgM reaction to the GSL glycans, whereas these glycans with their highly fucosylated GalNAcβ1-4(GlcNAcβ1)n stretches were bound by antibodies present in the skin LN ASC probes. For S. mansoni, it has been shown previously that multifucosylated GSL glycans remained expressed in later stages of schistosomula development (9) and are surface exposed (28), and they have been shown to be major targets of antibody response in sera of infected individuals (47–49, 51). The reason we did not observe significant binding for lung LN ASC probes might be the differences in the expression of these glycans between S. mansoni and S. japonicum. Since S. japonicum does not express multifucosylated motifs in the egg stages (36, 37), it is possible that multifucosylated motifs are shed by S. japonicum cercariae or schistosomula in the skin and are not further expressed in subsequent stages. There might be a gradual change during maturation, so that multifucosylated LDN motifs have already been replaced by other (less antigenic) structures when the schistosomula reach the lungs. For S. mansoni, it was determined that protein-linked multifucosylated glycans disappear from the schistosomula glycome after 3 days, whereas GSL-linked DF elements remain in the overall spectrum, as well as exposed at the surface (9, 28). If these expression patterns are similar for S. japonicum, an alternative explanation for the absence of reactivity in the lung could be the differential expression on protein and lipid carriers. Little is known about the mechanisms by which antibodies to protein-linked glycans are generated, and knowledge about how anti-GSL glycan antibodies are induced is even more negligible.
Through the combined use of ASC probes and shotgun glycan microarrays, we identified the antiglycan responses raised against migrating schistosomula in rats. These local recognition profiles are broad, given the variety of glycans expressed by Schistosoma (9). Our data indicate that N-glycans with terminal mannose residues, LacNAc motifs, and LeX motifs, abundantly present in secretions of cercariae and schistosomula, together with GSL glycans containing fucosylated LDN and GlcNAc motifs, were major targets of antibody responses raised in the skin LN ASC probes. Antibodies raised in the lung LN ASC probes were mainly directed toward N-glycans presenting LDN and GlcNAc motifs, most likely present at the surfaces of the migrating schistosomula. Since antibody-dependent mechanisms primarily mediate resistance to infection in rats (12), each of the glycans recognized by these local immune responses possibly plays a role in elimination of the parasite. Given the breadth of the recognition profile, it is impossible to define individual glycan structures that might be involved in protection. Antibodies raised against glycans are specific for certain glycan elements but might not be functional in inducing protection. In addition, some of the glycan structures and glycan elements recognized might also be expressed by the host and are therefore not considered good vaccine candidates. Since the expression of glycans and antibodies raised against these glycans is so broad, it might also be worthwhile testing whether this polyvalent expression plays a role in generating protective immune responses. Future studies are therefore needed to further investigate the specific roles of antigenic glycans and glycan elements in the parasite-host interaction and to explore the possible role of antibodies against parasite glycan motifs in antibody-mediated protection against S. japonicum infection.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B, Polman K, Clerinx J, Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, et al. 2012. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManus DP, Loukas A. 2008. Current status of vaccines for schistosomiasis. Clin Microbiol Rev 21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Diepen A, van der Velden NS, Smit CH, Meevissen MH, Hokke CH. 2012. Parasite glycans and antibody-mediated immune responses in schistosoma infection. Parasitology 139:1219–1230. doi: 10.1017/S0031182012000273. [DOI] [PubMed] [Google Scholar]
- 6.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. 2010. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol 8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 7.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick JM, Peak E, Perally S, Chalmers IW, Barrett J, Yoshino TP, Ivens AC, Hoffmann KF. 2009. Anti-schistosomal intervention targets identified by lifecycle transcriptomic analyses. PLoS Negl Trop Dis 3:e543. doi: 10.1371/journal.pntd.0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smit CH, van Diepen A, Nguyen DL, Wuhrer M, Hoffmann KF, Deelder AM, Hokke CH. 2015. Glycomic analysis of life stages of the human parasite Schistosoma mansoni reveals developmental expression profiles of functional and antigenic glycan motifs. Mol Cell Proteomics 14:1750–1769. doi: 10.1074/mcp.M115.048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McWilliam HE, Driguez P, Piedrafita D, Maupin KA, Haab BB, McManus DP, Meeusen EN. 2013. The developing schistosome worms elicit distinct immune responses in different tissue regions. Immunol Cell Biol 91:477–485. doi: 10.1038/icb.2013.33. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth AE, Hagan P. 1987. Immunity in human schistosomiasis. Parasitol Today 3:11–16. doi: 10.1016/0169-4758(87)90091-3. [DOI] [PubMed] [Google Scholar]
- 12.Capron A, Dessaint JP, Capron M, Ouma JH, Butterworth AE. 1987. Immunity to schistosomes: progress toward vaccine. Science 238:1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- 13.Khalife J, Dunne DW, Richardson BA, Mazza G, Thorne KJ, Capron A, Butterworth AE. 1989. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol 142:4422–4427. [PubMed] [Google Scholar]
- 14.Dunne DW, Richardson BA, Jones FM, Clark M, Thorne KJ, Butterworth AE. 1993. The use of mouse/human chimaeric antibodies to investigate the roles of different antibody isotypes, including IgA2, in the killing of Schistosoma mansoni schistosomula by eosinophils. Parasite Immunol 15:181–185. doi: 10.1111/j.1365-3024.1993.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 15.Capron M, Rousseaux J, Mazingue C, Bazin H, Capron A. 1978. Rat mast cell-eosinophil interaction in antibody-dependent eosinophil cytotoxicity to Schistosoma mansoni schistosomula. J Immunol 121:2518–2525. [PubMed] [Google Scholar]
- 16.Nash TE. 1978. Antibody response to a polysaccharide antigen present in the schistosome gut. I. Sensitivity and specificity. Am J Trop Med Hyg 27:939–943. [DOI] [PubMed] [Google Scholar]
- 17.Omer-Ali P, Magee AI, Kelly C, Simpson AJ. 1986. A major role for carbohydrate epitopes preferentially recognized by chronically infected mice in the determination of Schistosoma mansoni schistosomulum surface antigenicity. J Immunol 137:3601–3607. [PubMed] [Google Scholar]
- 18.Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW, Coulson PS, Wilson RA. 2001. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Infect Dis 183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- 19.Wuhrer M, Koeleman CA, Fitzpatrick JM, Hoffmann KF, Deelder AM, Hokke CH. 2006. Gender-specific expression of complex-type N-glycans in schistosomes. Glycobiology 16:991–1006. doi: 10.1093/glycob/cwl020. [DOI] [PubMed] [Google Scholar]
- 20.Khoo KH, Sarda S, Xu X, Caulfield JP, McNeil MR, Homans SW, Morris HR, Dell A. 1995. A unique multifucosylated -3GalNAc beta 1→4GlcNAc beta 1→3Gal alpha 1- motif constitutes the repeating unit of the complex O-glycans derived from the cercarial glycocalyx of Schistosoma mansoni. J Biol Chem 270:17114–17123. doi: 10.1074/jbc.270.29.17114. [DOI] [PubMed] [Google Scholar]
- 21.Huang HH, Tsai PL, Khoo KH. 2001. Selective expression of different fucosylated epitopes on two distinct sets of Schistosoma mansoni cercarial O-glycans: identification of a novel core type and Lewis X structure. Glycobiology 11:395–406. doi: 10.1093/glycob/11.5.395. [DOI] [PubMed] [Google Scholar]
- 22.Khoo KH, Huang HH, Lee KM. 2001. Characteristic structural features of schistosome cercarial N-glycans: expression of Lewis X and core xylosylation. Glycobiology 11:149–163. doi: 10.1093/glycob/11.2.149. [DOI] [PubMed] [Google Scholar]
- 23.Wuhrer M, Koeleman CA, Deelder AM, Hokke CH. 2006. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J 273:347–361. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 24.Jang-Lee J, Curwen RS, Ashton PD, Tissot B, Mathieson W, Panico M, Dell A, Wilson RA, Haslam SM. 2007. Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics 6:1485–1499. doi: 10.1074/mcp.M700004-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Frank S, van Die I, Geyer R. 2012. Structural characterization of Schistosoma mansoni adult worm glycosphingolipids reveals pronounced differences with those of cercariae. Glycobiology 22:676–695. doi: 10.1093/glycob/cws004. [DOI] [PubMed] [Google Scholar]
- 26.Hokke CH, Deelder AM, Hoffmann KF, Wuhrer M. 2007. Glycomics-driven discoveries in schistosome research. Exp Parasitol 117:275–283. doi: 10.1016/j.exppara.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Wuhrer M, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. 2000. Schistosoma mansoni cercarial glycolipids are dominated by Lewis X and pseudo-Lewis Y structures. Glycobiology 10:89–101. doi: 10.1093/glycob/10.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Smit CH, Homann A, van Hensbergen VP, Schramm G, Haas H, van Diepen A, Hokke CH. 2015. Surface expression patterns of defined glycan antigens change during S. mansoni cercarial transformation and development of schistosomula. Glycobiology 7:cwv066. [DOI] [PubMed] [Google Scholar]
- 29.Meeusen EN, Brandon M. 1994. The use of antibody-secreting cell probes to reveal tissue-restricted immune responses during infection. Eur J Immunol 24:469–474. doi: 10.1002/eji.1830240231. [DOI] [PubMed] [Google Scholar]
- 30.Meeusen EN, Brandon MR. 1994. Antibody secreting cells as specific probes for antigen identification. J Immunol Methods 172:71–76. doi: 10.1016/0022-1759(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 31.Capron M, Capron A. 1986. Rats, mice and men—models for immune effector mechanisms against schistosomiasis. Parasitol Today 2:69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- 32.Khalife J, Cêtre C, Pierrot C, Capron M. 2000. Mechanisms of resistance to S. mansoni infection: the rat model. Parasitol Int 49:339–345. [DOI] [PubMed] [Google Scholar]
- 33.McWilliam HE, Piedrafita D, Li Y, Zheng M, He Y, Yu X, McManus DP, Meeusen EN. 2013. Local immune responses of the Chinese water buffalo, Bubalus bubalis, against Schistosoma japonicum larvae: crucial insights for vaccine design. PLoS Negl Trop Dis 7:e2460. doi: 10.1371/journal.pntd.0002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Diepen A, Smit CH, van Egmond L, Kabatereine NB, Pinot de Moira A, Dunne DW, Hokke CH. 2012. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Negl Trop Dis 6:e1922. doi: 10.1371/journal.pntd.0001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Diepen A, van der Plas AJ, Kozak RP, Royle L, Dunne DW, Hokke CH. 2015. Development of a Schistosoma mansoni shotgun O-glycan microarray and application to the discovery of new antigenic schistosome glycan motifs. Int J Parasitol 45:465–475. doi: 10.1016/j.ijpara.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Khoo KH, Chatterjee D, Caulfield JP, Morris HR, Dell A. 1997. Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: identification of novel core structures and terminal sequences. Glycobiology 7:663–677. doi: 10.1093/glycob/7.5.663. [DOI] [PubMed] [Google Scholar]
- 37.Khoo KH, Chatterjee D, Caulfield JP, Morris HR, Dell A. 1997. Structural characterization of glycosphingolipids from the eggs of Schistosoma mansoni and Schistosoma japonicum. Glycobiology 7:653–661. doi: 10.1093/glycob/7.5.653. [DOI] [PubMed] [Google Scholar]
- 38.Meevissen MH, Balog CI, Koeleman CA, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Wuhrer M, Hokke CH. 2011. Targeted glycoproteomic analysis reveals that kappa-5 is a major, uniquely glycosylated component of Schistosoma mansoni egg antigens. Mol Cell Proteomics 10:M110. doi: 10.1074/mcp.M110.005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meevissen MH, Wuhrer M, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Hokke CH. 2010. Structural characterization of glycans on omega-1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J Proteome Res 9:2630–2642. doi: 10.1021/pr100081c. [DOI] [PubMed] [Google Scholar]
- 40.Wuhrer M, Balog CI, Catalina MI, Jones FM, Schramm G, Haas H, Doenhoff MJ, Dunne DW, Deelder AM, Hokke CH. 2006. IPSE/alpha-1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis X motif on core-difucosylated N-glycans. FEBS J 273:2276–2292. doi: 10.1111/j.1742-4658.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- 41.Wuhrer M, Kantelhardt SR, Dennis RD, Doenhoff MJ, Lochnit G, Geyer R. 2002. Characterization of glycosphingolipids from Schistosoma mansoni eggs carrying Fuc(alpha1-3)GalNAc-, GalNAc(beta1-4)[Fuc(alpha1-3)]GlcNAc- and Gal(beta1-4)[Fuc(alpha1-3)]GlcNAc- (Lewis X) terminal structures. Eur J Biochem 269:481–493. doi: 10.1046/j.0014-2956.2001.02673.x. [DOI] [PubMed] [Google Scholar]
- 42.Burke ML, McGarvey L, McSorley HJ, Bielefeldt-Ohmann H, McManus DP, Gobert GN. 2011. Migrating Schistosoma japonicum schistosomula induce an innate immune response and wound healing in the murine lung. Mol Immunol 49:191–200. doi: 10.1016/j.molimm.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Li YL, Fishelson Z, Kusel JR, Ruppel A. 2005. Schistosoma japonicum migration through mouse skin compared histologically and immunologically with S. mansoni. Parasitol Res 95:218–223. doi: 10.1007/s00436-004-1284-4. [DOI] [PubMed] [Google Scholar]
- 44.Linder E. 1990. Cercarial kissing marks—no superficial make-up. Parasitol Today 6:393–395. doi: 10.1016/0169-4758(90)90151-S. [DOI] [PubMed] [Google Scholar]
- 45.Fishelson Z, Amiri P, Friend DS, Marikovsky M, Petitt M, Newport G, McKerrow JH. 1992. Schistosoma mansoni: cell-specific expression and secretion of a serine protease during development of cercariae. Exp Parasitol 75:87–98. doi: 10.1016/0014-4894(92)90124-S. [DOI] [PubMed] [Google Scholar]
- 46.Riengrojpitak S, Anderson S, Wilson RA. 1998. Induction of immunity to Schistosoma mansoni: interaction of schistosomula with accessory leucocytes in murine skin and draining lymph nodes. Parasitology 117:301–309. doi: 10.1017/S0031182098003187. [DOI] [PubMed] [Google Scholar]
- 47.Naus CW, van Remoortere A, Ouma JH, Kimani G, Dunne DW, Kamerling JP, Deelder AM, Hokke CH. 2003. Specific antibody responses to three schistosome-related carbohydrate structures in recently exposed immigrants and established residents in an area of Schistosoma mansoni endemicity. Infect Immun 71:5676–5681. doi: 10.1128/IAI.71.10.5676-5681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Remoortere A, van dam GJ, Hokke CH, van den Eijnden DH, van Die I, Deelder AM. 2001. Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect Immun 69:2396–2401. doi: 10.1128/IAI.69.4.2396-2401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. 2003. Immunity to schistosomiasis: glycans are potential antigenic targets for immune intervention. Exp Parasitol 104:1–13. doi: 10.1016/S0014-4894(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 50.Nyame AK, DeBose-Boyd R, Long TD, Tsang VC, Cummings RD. 1998. Expression of Lex antigen in Schistosoma japonicum and S. haematobium and immune responses to Lex in infected animals: lack of Lex expression in other trematodes and nematodes. Glycobiology 8:615–624. doi: 10.1093/glycob/8.6.615. [DOI] [PubMed] [Google Scholar]
- 51.Van Remoortere A, Vermeer HJ, van Roon AM, Langermans JA, Thomas AW, Wilson RA, van Die I, van den Eijnden DH, Agoston K, Kerekgyarto J, Vliegenthart JF, Kamerling JP, van Dam GJ, Hokke CH, Deelder AM. 2003. Dominant antibody responses to Fucalpha1-3GalNAc and Fucalpha1-2Fucalpha1-3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp Parasitol 105:219–225. doi: 10.1016/j.exppara.2003.12.005. [DOI] [PubMed] [Google Scholar]