Abstract
Osteoarticular brucellosis is the most common localization of human active disease. Osteocytes are the most abundant cells of bone. They secrete factors that regulate the differentiation of both osteoblasts and osteoclasts during bone remodeling. The aim of this study is to determine if Brucella abortus infection modifies osteocyte function. Our results indicate that B. abortus infection induced matrix metalloproteinase 2 (MMP-2), receptor activator for NF-κB ligand (RANKL), proinflammatory cytokines, and keratinocyte chemoattractant (KC) secretion by osteocytes. In addition, supernatants from B. abortus-infected osteocytes induced bone marrow-derived monocytes (BMM) to undergo osteoclastogenesis. Using neutralizing antibodies against tumor necrosis factor alpha (TNF-α) or osteoprotegerin (OPG), RANKL's decoy receptor, we determined that TNF-α and RANKL are involved in osteoclastogenesis induced by supernatants from B. abortus-infected osteocytes. Connexin 43 (Cx43) and the integrins E11/gp38, integrin-α, integrin-β, and CD44 are involved in cell-cell interactions necessary for osteocyte survival. B. abortus infection inhibited the expression of Cx43 but did not modify the expression of integrins. Yet the expression of both Cx43 and integrins was inhibited by supernatants from B. abortus-infected macrophages. B. abortus infection was not capable of inducing osteocyte apoptosis. However, supernatants from B. abortus-infected macrophages induced osteocyte apoptosis in a dose-dependent manner. Taken together, our results indicate that B. abortus infection could alter osteocyte function, contributing to bone damage.
INTRODUCTION
Brucella spp. are Gram-negative facultative intracellular bacteria that cause a debilitating and chronic zoonotic disease (1). Osteoarticular complications are important due to their high prevalence and also to the associated functional sequelae (2–4). Bone loss has been consistently reported in the three most frequent forms of osteoarticular brucellosis (sacroiliitis, spondylitis, and peripheral arthritis) (5–8).
Although the ability of Brucella to cause bone loss is well documented, the molecular mechanisms implicated have not been completely deciphered yet. We have recently described a putative immune mechanism for inflammatory bone loss that may occur in response to infection by B. abortus. Our results revealed an important contribution of the macrophages, osteoblasts, and T lymphocytes in response to B. abortus infection and the resulting induction of osteoclast differentiation (9–11).
For many years the bone-bound osteocyte has been considered a relatively inactive cell with a broadly unknown role in the bone. But osteocytes are not only the most abundant bone cells and comprise up to 95% of the bone cells in the adult skeleton but also the central regulators of the differentiation and activity of both osteoblasts and osteoclasts during bone remodeling (12). Primary osteocytes and the osteocyte cell line MLO-Y4 secrete macrophage colony-stimulating factor (M-CSF) and RANKL, both necessary for osteoclast formation (13), and recent studies showed that osteocytes are the major regulators of osteoclast formation and activation (14). In addition to the role of osteocytes in regulating bone remodeling, emerging evidence suggests an important role for the gap junction in osteoclast-osteocyte communication (15). Connexin 43 (Cx43) is the most prominent gap junction protein expressed in osteocytes (15), and deficient mice have increased bone resorption and osteoclast numbers (16, 17). In vitro studies revealed that Cx43-deficient MLO-Y4 cells display an increase in the RANKL/osteoprotegerin (OPG) ratio compared to control MLO-Y4 cell levels, indicating that loss of Cx43 in osteocytes promotes osteoclastogenesis (17, 18). On the other hand, it has been reported that mice lacking Cx43 in osteoblasts/osteocytes or only in osteocytes exhibit increased osteocyte apoptosis (18). Moreover, integrins can link the cellular cytoskeletal network to the extracellular matrix (19). Integrins are essential determinants of cell survival, and, in many cases, prevention or alteration of integrin adhesion triggers a form of apoptosis known as anoikis (20). In this way osteocyte cell death has been shown to be important for disease progression and bone loss (21).
We have previously demonstrated that Brucella spp. can infect and survive within human osteoblasts and that this infection elicits the secretion of RANKL, proinflammatory cytokines, and chemokines that might be involved in the osteoarticular manifestations of brucellosis. Such a response was further amplified by subsequent interactions between osteoblasts and monocytes in the face of B. abortus infection (9, 10). Then, B. abortus infection might create a microenvironment that would promote alterations of osteocyte biology. This could have an important contribution in the bone damage observed in patients with osteoarticular brucellosis.
MATERIALS AND METHODS
Bacterial culture.
Brucella abortus S2308 and its isogenic virB10 mutant were grown overnight in 10 ml of tryptic soy broth (Merck, Buenos Aires, Argentina) with constant agitation at 37°C. Bacteria were harvested, and the inocula were prepared as described previously (10). All live-Brucella manipulations were performed in biosafety level 3 facilities located at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA.
Cellular infection.
The MLO-Y4 cell line, kindly provided by Lynda Bonewald (University of Missouri—Kansas City), was infected with B. abortus at different multiplicities of infection (MOIs); J774. A1 cells were infected at an MOI of 100. After the bacterial suspension was dispensed, the plates were centrifuged for 10 min at 2,000 rpm and then incubated for 2 h at 37°C under a 5% CO2 atmosphere. Cells were extensively washed with Dulbecco's modified Eagle's medium (DMEM) to remove extracellular bacteria and incubated in medium supplemented with 100 μg/ml of gentamicin and 50 μg/ml of streptomycin to kill extracellular bacteria.
MLO-Y4 cells were harvested at different times (see below) to determine intracellular replication, cytokine production, matrix metalloproteinase (MMP) secretion, Cx43, E11/gp38, integrin-α and -β, and CD44 expression, and apoptosis and to obtain culture supernatants to perform osteoclastogenesis assays. Supernatants from J774.A1 cells were harvested at 24 h postinfection (p.i.) to be used as conditioned medium.
Zymography.
Gelatinase activity was assayed as described previously (10, 22).
Measurement of cytokine concentrations.
Secretion of interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor alpha (TNF-α), and monocyte chemotactic protein 1 (MCP-1) was quantified by enzyme-linked immunosorbent assays (ELISAs) (BD Biosciences) of culture supernatants.
Apoptosis assays.
MLO-Y4 cells were infected at different MOIs; 24 h after infection cells were washed, and the percentage of apoptotic cells was assessed by annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (BD) for 10 min on ice. Apoptosis was analyzed on a FACSCalibur flow cytometer. Data were processed using FlowJo software (Tree Star). Apoptosis was also assessed quantitatively either by a terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay using a fluorescein FragEL DNA fragmentation detection kit (Calbiochem, San Diego, CA), by Hoechst dye 33342 (which visualized nuclear morphology), or by annexin V-FITC/PI by reseeding osteocytes on Permanox chamber slides (Nunc, Roskilde, Denmark). As a positive control, cells were treated with H2O2 (200 μM).
Stimulation with conditioned medium.
Culture supernatants from B. abortus-infected J774.A1 macrophage cells were harvested at 24 h p.i., sterilized by filtration through a 0.22-μm-pore-size nitrocellulose filter, and used to stimulate noninfected MLO-Y4 cells. Supernatants were used diluted 1/2, 1/5, or 1/10 in complete medium. After 24 h the cells were harvested to determine Cx43, E11/gp38, integrin-α and -β, tubulin-α, and CD44 expression and apoptosis.
Osteoclast formation assay.
Bone marrow-derived monocytes (BMM) were induced to undergo osteoclastogenesis as described previously (23). Briefly, bone marrow cells from BALB/c mice were cultured in complete medium containing 5 ng/ml murine recombinant M-CSF (rM-CSF) (R&D Systems, Minneapolis, MN, USA) for 12 h in 24-well plates. Nonadherent cells were harvested and cultured with 30 ng/ml M-CSF in 24-well plates for an additional 24 h. Nonadherent cells were washed out, and adherent cells were collected and used as BMM (5 × 104 cells/well), which were seeded onto glass coverslips in 24-well plates for 7 days and cultured in complete medium containing 30 ng/ml M-CSF and 0.2 ml of culture supernatants from MLO-Y4 osteocytes infected with B. abortus. As positive controls of osteoclast formation, BMM cultures received 50 ng/ml murine TNF-α or RANKL. On day 3, the culture medium and all reagents were replaced. To identify osteoclasts, cells were fixed in 4% paraformaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) (Sigma-Aldrich, St. Louis, MO, USA). TRAP-positive, multinucleated (more than three nuclei) cells were defined as osteoclasts, and the number was determined by microscopic counts.
Immunofluorescence.
B. abortus-infected MLO-Y4 cells or MLO-Y4 cells treated with supernatants from B. abortus-infected J774.A1 macrophages were fixed in 3% paraformaldehyde for 10 min at room temperature and permeabilized with 0.3% Triton X-100 for 10 min. Cells were first incubated with mouse anti-Cx43 (Invitrogen) diluted in phosphate-buffered saline (PBS)–0.1% Tween for 30 min and then with Alexa 488 anti-mouse (Invitrogen). DAPI (4′,6′-diamidino-2-phenylindole) was used for nuclear staining for 30 min. After cells were washed in PBS, they were mounted and analyzed by fluorescence microscopy.
mRNA preparation and quantitative reverse transcription-PCR (qRT-PCR).
RNA was extracted using a Quick-RNA MiniPrep Kit (Zymo Research), and 1 μg of RNA was subjected to reverse transcription using Improm-II reverse transcriptase (Promega). PCR analysis was performed with an Mx3000P real-time PCR detection system (Stratagene) using SYBR green as a fluorescent DNA binding dye. The primer sets used for amplification were as follows: Cx43 sense, 5′-TACCACGCCACCACCGGCCCA, and antisense, 5′-GGCATTTTGGCTGTCGTCAGGGAA; E11/gp38 sense, 5′-CGACCAGTTTCTAACACCTGCCTTCT, and antisense, 5′-CTGTCCCAGCAACACTGAGTCCC; integrin-α sense, 5′-GAATGGCGAAGGAAACTCTGAAA, and antisense, 5′-ATAAACTGAGACTGCTGGGTGCT; integrin-β sense, 5′-CCACCTTCACCAATATCAC, and antisense, 5′-CCAAATCCCACCCATACAC; CD44 sense, 5′-GGATTCATCCCAACGCTAT, and antisense, 5′-ACTCGCCCTTCTTGCTGT; β-actin sense, 5′-AACAGTCCGCCTAGAAGCAC, and antisense, 5′-CGTTGACATCCGTAAAGACC.
The amplification cycle consisted of 95°C for 30 s, 55°C for 30 s, and 72°C for 60 s. All primer sets yielded a single product of the correct size. Relative expression levels were normalized against the level of β-actin.
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance (ANOVA), followed by a post hoc Tukey test using GraphPad Prism, version 4.0, software. Data are represented as means ± standard deviations (SD).
RESULTS
B. abortus invades and multiplies in osteocytes.
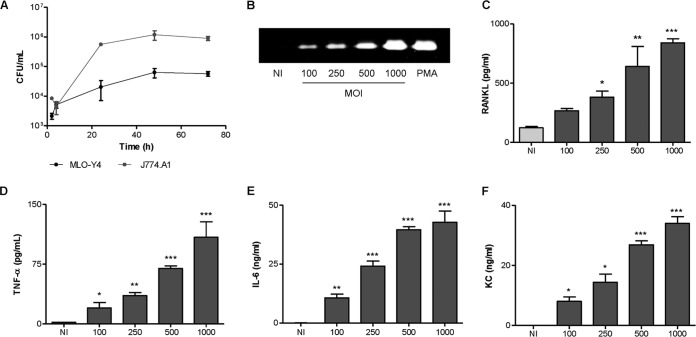
Infection experiments showed that Brucella abortus is internalized by murine osteocytes (MLO-Y4 cell line) in vitro. Follow-up of infected cultures revealed that B. abortus can replicate inside murine osteocytes. The magnitude of infection (intracellular CFU) increased by about 1 log during the first 48 h p.i., and then the number of intracellular bacteria was maintained during the next 24 h. As a control, J774.A1 cells, a macrophage cell line that was consistently reported to support Brucella infection and growth, were infected in parallel. At any time tested, the number of bacteria was higher in J774.A1 cells than in MLO-Y4 cells (Fig. 1A). This is a consistent result because it has been reported that macrophages are the preferential cells that support Brucella replication (24, 25).
FIG 1.
B. abortus invades and multiplies in murine osteocytes cells, inducing MMP-9 and cytokine secretion. (A) Osteocytes (MLO-Y4 cell line) and monocytes (J774.A1 cell line) were infected with B. abortus at an MOI of 100, and replication in each cell type was assessed by determining CFU counts after 2, 4, 24, 48, and 72 h. (B) MLO-Y4 cells were infected with B. abortus at different MOIs, and 24 h after infection MMP-9 production was determined by zymography. (C to F) Levels of RANKL, the cytokines TNF-α and IL-6, and KC, as indicated, were evaluated by ELISA in culture supernatants harvested at 24 h (RANKL, IL-6, and KC) or 8 h (TNF-α) postinfection. PMA, phorbol myristate acetate. Data are given as the means ± SD from at least three individual experiments. *, P < 0.1; **, P < 0.01; ***, P < 0.001, for results versus noninfected (NI) cells.
B. abortus-infected osteocytes secrete proinflammatory mediators.
Several soluble mediator/effector molecules have been implicated in bone resorption. RANKL, MMP-2, and proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been shown to be important (26–28). In addition, chemokines could contribute to bone damage by attracting inflammatory cells to the sites of infection that secrete inflammatory cytokines. Thus, we decided to investigate the ability of mouse MLO-Y4 osteocytes to secrete cytokines, chemokines, and MMPs upon infection with B. abortus. B. abortus infection of MLO-Y4 osteocytes elicited a significant secretion of MMP-2, RANKL, TNF-α, IL-6, and keratinocyte chemoattractant (KC) in an MOI-dependent manner (Fig. 1B to F). However, B. abortus-infected MLO-Y4 osteocytes were unable to secrete IL-1β (data not shown). Altogether, these results indicate that B. abortus can infect and replicate in osteocytes, and, as a result of this infection, proinflammatory mediators are secreted.
B. abortus infection reduced Cx43 expression but did not modify the expression of E11/gp38, integrin-α and -β, and CD44.
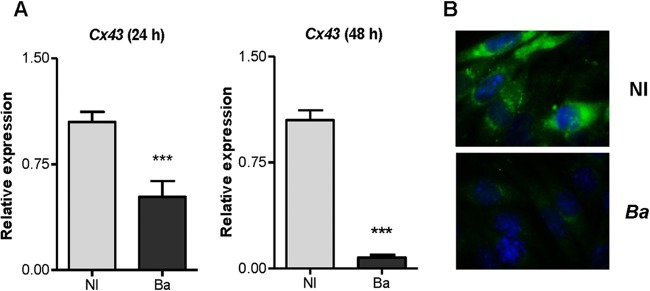
Cx43 is the predominant gap junction protein in bone cells. It facilitates the communication of cellular signals between cells that are required to maintain viability of osteocytes (18). To determine if B. abortus infection could modulate Cx43 expression in osteocytes, these cells were infected with B. abortus; then the expression of the Cx43 gene was determine by qRT-PCR, and Cx43 protein expression was assessed by immunofluorescence using specific antibodies. B. abortus infection reduced Cx43 expression in a time-dependent fashion (Fig. 2A and B), indicating that the infection could alter the gap junction in osteocytes. On the other hand, integrins can link the cellular cytoskeletal network to the extracellular matrix (19), and they are essential determinants of cell survival (20). Therefore, experiments were conducted to determine whether B. abortus infection could affect the expression of integrins. Our results indicated that B. abortus infection did not modify mRNA expression of E11/gp38, integrin-α and -β, and CD44 (data not shown).
FIG 2.
B. abortus infection reduced Cx43 expression in osteocytes. (A) Relative expression of the Cx43 gene was assessed by qRT-PCR in noninfected (NI) and B. abortus (Ba)-infected MLO-Y4 cells. (B) Cx43 was revealed by immunofluorescence with a specific antibody at 48 h postinfection. Data are given as the means ± SD from at least three individual experiments. Data shown are from a representative experiment of three performed. ***, P < 0.001, versus results in noninfected cells.
Culture supernatants from B. abortus-infected macrophages inhibit Cx43 and integrin expression in osteocytes.
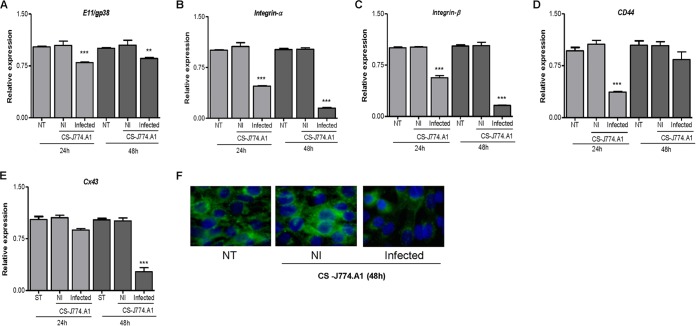
Macrophages secrete proinflammatory cytokines upon infection with B. abortus (29, 30). These cytokines may have different effects on Cx43 and integrin expression (31–34). Thus, we decided to investigate the effect of cytokines present in supernatants from B. abortus-infected macrophages on Cx43 and integrin expression. To this end, supernatants from B. abortus-infected J774.A1 macrophages were harvested at 24 h p.i., sterilized by filtration, and used at 1/2 dilutions to stimulate osteocytes. Culture supernatants from B. abortus-infected macrophages inhibited the mRNA expression of CD44 and integrin-α and -β and, to a lower extent, the mRNA expression of E11/gp38 (Fig. 3A to D). They also inhibited mRNA and protein expression of Cx43 (Fig. 3E and F). The magnitude of inhibition was higher at 48 h posttreatment for all molecules except for CD44, which showed greater inhibition at 24 h and the expression of which was restored at 48 h posttreatment. Supernatants from noninfected macrophages had no effect on the expression of these molecules.
FIG 3.
Supernatants from B. abortus-infected macrophages inhibit Cx43 and integrin expression in osteocytes. MLO-Y4 cells were stimulated with supernatants (added at a 1:2 proportion) from noninfected (NI) or B. abortus-infected macrophages (murine J774.A1 cell line), and the relative expression levels of the of E11/gp38 (A), integrin-α (B), integrin-β (C), CD44 (D), and Cx43 (E) genes were assessed by qRT-PCR at 24 and 48 h poststimulation. (F) Cx43 production was revealed by immunofluorescence with a specific antibody at 48 h poststimulation. Data are given as the means ± SD from at least three individual experiments. **, P < 0.01; ***, P < 0.001, versus results in noninfected cells. CS, culture supernatant.
Taking into account that Cx43 and integrins are involved in sensing and signaling mechanisms in response to mechanical forces, osteoclast differentiation, osteoclast migration, apoptosis of osteocytes, and MMP secretion (35–37), these results suggest that macrophage infection could alter bone homeostasis by modulating Cx43 and integrin expression in osteocytes.
B. abortus infection does not induce apoptosis of osteocytes, but it is induced by supernatants from B. abortus-infected macrophages.
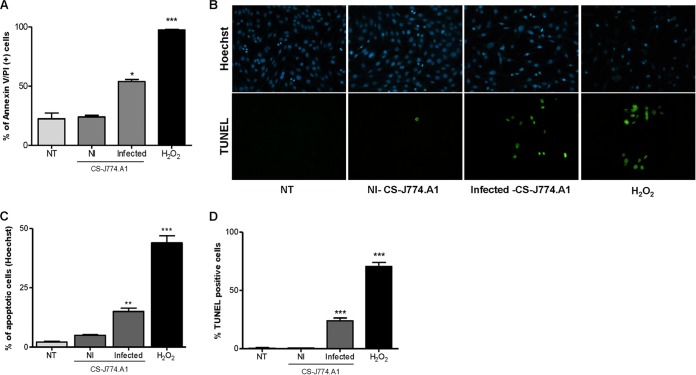
It has been reported that mice lacking Cx43 in osteoblasts/osteocytes or only in osteocytes exhibit increased osteocyte apoptosis (18). Taking into account that B. abortus induced a reduction in Cx43 expression, we decided to investigate whether B. abortus infection induces osteocyte apoptosis. To this end, osteocytes were infected with B. abortus, and the presence of apoptotic cells was determined by annexin V-FITC and propidium iodide staining, TUNEL assay, and Hoechst 33342 staining. Our results indicated that B. abortus infection did not induce osteocyte apoptosis at any MOI tested (data not shown). These results indicated that, by itself, the reduction in the expression of Cx43 induced by B. abortus infection was not sufficient to induce apoptosis in osteocytes. On the other hand, Brucella could also modulate apoptotic and antiapoptotic factors during osteocyte infection as occurs in macrophages and synovial fibroblast infection (38, 39). Yet integrins are also involved in promoting cell viability (20). Since supernatants from B. abortus-infected macrophages inhibited the expression of these molecules, experiments were conducted to determine whether supernatants from B. abortus-infected macrophages induce osteocyte apoptosis. To this end, MLO-Y4 cells were stimulated with a 1/2 dilution of supernatants from B. abortus-infected J774.A1 macrophages or supernatants from uninfected macrophages as a control. After 24 h cells were stained with annexin V-FITC and analyzed by flow cytometry. Osteocytes stimulated with supernatants from B. abortus-infected macrophages exhibited significantly higher annexin V-FITC binding than cells treated with supernatants from uninfected macrophages or untreated cells (Fig. 4A), suggesting a proapoptotic effect of Brucella-infected supernatants. The occurrence of apoptosis was confirmed by Hoechst 33342 staining (Fig. 4B and C) and TUNEL assay (Fig. 4B and D). These results indicated that supernatants from B. abortus-infected macrophages induce osteocyte apoptosis, contributing in this way to bone loss.
FIG 4.
Supernatants from B. abortus-infected macrophages induce apoptosis of osteocytes. MLO-Y4 cells were treated with supernatants (added at a 1:2 proportion) from noninfected (NI) or B. abortus-infected macrophages (J774.A1 cell line), and 24 h after stimulation apoptosis was evaluated by annexin V/PI, TUNEL, and Hoechst 33342 assays. (A) Flow cytometry analysis of apoptotic cells by annexin V/PI. (B) Hoechst 33342 staining and TUNEL assays using fluorescence microscopy. (C and D) Quantitative analysis of experiments presented in panel B. H2O2 (200 μM) was used as a positive control. NT, nontreated cells. Data are given as the means ± SD from at least three individual experiments. *, P < 0.1; **, P < 0.01; ***, P < 0.001, versus results in noninfected cells.
Supernatants from B. abortus-infected osteocytes induce BMM-derived osteoclastogenesis.
Osteoclasts play an important role in bone resorption and originate from fusion of precursor cells that belong to the monocyte macrophage linage in the bone marrow (40, 41). This process is mediated by RANKL, but under inflammatory conditions it could be mediated by proinflammatory cytokines in conjunction with M-CSF (23). To determine if factors produced by B. abortus-infected osteocytes could induce osteoclast formation from BMM, these cells were stimulated with M-CSF in conjunction with supernatants from osteocytes infected with B. abortus, and osteoclastogenesis was determined by the generation of multinucleated TRAP-expressing cells. The formation of osteoclast-like cells was induced by supernatants from B. abortus-infected MLO-Y4 cells in a dose-dependent manner but not by those from uninfected cells (Fig. 5). These results indicate that infection of osteocytes with B. abortus promotes osteoclast formation.
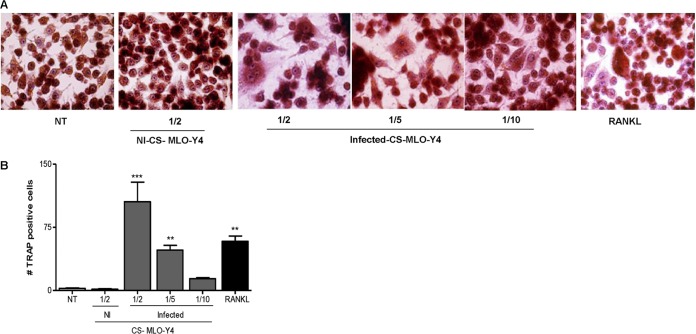
FIG 5.
Supernatants from B. abortus-infected osteocytes induce BMM-derived osteoclastogenesis. BMM cells were either not treated (NT) or stimulated with culture supernatants (CS) from B. abortus-infected MLO-Y4 cells (added at a 1:2, 1:5, or 1:10 proportion) or with culture supernatants from noninfected (NI) MLO-Y4 cells (added at a 1:2 proportion) in conjunction with M-CSF. After 7 days, osteoclastogenesis was determined by the generation of multinucleated TRAP-positive cells. Representative digital images were taken by light microscopy (A), and multinucleated TRAP-positive cells were identified and counted (B). RANKL was used as a positive control. Data are given as the means ± SD from at least three individual experiments. **, P < 0.01; ***, P < 0.001, versus results in noninfected cells.
Supernatant from B. abortus wild-type- or virB10 mutant-infected osteocytes induces osteoclastogenesis in a mechanism dependent on the presence of RANKL and TNF-α.
RANKL and TNF-α are abundant in sites of inflammatory bone erosion (42). Because these cytokines are potent osteoclastogenic factors and because their signaling pathways are considerably overlapping under proinflammatory conditions, RANKL and TNF-α might synergistically orchestrate enhanced osteoclastogenesis via cooperative mechanisms (42). Since these cytokines were secreted upon infection of MLO-Y4 cells, experiments were conducted to determine their contribution in the osteoclastogenesis induced by supernatants from B. abortus-infected MLO-Y4 cells. To this end, BMM cells were cultured with M-CSF and supernatants from B. abortus-infected MLO-Y4 cells in the presence of anti-TNF-α antibody or OPG, a RANKL decoy receptor, and osteoclastogenesis was evaluated by the generation of TRAP-expressing cells. Compared with untreated cells, TNF-α neutralizing antibody significantly reduced osteoclastogenesis induced by supernatants from Brucella-infected MLO-Y4 osteocytes. In addition, OPG also reduced the formation of osteoclast-like cells. As expected, RANKL and TNF-α induced TRAP expression in cells (see Fig. 7).
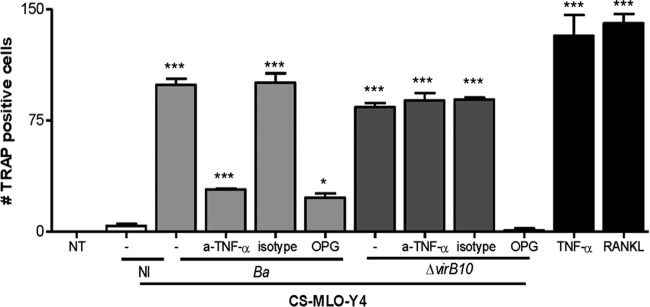
FIG 7.
Supernatants from B. abortus wild-type- or virB10 mutant-infected osteocytes inhibit osteoclast differentiation in the presence of anti-TNF-α or OPG. BMM cells were stimulated with culture supernatants (CS) from B. abortus (Ba)-infected or virB10 mutant (ΔvirB10)-infected MLO-Y4 cells or with culture supernatants from noninfected (NI) MLO-Y4 cells (added at a 1:2 proportion) in conjunction with M-CSF. After 7 days, osteoclastogenesis was determined by the generation of multinucleated TRAP-positive cells. Supernatants from infected MLO-Y4 cells were preincubated with OPG, an anti-TNF-α neutralizing antibody (a-TNF-α), or an isotype control. RANKL and TNF-α were used as positive controls. Data are given as the means ± SD from at least three individual experiments. *, P < 0.1; ***, P < 0.001, versus results in nontreated (NT) cells.
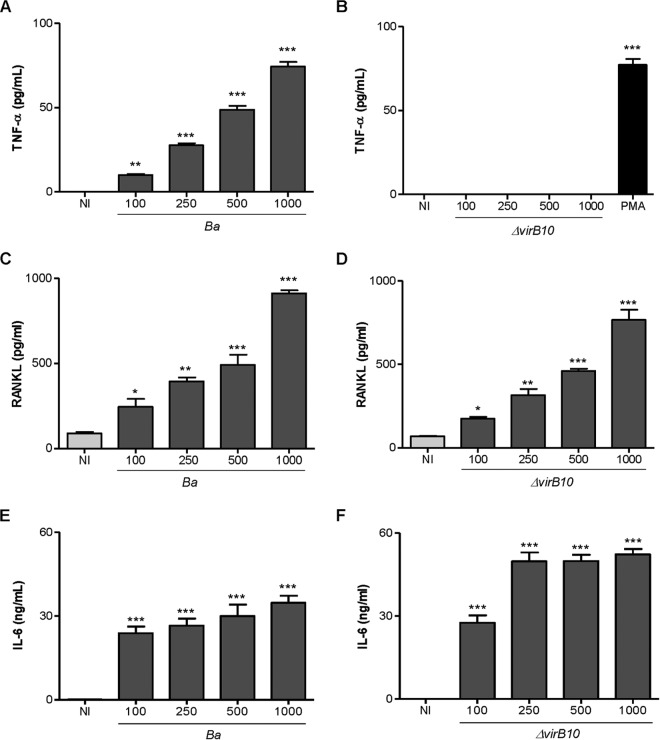
Since the type four secretion system (T4SS) VirB from B. abortus has been involved in the induction of inflammatory responses upon infection (43, 44), experiments were conducted to determine the role of the T4SS in the secretion of TNF-α, IL-6, and RANKL by osteocytes. To this end, MLO-Y4 osteocytes were infected with B. abortus or the isogenic mutant virB10 strain, and the B. abortus virB10 mutant was unable to induce significant levels of TNF-α secretion (Fig. 6A and B). However, levels of RANKL (Fig. 6C, D, E, and F) produced by the virB10 mutant were comparable at all MOIs to the levels induced by the B. abortus wild-type strain. In contrast to the levels of IL-6 observed in macrophages (44), the B. abortus virB10 mutant induced higher levels of IL-6 than the wild type. Together, these results indicate that B. abortus induces TNF-α secretion by a mechanism that is dependent on a functional T4SS. Since under inflammatory conditions TNF-α is the key proinflammatory cytokine involved in osteoclastogenesis (45, 46), experiments were conducted to determine the role of the T4SS in osteoclastogenesis induction. To this end, supernatants from B. abortus virB10 mutant-infected osteocytes were used to determine osteoclast differentiation from BMM in the presence of M-CSF. Our results indicated that supernatants from virB10 mutant-infected osteocytes, which do not induce TNF-α secretion, were able to induce osteoclast differentiation from BMM at a degree similar to that induced by supernatants from B. abortus wild-type-infected osteocytes. When osteoclastogenesis experiments were performed in the presence of OPG, osteoclastogenesis induced by supernatants from virB10 mutant-infected osteocytes was abrogated (Fig. 7).
FIG 6.
B. abortus virB10 mutant does not induce TNF-α secretion by osteocytes. Levels of TNF-α (A and B), RANKL (C and D), and IL-6 (E and F) were measured in culture supernatants from noninfected (NI) and B. abortus (Ba)-infected or B. abortus virB10 mutant (ΔvirB10)-infected MLO-Y4 cells by ELISA at 24 and 48 h postinfection. PMA, phorbol myristate acetate. Data are given as the means ± SD from at least three individual experiments. *, P < 0.1; **, P < 0.01; ***, P < 0.001, versus results in noninfected cells.
Taken together, these results indicated that both RANKL and TNF-α contribute to the generation of osteoclast-like cells.
DISCUSSION
Osteoarticular brucellosis is the most common presentation of human active brucellosis disease (5, 7, 47–49). In this paper, we studied the role played by osteocytes in this form of the disease as they are the most abundant cells in the bone (12). Yet their functional role was unknown for a long time. In part, it is because osteocytes are deeply embedded in the mineralized bone matrix and are not readily accessible for many experimental approaches. The establishment of the osteocyte-like cell line MLO-Y4 has made experiment possible because these cells have been shown to have characteristics of primary osteocytes (50). In this paper, we studied the role played by osteocytes in osteoarticular brucellosis as they have been recognized as central mediators involved in osteoblast and osteoclast homeostasis that is disrupted in inflammatory bone disease of either infectious or noninfectious origin (51).
We demonstrate that B. abortus may invade and replicate in osteocytes. The ability of B. abortus to invade, survive, and replicate within osteocytes is in line with its capacity to replicate in other nonphagocytic cells, including hepatocytes, astrocytes, synoviocytes, and osteoblasts (10, 52–54). Our results also indicate that B. abortus infection induces the secretion of MMP-2, RANKL, and proinflammatory cytokines. Increases in MMP levels may cause tissue damage. Indeed, locally increased levels of MMP have been found in several osteoarticular diseases, including rheumatic conditions (rheumatoid arthritis, osteoarthritis, and spondyloarthritis) and infectious arthritis such as that observed in Lyme disease (55–57). Furthermore, RANKL is a homotrimeric molecule displayed on the membrane of osteoblasts that stimulates differentiation of osteoclasts and is a key molecule involved in bone resorption and, under pathological conditions, increased expression levels, leading to bone destruction (58). In addition, proinflammatory cytokines could contribute to bone damage, inducing osteoclastogenesis (26, 27, 59–61). In chronic inflammatory bone diseases, such as rheumatoid arthritis, the proinflammatory cytokines TNF-α, IL-1β, and IL-6 have been shown to be important for disease progression and osteoclastogenesis (23, 26, 27, 59–61). Our results using the neutralizing antibody anti-TNF-α and OPG indicate that RANKL and TNF-α are both the key cytokines involved in osteoclastogenesis induced by B. abortus-infected osteocytes. In addition, TNF-α secretion was shown to be dependent on the expression of a functional T4SS. This secretion system has been reported previously to be involved in the inflammatory response induced by B. abortus infection (43, 44).
Channels formed by Cx43, the most abundant member of the connexin family of proteins expressed in bone cells, mediate the communication among osteocytes and between osteocytes and cells on the bone surface (62). Cx43 deletion in cultured osteocytic cells resulted in increased apoptosis (18). Integrins also control the fate and function of cells by influencing not only their proliferation and differentiation but also apoptosis (63). B. abortus infection reduced the expression of Cx43 expression but did not modify integrin expression, which resulted in the absence of apoptosis. However, taking into account that monocytes/macrophages are the main replication niche for Brucella, experiments were conducted to determine whether B. abortus-infected macrophages could modulate Cx43 and integrin expression in osteocytes. Supernatants from B. abortus-infected macrophages significantly reduced the expression of Cx43 and integrins, with concomitant apoptotic cell death.
Based on the results obtained in the present study, we hypothesize that B. abortus may harm osteocyte function and viability directly and indirectly, contributing to the bone and joint destruction observed in patients with osteoarticular complications of brucellosis.
ACKNOWLEDGMENTS
We thank Lynda Bonewald for MLO-Y4 cells and also Horacio Salomón and the staff of the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA for their assistance with biosafety level 3 laboratory use.
Funding Statement
This work was supported by grants PICT 2010-0023, PICT 2011-1501, PICT 2011-1200, and PICT 2012-2252 from Agencia Nacional of Promoción Científica y Tecnológica (ANPCyT, Argentina) and by grants UBACYT 20020090200012 and 20020120100128 from Universidad de Buenos Aires. Funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Glasgow MM. 1976. Brucellosis of the spine. Br J Surg 63:283–288. doi: 10.1002/bjs.1800630408. [DOI] [PubMed] [Google Scholar]
- 3.Keenan JD, Metz CW Jr. 1972. Brucella spondylitis. A brief review and case report. Clin Orthop Relat Res 82:87–91. [PubMed] [Google Scholar]
- 4.Geyik MF, Gur A, Nas K, Cevik R, Sarac J, Dikici B, Ayaz C. 2002. Musculoskeletal involvement of brucellosis in different age groups: a study of 195 cases. Swiss Med Wkly 132:98–105. [DOI] [PubMed] [Google Scholar]
- 5.Aydin M, Fuat Yapar A, Savas L, Reyhan M, Pourbagher A, Turunc TY, Ziya Demiroglu Y, Yologlu NA, Aktas A. 2005. Scintigraphic findings in osteoarticular brucellosis. Nucl Med Commun 26:639–647. doi: 10.1097/01.mnm.0000167651.52724.68. [DOI] [PubMed] [Google Scholar]
- 6.Colmenero JD, Ruiz-Mesa JD, Plata A, Bermudez P, Martin-Rico P, Queipo-Ortuno MI, Reguera JM. 2008. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin Infect Dis 46:426–433. doi: 10.1086/525266. [DOI] [PubMed] [Google Scholar]
- 7.Madkour MM. 2001. Madkour's brucellosis, 2nd ed Springer-Verlag, Berlin, Germany. [Google Scholar]
- 8.Young EJ. 1989. Clinical manifestations of human brucellosis, p 97–126. In Young EJ, Corbel MJ (ed), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL. [Google Scholar]
- 9.Delpino MV, Fossati CA, Baldi PC. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect Immun 77:984–995. doi: 10.1128/IAI.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scian R, Barrionuevo P, Giambartolomei GH, Fossati CA, Baldi PC, Delpino MV. 2011. Granulocyte-macrophage colony-stimulating factor- and tumor necrosis factor alpha-mediated matrix metalloproteinase production by human osteoblasts and monocytes after infection with Brucella abortus. Infect Immun 79:192–202. doi: 10.1128/IAI.00934-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giambartolomei GH, Scian R, Acosta-Rodriguez E, Fossati CA, Delpino MV. 2012. Brucella abortus-infected macrophages modulate T lymphocytes to promote osteoclastogenesis via IL-17. Am J Pathol 181:887–896. doi: 10.1016/j.ajpath.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Lanyon LE. 1993. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int 53(Suppl 1):S102–S106. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. 2002. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res 17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien CA, Nakashima T, Takayanagi H. 2013. Osteocyte control of osteoclastogenesis. Bone 54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donahue HJ. 2000. Gap junctions and biophysical regulation of bone cell differentiation. Bone 26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 16.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. 2011. Osteoblast connexin 43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell 22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S, Srinivasan S, Gross TS, Donahue HJ. 2011. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One 6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. 2012. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int 91:215–224. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiger B, Bershadsky A, Pankov R, Yamada KM. 2001. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore AP. 2005. Anoikis. Cell Death Differ 12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein RS, Nicholas RW, Manolagas SC. 2000. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab 85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 22.Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. 1985. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem 260:2493–2500. [PubMed] [Google Scholar]
- 23.Ukai T, Yumoto H, Gibson FC III, Genco CA. 2008. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-alpha production. Infect Immun 76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roop RM II, Bellaire BH, Valderas MW, Cardelli JA. 2004. Adaptation of the brucellae to their intracellular niche. Mol Microbiol 52:621–630. doi: 10.1111/j.1365-2958.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 25.Celli J. 2006. Surviving inside a macrophage: the many ways of Brucella. Res Microbiol 157:93–98. doi: 10.1016/j.resmic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Haynes DR. 2004. Bone lysis and inflammation. Inflamm Res 53:596–600. doi: 10.1007/s00011-004-1303-z. [DOI] [PubMed] [Google Scholar]
- 27.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. 1996. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 64:2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu M, Rabie AB. 2006. Matrix metalloproteinase-9 regulates graft bone resorption. Angle Orthod 76:598–604. [DOI] [PubMed] [Google Scholar]
- 29.Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol 173:4635–4642. doi: 10.4049/jimmunol.173.7.4635. [DOI] [PubMed] [Google Scholar]
- 30.Delpino MV, Barrionuevo P, Macedo GC, Oliveira SC, Genaro SD, Scian R, Miraglia MC, Fossati CA, Baldi PC, Giambartolomei GH. 2012. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J Leukoc Biol 91:285–298. doi: 10.1189/jlb.04111185. [DOI] [PubMed] [Google Scholar]
- 31.Heinrich M, Oberbach A, Schlichting N, Stolzenburg JU, Neuhaus J. 2011. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS One 6:e20792. doi: 10.1371/journal.pone.0020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illario M, Amideo V, Casamassima A, Andreucci M, di Matola T, Miele C, Rossi G, Fenzi G, Vitale M. 2003. Integrin-dependent cell growth and survival are mediated by different signals in thyroid cells. J Clin Endocrinol Metab 88:260–269. doi: 10.1210/jc.2002-020774. [DOI] [PubMed] [Google Scholar]
- 33.Vitale M, Casamassima A, Illario M, Bassi V, Fenzi G, Rossi G. 1995. Cell-to-cell contact modulates the expression of the beta 1 family of integrins in primary cultures of thyroid cells. Exp Cell Res 220:124–129. doi: 10.1006/excr.1995.1298. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Ramirez MA, Male DK, Wang C, Sharrack B, Wu D, Romero IA. 2013. Cytokine-induced changes in the gene expression profile of a human cerebral microvascular endothelial cell-line, hCMEC/D3. Fluids Barriers CNS 10:27. doi: 10.1186/2045-8118-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plotkin LI. 2014. Connexin 43 hemichannels and intracellular signaling in bone cells. Front Physiol 5:131. doi: 10.3389/fphys.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chellaiah MA, Hruska KA. 2003. The integrin alphavbeta3 and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int 72:197–205. doi: 10.1007/s00223-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Cao W, Chellaiah MA. 2012. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis. Mol Cancer 11:66. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskra L, Mathison A, Splitter G. 2003. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect Immun 71:1125–1133. doi: 10.1128/IAI.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scian R, Barrionuevo P, Rodriguez AM, Arriola Benitez PC, Garcia Samartino C, Fossati CA, Giambartolomei GH, Delpino MV. 2013. Brucella abortus invasion of synoviocytes inhibits apoptosis and induces bone resorption through RANKL expression. Infect Immun 81:1940–1951. doi: 10.1128/IAI.01366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheven BA, Visser JW, Nijweide PJ. 1986. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature 321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 41.Ibbotson KJ, Roodman GD, McManus LM, Mundy GR. 1984. Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol 99:471–480. doi: 10.1083/jcb.99.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. 2001. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 43.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. 2013. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol 190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- 44.Roux CM, Rolan HG, Santos RL, Beremand PD, Thomas TL, Adams LG, Tsolis RM. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell Microbiol 9:1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 45.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. 2000. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotuzzo E, Alarcon GS, Bocanegra TS, Carrillo C, Guerra JC, Rolando I, Espinoza LR. 1982. Articular involvement in human brucellosis: a retrospective analysis of 304 cases. Semin Arthritis Rheum 12:245–255. doi: 10.1016/0049-0172(82)90064-6. [DOI] [PubMed] [Google Scholar]
- 48.Khateeb MI, Araj GF, Majeed SA, Lulu AR. 1990. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis 49:994–998. doi: 10.1136/ard.49.12.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. 1999. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis 29:1440–1449. doi: 10.1086/313524. [DOI] [PubMed] [Google Scholar]
- 50.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. 1997. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res 12:2014–2023. [DOI] [PubMed] [Google Scholar]
- 51.Nakashima T, Tomita H, Chitose S. 2011. Evidence of the local immune status in the human larynx. Adv Otorhinolaryngol 72:100–102. doi: 10.1159/000324628. [DOI] [PubMed] [Google Scholar]
- 52.Garcia Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH. 2010. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am J Pathol 176:1323–1338. doi: 10.2353/ajpath.2010.090503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delpino MV, Barrionuevo P, Scian R, Fossati CA, Baldi PC. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J Hepatol 53:145–154. doi: 10.1016/j.jhep.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Scian R, Barrionuevo P, Giambartolomei GH, De Simone EA, Vanzulli SI, Fossati CA, Baldi PC, Delpino MV. 2011. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect Immun 79:3619–3632. doi: 10.1128/IAI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behera AK, Hildebrand E, Scagliotti J, Steere AC, Hu LT. 2005. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme arthritis. Infect Immun 73:126–134. doi: 10.1128/IAI.73.1.126-134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rengel Y, Ospelt C, Gay S. 2007. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther 9:221. doi: 10.1186/ar2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandooren B, Kruithof E, Yu DT, Rihl M, Gu J, De Rycke L, Van Den Bosch F, Veys EM, De Keyser F, Baeten D. 2004. Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum 50:2942–2953. doi: 10.1002/art.20477. [DOI] [PubMed] [Google Scholar]
- 58.Suda T, Kobayashi K, Jimi E, Udagawa N, Takahashi N. 2001. The molecular basis of osteoclast differentiation and activation. Novartis Found Symp 232:235–247, discussion 247–250. doi: 10.1002/0470846658.ch16. [DOI] [PubMed] [Google Scholar]
- 59.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. 1999. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol 154:203–210. doi: 10.1016/S0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. 2005. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115:282–290. doi: 10.1172/JCI200523394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Tashiwazaki S. 1996. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res 11:88–95. [DOI] [PubMed] [Google Scholar]
- 62.Civitelli R. 2008. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys 473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Streuli CH. 2009. Integrins and cell-fate determination. J Cell Sci 122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]