Abstract
Mucins secreted by intestinal goblet cells are considered an important component of innate defense in a number of enteric infections, including many parasitic infections, but also likely provide protection against the gut microbiota. Nod proteins are intracellular receptors that play key roles in innate immune response and inflammation. Here, we investigated the role of Nod proteins in regulation of intestinal goblet cell response in naive mice and mice infected with the enteric parasite Trichuris muris. We observed significantly fewer periodic acid-Schiff (PAS)-stained intestinal goblet cells and less mucin (Muc2) in Nod1 and Nod2 double-knockout (Nod DKO) mice after T. muris infection than in wild-type (WT) mice. Expulsion of parasites from the intestine was significantly delayed in Nod DKO mice. Treatment of naive WT mice with Nod1 and Nod2 agonists simultaneously increased numbers of PAS-stained goblet cells and Muc2-expressing cells, whereas treatment with Nod1 or Nod2 separately had no significant effect. Stimulation of mucin-secreting LS174T cells with Nod1 and Nod2 agonists upregulated core 3 β1,3-N-acetylglucosaminyltransferase (C3GnT; an important enzyme in mucin synthesis) and MUC2. We also observed lower numbers of PAS-stained goblet cells and less Muc2 in germfree mice. Treatment with Nod1 and Nod2 agonists enhanced the production of PAS-stained goblet cells and Muc2 in germfree mice. These data provide novel information on the role of Nod proteins in goblet cell response and Muc2 production in relation to intestinal innate defense.
INTRODUCTION
Goblet cells are the main source of mucins, and the mucus layer coating the gastrointestinal (GI) tract and containing mucins represents the front line of innate defense in the GI tract (1–3). Mucins act as the main structural component of the mucus, giving rise to its polymeric, viscoelastic, and protective properties. Up to 21 different mucin genes have been identified, cloned, and partially sequenced in humans, and the majority of their homologues have been identified in mice (4). The mucin genes MUC2/Muc2 and MUC3/Muc3 are found in large amounts in the GI tract of mice and play the key role in mucin production (1, 5). (MUC and MUC refer to the human gene and protein, whereas Muc and Muc refer to the mouse counterparts [6, 7].) Core 3 β1,3-N-acetylglucosaminyltransferase (C3GnT) is an important enzyme in the synthesis of core 3-derived O-glycans, and it has been shown that disruption of the C3GnT gene reduces levels of colon-specific Muc2 protein (8). Hyperplasia of goblet cells has been observed in a number of parasitic infections, including infection with Nippostrongylus brasiliensis, Trichinella spiralis, Hymenolepis diminuta, and Trichuris muris (5, 9–11). Putative mechanisms underlying the protective role of mucins against parasites include the trapping of worms in the mucus and inhibition of parasite motility and feeding capacity (5, 9, 11). Recently, we showed that in T. muris infection, worm expulsion in the initial stage of infection is Muc2 dependent, whereas worm expulsion in the late stage is Muc2 independent (12). Goblet cell response in nematode infection is thought to be under the control of the T helper 2 (Th2)-type response and is considered a potential effector mechanism (3, 5). However, a precise mechanism by which goblet cell response is modulated in intestinal parasite infection remains to be elucidated.
The gut is colonized by a complex, dynamic microbial ecosystem. The resident microbiota in the gut constitutes a heterogeneous microbial ecosystem containing up to 1 × 1014 CFU of bacteria (13). Pattern recognition receptors (PRRs), such as cell surface Toll-like receptors (TLRs) and cytoplasmic nucleotide-binding oligomerization domain-like receptors (NLRs), have a crucial role in innate defense, that of recognizing pathogen-associated molecular patterns (PAMPs) (14). Studies have identified a role of Nod proteins in recognizing bacterial infection through their detection of peptidoglycan, which can enter the cell either through specific transporters or as a consequence of infection with certain pathogenic bacteria (15). Once triggered, Nod proteins commence a pattern of gene expression in cells that help to clear infection. There are two Nod proteins, Nod1 and Nod2, and while Nod1 senses diaminopimelic acid (DAP)-containing peptidoglycan, which is found mainly in Gram-negative bacteria, Nod2 senses muramyl dipeptide (MDP), which is present in both Gram-positive and Gram-negative bacteria (16). Despite a significant increase in knowledge of Nod proteins in bacterial infections in recent years, the precise role of these innate receptors in intestinal cellular and immune responses in enteric parasitic infections remains unexplored.
Gut microbes can regulate mucin production by activating different signaling cascades and secretory elements. Probiotics such as Lactobacillus plantarum were reported to induce MUC2 and MUC3 and inhibit the adherence of enteropathogenic Escherichia coli, indicating that the enhanced mucus layer and glycocalyx overlying the intestinal epithelium and the occupancy of the microbial binding sites by Lactobacillus spp. provide protection against invasion by the pathogens (17). Bacterial products such as lipopolysaccharides (LPS) and flagellin A from Gram-negative bacteria and lipoteichoic acids (LTA) from Gram-positive bacteria are the most common modulators of mucin production, affecting mainly Muc2 and Muc5AC. Altered goblet cell response is also observed in germfree animals (18, 19). Due to the strategic location of goblet cells in the intestinal mucosa, it is very likely that the gut microbiota-Nod axis plays an important role in goblet cell response and that infections with enteric parasites utilize this axis to modulate mucin production in the gut.
In this study, we investigated the role of Nod proteins in regulation of goblet cell response in the context of T. muris infection. For the first time, we demonstrate that Nod proteins play an important role in regulation of intestinal goblet cell hyperplasia and mucin production in the context of innate defense in T. muris infection.
MATERIALS AND METHODS
Animals.
Breeding pairs of Nod1 and Nod2 double-knockout (Nod DKO) mice on a C57BL/6 background were provided by D. Philpott (University of Toronto). Nod DKO mice were originally generated by crossing Nod1 single-knockout mice and Nod2 single-knockout mice (20). Breeding pairs of C3GnT-deficient (C3GnT−/−) mice on a C57BL/6 background were provided by L. Xia (University of Oklahoma). C3GnT−/− mice were generated by targeted homologous recombination in mouse embryonic stem cells (8). Nod DKO and C3GnT−/− mice were kept in sterilized, filter-topped cages and fed autoclaved food under specific-pathogen-free (SPF) conditions in the animal facility at McMaster University, Hamilton, Ontario, Canada. Germfree C57BL/6 mice were acquired from the Farncombe Axenic Gnotobiotic Unit (AGU) at McMaster University. In some experiments, C57BL/6 mice received nonabsorbable antimicrobials (neomycin [5 mg/ml] or rifampin [0.2 mg/ml]) in drinking water for 7 days. Control mice received sterile water. All animal experiments were approved by the McMaster University Animal Care Committee and conducted in accordance with guidelines set by the Canadian Council on the Use of Laboratory Animals.
Parasite infection.
Trichuris muris parasites were harvested and ova were collected and maintained as previously described (21). All infected mice received approximately 300 T. muris ova in 200 μl distilled water by oral gavage. Mice were sacrificed at various time points postinfection, and worm burden was assessed by counting the number of worms present in the cecum.
Histological analysis and immunohistochemistry.
Formalin-fixed, paraffin-embedded sections of intestines were stained with periodic acid-Schiff (PAS) stain to detect intestinal goblet cells. The number of PAS+ goblet cells was expressed per 10 crypts. For immunohistochemistry, formalin-fixed, paraffin-embedded colonic segments were sectioned to 5 μm in thickness, deparaffinized by heating at 60°C for 30 min, cleared with CitriSolv (Fisher Scientific, ON, Canada), and rehydrated in a graded ethanol series of decreasing ethanol concentrations. Sections were subjected to heat-induced epitope retrieval (10 mM sodium citrate buffer–0.05% Tween 20, pH 6.0), blocked, and incubated with a polyclonal antibody raised against Muc2 (1:75 dilution; sc-15334; Santa Cruz Biotech) overnight at 4°C. Sections were washed with phosphate-buffered saline (PBS)–0.5% Tween 20 and incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:1,000) (Molecular Probes/Invitrogen). Sections were mounted using ProLong Gold antifade reagent containing 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes/Invitrogen). Images were captured using a Nikon Eclipse 80i microscope and NIS-Elements Basic Research imaging software. The number of Muc2+ cells was expressed per crypt. Investigators were blinded to the study groups.
Treatment with Nod agonists both in vitro and in vivo.
Naive C57BL/6, C3GnT−/−, and germfree mice (n = 5 per group) were intrarectally administered Nod1 ligand (C12-iE-DAP), Nod2 ligand (L18-MDP), both Nod1 and Nod2, or vehicle (PBS) at a dose of 50 μg per mouse. All mice were sacrificed 72 h later to assess colonic goblet cell numbers and expression levels of Muc2 and C3GnT in colonic tissue via real-time PCR.
Cell culture.
LS174T cells were obtained from Kris Chadee (Gastrointestinal Research Group, University of Calgary, Canada). Cells were cultured in T75 tissue culture flasks (Costar, Cambridge, MA, USA) in Dulbecco's modified Eagle medium–nutrient mixture F-12 (DMEM–F-12) medium (Gibco BRL Life Technologies, Burlington, Canada) containing 10% fetal bovine serum, 100 units ml−1 penicillin-streptomycin, 100 μg ml−1 streptomycin, and 20 mM HEPES (all purchased from Invitrogen Life Technologies). Cells were maintained at 37°C in a humidified incubator at 5% CO2. Culture medium was replaced with prewarmed medium every 2 days. Confluent cultures (80%) were harvested by trypsin-EDTA digest. Cells from passages 3 to 5 were used in this study.
PCR.
Colon tissue samples were stored in RNAlater RNA stabilization reagent (Qiagen) and stored at −80°C. Total RNA was isolated from tissue or cultured cells using an RNeasy midikit (Qiagen) and reverse transcribed into cDNA using a Omniscript reverse transciprtion (RT) kit or QuantiTect RT-PCR kit (Qiagen), as per the manufacturer's instructions. Total RNA was quantified using a NanoDrop 1000 spectrophotometer. Real-time PCR studies were performed with SsoFast EvaGreen Supermix (Bio-Rad) using a CFX96 real-time system (Bio-Rad). By using multiple classical internal control genes, the variability of each gene using a random subset of cDNA samples was assessed and the stability of the reference gene was assessed using geNorm (https://genorm.cmgg.be/) and as previously described by Vandesompele et al (22). 18S rRNA was used as an internal standard. Primer sequences for Muc2, C3GnT, and 18S rRNA are shown in Table 1. A commercially available prevalidated primer was used to determine the expression of MUC2 following the manufacturer's instructions (Bio-Rad assay ID qHsaCID0011696; NCBI RefSeq NC_000011.9). Each sample was run in triplicate. The cycling conditions were as follows: for Muc2, initial denaturation at 95°C for 5 min followed by 30 amplification cycles (94°C for 30 s, 55°C for 30 s, 72°C for 45 s) with an extension step of 72°C for 10 min following the final cycle; for C3GnT, initial denaturation at 95°C for 5 min followed by 30 amplification cycles (94°C for 30 s, 53°C for 30 s, 72°C for 30 s). Data were analyzed by using Gene Expression Macro OM 3.0 software (Bio-Rad).
TABLE 1.
Primers used for real-time RT-PCR
| Gene | Orientation | Sequence (5′-3′) |
|---|---|---|
| Muc2 (mouse) | Forward | CTGACCAAGAGCGAACACAA |
| Reverse | CATGACTGGAAGCAACTGGA | |
| C3GnT (mouse) | Forward | GGCCAGATTCTCCTCTCTCAAACG |
| Reverse | AGTGCTCCGCTGTCCAGTCCA | |
| C3GnT (human) | Forward | GGGGATGGCTCCTGTCTATT |
| Reverse | TAAGAAACTCACGCCCACCAG | |
| 18S rRNA (mouse) | Forward | GTAACCCGTTGAACCCCATT |
| Reverse | CCATCCAATCGGTAGTAGCG | |
| 18S rRNA (human) | Forward | TCCACAGGAGGCCTACACGCC |
| Reverse | TTTCCGCCGCCCATCGATGTT |
Colonic cytokines.
To assess colonic tissue cytokine levels, colon samples were homogenized in Tris-buffered saline (TBS) containing a protease inhibitor mixture (Sigma). Total protein levels in colon homogenates were quantified using the Bio-Rad DC protein assay kit (Bio-Rad). Cytokine levels were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quantikine murine; R&D Systems, Minneapolis, MN, USA) or the Bio-Plex Luminex system with a Bio-Rad mouse cytokine multiplex assay kit (Bio-Rad, Canada). In the latter, fluorescence data were acquired and analyzed by Bio-Plex Manager software (version 5.0; Bio-Rad Laboratories).
Statistical analysis.
All data are presented as means and standard errors of the means (SEM). An unpaired t test or one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison post hoc test or Mann-Whitney U test was performed using GraphPad Prism version 6.0b for Mac OS X (GraphPad Software, La Jolla, CA). An associated P value of <0.05 was considered statistically significant.
RESULTS
Nod proteins play an important role in the development of T. muris-induced intestinal goblet cell hyperplasia.
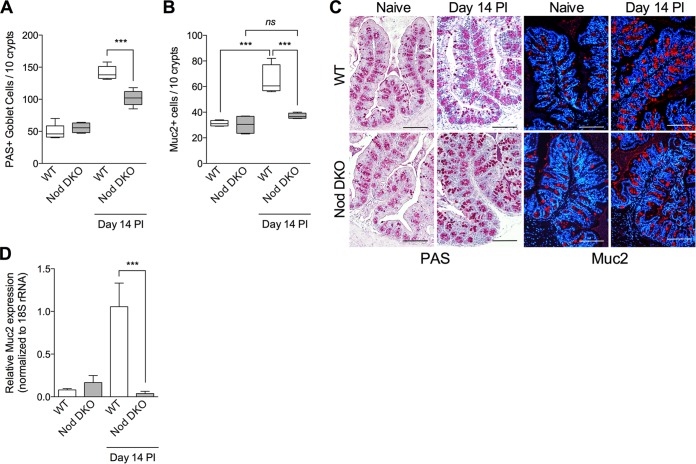
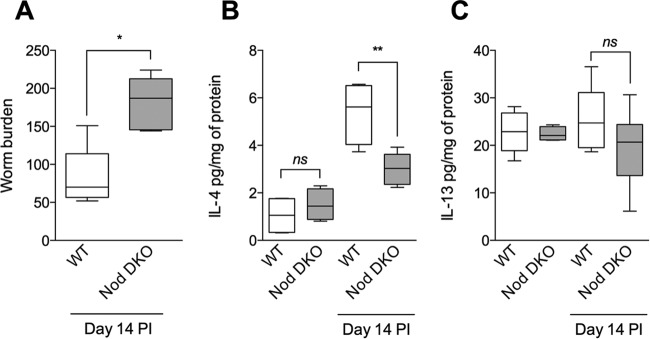
To investigate the role of Nod proteins in generation of T. muris-induced intestinal goblet cell hyperplasia, we infected Nod DKO mice with T. muris and assessed the numbers of PAS+ goblet cells and Muc2 production. We observed significantly fewer PAS+ intestinal goblet cells on day 14 postinfection (p.i.) in Nod DKO mice than in wild-type (WT) C57BL/6 mice (Fig. 1A and C). We also observed significant downregulation of Muc2+ cells and Muc2 expression in intestinal tissues, as assessed by immunohistochemical staining and RT-PCR analysis, respectively, in Nod DKO mice compared to WT mice after infection (Fig. 1B, C, and D). Goblet cells are considered a key component of host defense in T. muris infection. We observed significantly more worms in Nod DKO mice on day 14 p.i. than in WT mice (Fig. 2A). As T. muris infection induces Th2-type immune response in C57BL/6 mice, we investigated interleukin 4 (IL-4) and IL-13 levels in colonic tissues and observed significantly lower IL-4 levels in Nod DKO mice than WT mice after infection (Fig. 2B). There was no significant difference in IL-13 levels between the Nod DKO and WT mice with or without infection (Fig. 2C).
FIG 1.
Intestinal goblet cells and Muc2 production in Nod DKO and wild-type (WT) mice with or without T. muris infection. Nod DKO and WT mice were infected with T. muris and were sacrificed on day 14 p.i. (A) Numbers of PAS-stained goblet cells. (B) Numbers of Muc2+ cells, as assessed by immunohistochemistry. (C) Representative images of PAS- and Muc2-stained intestinal sections in Nod DKO and WT mice with or without T. muris infection. (D) Muc2 expression in intestinal tissue using real-time PCR. Data are presented as means, with error bars representing SEM, or box and whisker plots. Magnification, ×200; bars, 100 μm. Groups were compared using Tukey's multiple comparison posttest. For box and whisker plots, the horizontal line within the box indicates the median, boundaries of the box indicates the 25th and 75th percentiles, and whiskers indicate the highest and lowest values of the results. ***, P < 0.001 (n = 5 for uninfected groups; n = 7 for infected groups); ns, not significant.
FIG 2.
Intestinal worm burden and cytokines in Nod DKO and wild-type (WT) mice with or without T. muris infection. Nod DKO and WT mice were infected with T. muris and were sacrificed on day 14 p.i. (A) Worm burden. (B) IL-4 levels in intestinal tissues. (C) IL-13 levels in intestinal tissues. Data are presented as box and whisker plots. Groups were compared using Tukey's multiple comparison posttest. The horizontal line within the box indicates the median, boundaries of the box indicates the 25th and 75th percentiles, and whiskers indicate the highest and lowest values of the results. **, P < 0.01; *, P < 0.05; ns, not significant (n = 5 for uninfected groups; n = 7 for infected groups).
Treatment with Nod agonists increases intestinal goblet cell numbers in naive mice.
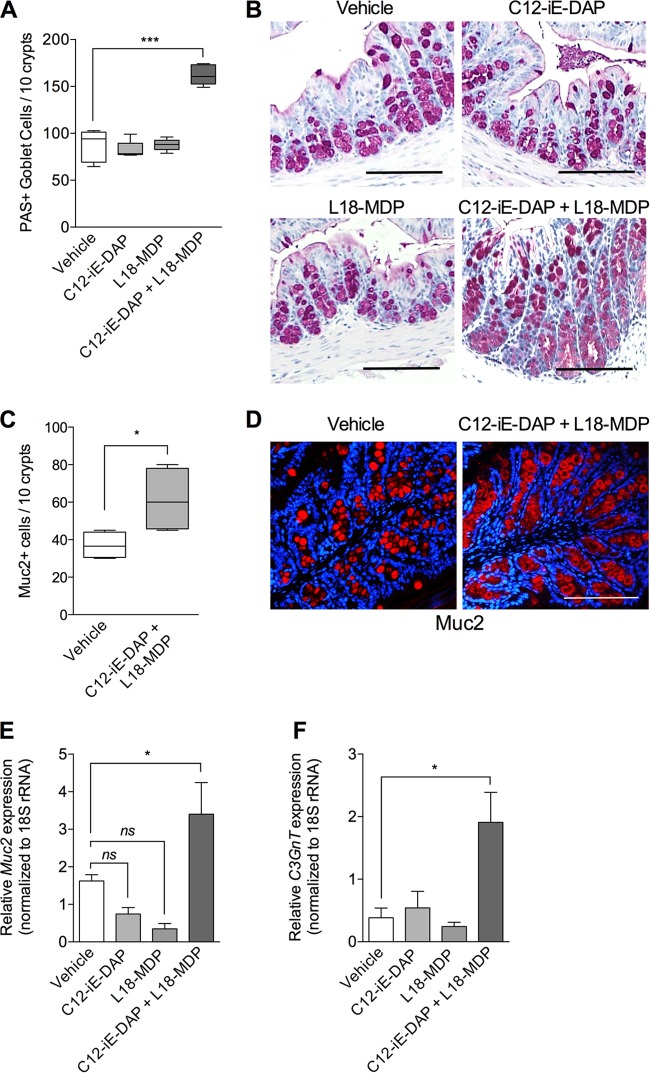
As we observed significantly lower numbers of PAS+ intestinal goblet cells and Muc2+ cells in Nod DKO mice after T. muris infection, next, we investigated whether treatment with a Nod1 (C12-iE-DAP) or Nod2 (L18-MDP) agonist has an effect on intestinal goblet cell response in naive wild-type mice. Naive C57BL/6 mice were treated with either C12-iE-DAP or L18-MDP or both (Nod1 plus Nod2), and colonic PAS-stained goblet cells were investigated 72 h posttreatment. Control mice received vehicle (PBS). Treatment with C12-iE-DAP or L18-MDP alone had no significant effect on the number of PAS-stained goblet cell numbers compared with controls. Treatment with both agonists, however, resulted in a significant increase in numbers of PAS-stained goblet cells (Fig. 3A and B). In addition, by immunohistochemical staining and RT-PCR analysis, we observed a significant upregulation of Muc2 in mice treated with both agonists (C12-iE-DAP and L18-MDP) simultaneously compared to that in mice treated with vehicle or with a single agonist (Fig. 3C, D, and E).
FIG 3.
Numbers of intestinal goblet cells and levels of Muc2 and C3Gnt expression in naive mice after treatment with Nod1 and Nod2 agonists. Naive C57BL/6 mice were treated (intrarectally, single dose) with either a Nod1 agonist (C12-iE-DAP), a Nod2 agonist (L18-MDP), both Nod1 and Nod2 agonists, or vehicle (PBS). Mice were sacrificed 72 h posttreatment. (A) Numbers of PAS-stained goblet cells in colon tissue. (B) Representative images of PAS-stained intestinal sections. (C) Numbers of Muc2+ cells, as assessed by immunohistochemistry. (D) Representative immunostaining for murine Muc2 (red) and counterstaining with DAPI (blue) in vehicle-treated mice and mice treated with both Nod1 and Nod2 agonists (C12-iE-DAP + L18-MDP. (E) Muc2 gene expression in intestinal tissue sections, assessed by using real-time PCR. (F) C3GnT gene expression in colon tissue sections, assessed by using real-time PCR. Magnification, ×200; bars, 100 μm. Groups were compared using Tukey's multiple comparison posttest. Data are represented as means, and error bars represent SEM (5 mice per group). ***, P < 0.001; *, P < 0.05; ns, not significant.
Treatment with Nod agonists upregulates C3GnT expression both in vivo and in vitro.
C3GnT is an important enzyme in mucin (Muc2) synthesis. As treatment with Nod1 and Nod2 agonists upregulated intestinal goblet cell numbers, we next investigated whether treatment had any effect on C3GnT gene expression using both in vivo and in vitro systems. In order to investigate whether Nod1 and Nod2 agonists are able to upregulate C3GnT expression in vivo, we investigated C3GnT expression in naive mice treated with C12-iE-DAP and L18-MDP intrarectally. Treatment with both agonists simultaneously induced significant upregulation of colonic C3GnT expression (Fig. 3F), consistent with the results of upregulation in Muc2 production (Fig. 3C, D, and E). In addition, stimulation of mucin-producing LS 174T cells in vitro with both C12-iE-DAP and L18-MDP significantly upregulated C3GnT expression compared to that in unstimulated cells or single-agonist-stimulated cells (Fig. 4A). We also observed an increase in MUC2 gene expression in LS 174T cells after simultaneous stimulation with C12-iE-DAP and L18-MDP (Fig. 4B). Together, these observations suggest that Nod proteins play an important role in regulating mucin production by modulating C3GnT gene expression.
FIG 4.
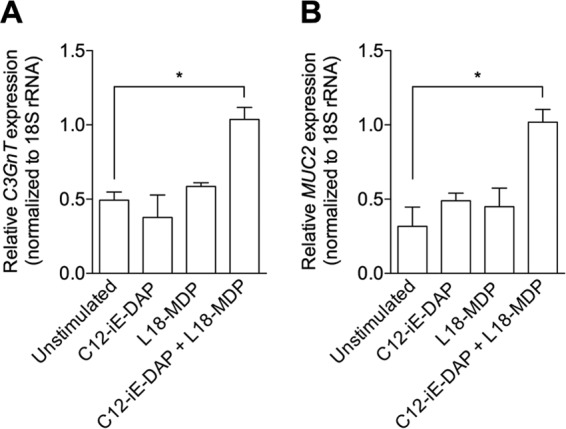
Treatment with Nod1 and Nod2 agonists simultaneously in vitro increases C3GnT (A) and MUC2 (B) expression. Mucin-producing LS 174T cells were stimulated with a Nod1 agonist (C12-iE-DAP), a Nod2 agonist (L18-MDP), or both Nod1 and Nod2 agonists for investigation of C3GnT and MUC2. C3GnT and MUC2 expression was assessed by using real-time PCR. Data are represented as means, and error bars represent SEM. Groups were compared using Tukey's multiple comparison posttest. *, P < 0.05.
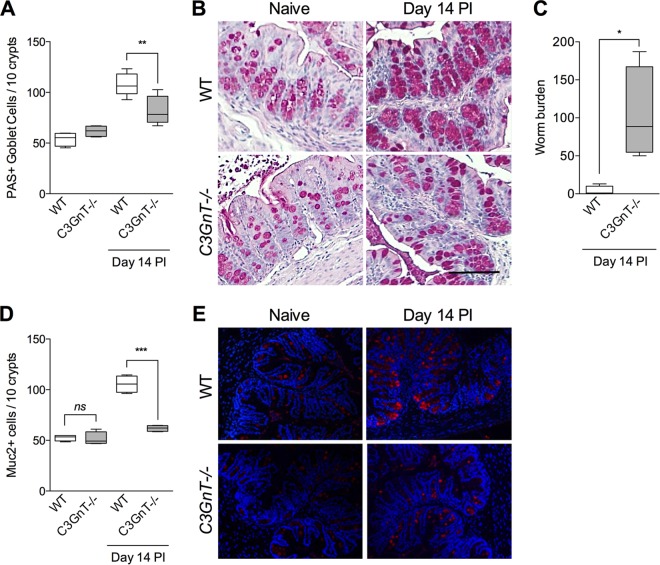
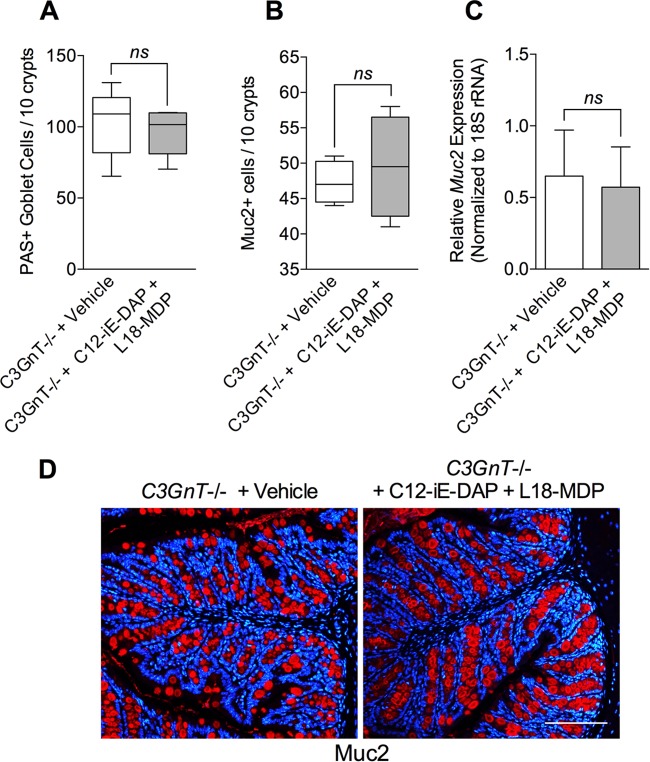
Nod agonists fail to enhance goblet cell numbers and Muc2 production in C3GnT deficient mice.
To extend our studies and investigate whether Nod1 and Nod2 agonists are able to upregulate intestinal goblet cell numbers and mucin production by influencing C3GnT gene expression, we repeated these experiments using C3GnT gene deficient (C3GnT−/−) mice with or without T. muris infection. We observed significantly fewer PAS+ intestinal goblet cells (Fig. 5A and B) and Muc2+ cells (Fig. 5D and E) in C3GnT−/− mice than in the WT mice after T. muris infection, implying an important role of this enzyme in goblet cell function in this model. There was modest but statistically significant difference between PAS+ goblet cell numbers in uninfected C3GnT −/− mice and T. muris-infected C3GnT−/− mice, suggesting the presence of additional factors in regulation of goblet cell biology during this infection. The reduced numbers of PAS+ goblet cells and Muc2+ cells in C3GnT−/− were associated with increased worm burden on day 14 p.i. (Fig. 5C). We also observed that simultaneous treatment of naive C3GnT−/− mice with agonists of Nod1 and Nod2 had no effect on PAS+ goblet cell numbers, Muc2+ cell numbers, or Muc2 expression in intestinal tissues (Fig. 6). These findings suggest that C3GnT plays an important role in regulation of Nod protein-stimulated intestinal goblet cell response and mucin production.
FIG 5.
Numbers of PAS+ and Muc2+ cells were significantly lower in C3GnT−/− mice after T. muris infection. C3GnT−/− mice were infected with T. muris and sacrificed on day 14 p.i. (A) PAS+ goblet cell numbers in C3GnT−/− and wild-type mice on day 14 p.i. Groups were compared using Tukey's multiple comparison posttest. (B) Representative micrograph of PAS-stained goblet cells in colon sections. Magnification, ×200; bars, 100 μm. (C) Worm burden in C3GnT−/− and wild-type (WT) mice on day 14 p.i. The Mann-Whitney U test was used to analyze statistical differences among the groups. (D) Muc2+ cells as assessed by immunohistochemistry. (E) Representative images of Muc2-stained intestinal sections in C3GnT−/− and WT mice with or without T. muris infection. Groups were compared using Tukey's multiple comparison posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant (5 mice per group).
FIG 6.
Nod agonists fail to increase goblet cell numbers and Muc2 production in naïve, C3GnT-deficient mice. (A) Numbers of PAS+ goblet cells in C3GnT−/− mice treated with both Nod1 and Nod2 agonists (C12-iE-DAP + L18-MDP). There was no significant difference between agonist-treated groups and vehicle-treated controls in C3GnT−/− mice. (B) Numbers of Muc2+ cells, as assessed by immunohistochemistry. (C) Muc2 expression in C3GnT−/− mouse intestinal tissue following treatment with Nod1 and Nod2 agonists (C12-iE-DAP + L18-MDP) as determined by real-time PCR. (D) Representative immunostaining for murine Muc2 (red) and counterstaining with DAPI (blue) in C3GnT−/− mice treated with vehicle or Nod1 and Nod2 agonists (C12-iE-DAP + L18-MDP). Magnification, ×200; bars, 100 μm. The Mann-Whitney U test was used to analyze statistical differences among the groups. ns, not significant (5 mice per group).
Treatment with Nod agonists upregulates intestinal goblet cell numbers and Muc2 in germfree mice.
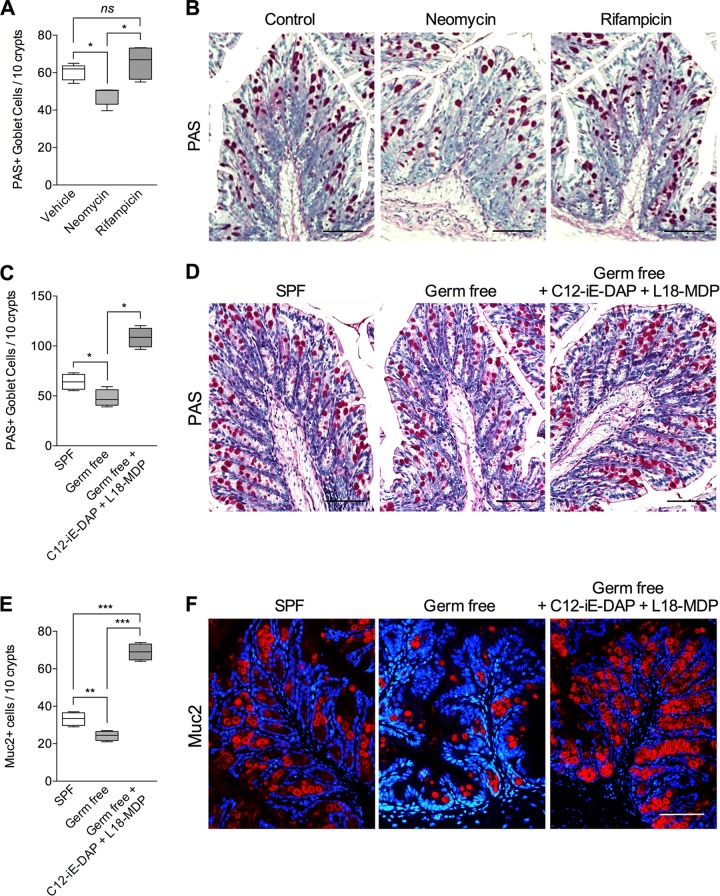
Nod proteins are important innate receptors for bacterial products. As we observed an upregulation of goblet cells in response to Nod agonists, we next tested the hypothesis that the gut microbiota plays an important role in mediating the Nod protein-mediated changes in intestinal goblet cell responses. To understand the role of the gut microbiota in intestinal goblet cell biology, we investigated colonic goblet cell numbers in germfree C57BL/6 mice and in mice with perturbation of the microbiota by oral administration of antimicrobials which have been previously shown to alter the bacterial composition of the gut (23). We observed significantly reduced numbers of PAS+ intestinal goblet cells in naive mice treated with the broad-spectrum antibiotic neomycin (Fig. 7A and B). However, there was no significant difference in PAS+ goblet cell numbers after treatment with another antibiotic, rifampin. The role of gut microbiota in intestinal goblet cells response was further investigated in germfree mice. The numbers of PAS+ intestinal goblet cells were significantly lower in germfree mice than in SPF mice (Fig. 7C and D). Immunohistochemical studies revealed the presence of less Muc2+ cells in germfree mice (Fig. 7E and F). Importantly, when we treated the germfree mice simultaneously with C12-iE-DAP and L18-MDP, we observed a significant increase in PAS+ intestinal goblet cells and Muc2+ cells (Fig. 7C to F). These observations suggest a potential important role of the gut microbiota-Nod axis in the intestinal goblet cell response.
FIG 7.
Treatment with Nod agonists upregulates intestinal goblet cell numbers and Muc2 in germfree mice. (A) Numbers of PAS+ goblet cells in naive C57BL/6 mice treated with vehicle or the broad-spectrum antibiotic neomycin or rifampin. (B) Representative micrographs of PAS-stained intestinal sections in vehicle-, neomycin-, or rifampin-treated mice. (C) Numbers of PAS+ intestinal goblet cells in specific-pathogen-free (SPF) mice, germfree mice, and germfree mice treated with both Nod1 and Nod2 agonists (C12-iE-DAP + L18-MDP). (D) Representative micrographs of PAS-stained intestinal sections from the same mice. (E) Numbers of Muc2+ cells, as assessed by immunohistochemistry. (F) Representative micrographs of Muc2-stained intestinal sections. Magnification, ×200; bars, 100 μm. Groups were compared using Tukey's multiple comparison posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant (5 mice per group).
DISCUSSION
Goblet cells are the main source of mucins in the gut and are considered a key component of innate defense mechanisms against various enteric infections (9). Despite a significant increase in knowledge regarding Nod proteins in bacterial infections and mucosal immunity in recent years, the role of these innate receptors in goblet cell responses remains unexplored. In the present study, we demonstrate an important role of Nod proteins in intestinal goblet function and mucin production in the context of an intestinal nematode parasite infection.
The innate immune receptors Nod1 and Nod2 are important components of the first line of host defense for detection of bacterial peptidoglycan (PG) within the cytoplasm of intestinal epithelial cells. Recently, it was shown that Nod2 plays an important role in colonic epithelial cells responses to T. muris infection and subsequent recruitment of dendritic cells following infection (24). Goblet cells are considered a key component of innate defense in many nematode parasite infections, including T. muris infection (12, 25–27), but the role of Nod proteins in the regulation of these cells has not been investigated. In this study, we observed downregulation of intestinal goblet cell numbers and Muc2 expression following T. muris infection in Nod DKO mice compared to WT mice, suggesting an important role of Nod proteins in generation of intestinal goblet cell hyperplasia and mucin production in this infection. We also observed a significant difference in PAS+ goblet cell numbers in infected Nod DKO mice on day 14 p.i. compared to those in uninfected mice. Previous studies have shown that Th2 cells play an important role in goblet cell response in intestinal nematode infections, including T. muris infection (3, 28, 29). In addition, recent studies have shown a role of TLR4 in goblet cell response in Citrobacter rodentium infection (30) and in differentiation of goblet cells in intestinal organoid and enterocyte cell cultures (31). Therefore, it seems likely that in addition to Nod receptors, other innate receptors, such as TLR, and adaptive immune cells, such as T cells, can also influence the goblet cell response after T. muris infection.
We also observed that simultaneous treatment of naive mice with Nod1 and Nod2 agonists significantly upregulated PAS+ intestinal goblet cell numbers and Muc2 production, whereas treatment with individual agonists had no significant effect. In addition, treatment of naive mice with Nod1 and Nod2 agonists significantly upregulated the expression of C3GnT (the enzyme responsible for synthesizing the major component of Muc2) (32). To further understand the role of Nod proteins in intestinal goblet cell biology, we utilized the mucin-producing cell line LS 174T, and consistent with in vivo findings, we observed that simultaneous stimulation with agonists for Nod1 and Nod2 significantly upregulated the expression of MUC2 and C3GnT in mucin-producing cells. Individual treatment with Nod1 or Nod2 agonists had no significant effect on MUC2 or C3GnT expression. As we observed that the absence of Nod1 and Nod2 in Nod DKO mice inhibited the upregulation of T. muris-induced goblet cell hyperplasia, and as C3GnT is a major enzyme in Muc2 synthesis, we next investigated whether C3GnT has any role in goblet cell response or mucin production by utilizing C3GnT−/− mice. C3GnT−/− mice lack the core 3 O-glycans of colonic mucins. These mice have a modest reduction in glycosylation and are susceptible to experimental triggers of colitis (8). We observed significantly fewer PAS+ goblet cells and less Muc2 expression in C3GnT−/− mice after T. muris infection. The reduced numbers of PAS+ goblet cells and smaller amounts of Muc2 in C3GnT−/− were associated with increased worm burden. We did not observe any significant difference in intestinal IL-4 and IL-13 levels between C3GnT−/− mice and WT mice after T. muris infection (data not shown). Thus, in spite of the delay in worm expulsion, C3GnT deficiency had no significant effect on generation of the Th2-type immune response in T. muris infection. Moreover, simultaneous treatment with Nod1 and Nod2 agonists in naive C3GnT−/− mice failed to upregulate goblet cell numbers and Muc2 production suggesting an important role for C3GnT in Nod protein-mediated goblet cell hyperplasia and mucin production. Together, these findings suggest that Nod proteins play an important role in goblet cell response and mucin production and in generation of T. muris-induced intestinal goblet cell hyperplasia in relation to host defense. The mechanisms underlying the synergistic roles of Nod1 and Nod2 in increasing goblet cell numbers and mucin production in T. muris infection are not known. However, there are studies that report that both Nod1 and Nod2 function in a synergistic fashion to tune the appropriate responses to certain pathogens. For example, Nod1 and Nod2 DKO mice showed a significant reduction in the production of inflammatory cytokines and an increase in the bacterial colonization of the mucosal tissue in a Salmonella model of colitis. These phenotypes were not observed in Nod1 and Nod2 single knockouts (33). Synergism between Nod1 and Nod2 was also reported in a murine model of Bacillus anthracis. Mice deficient for both Nod1 and Nod2 were more susceptible to lethal challenge with B. anthracis and produced lower levels of proinflammatory molecules than single knockouts (34). Th2 cells play an important role in development of goblet cell hyperplasia in infections with many intestinal nematode parasites, including T. muris (3, 28). Therefore, since stimulation of Nod1 and that of Nod2 drive similar activation pathways, it is likely that synergistic contributions of Nod1 and Nod2 are required for the development of Th2 immune response and subsequent goblet cell hyperplasia during T. muris infection. We also observed that treatment of noninfected mice with Nod1 agonist or Nod2 agonist alone had no significant effect on goblet cell numbers or mucin production. However, simultaneous treatment with both agonists resulted in a significant increase in goblet cell numbers and Muc2 production. Therefore, another possibility is the direct stimulation of Nod proteins by gut microbiota in goblet cell biology. Combined stimulation of both Nod1 and Nod2 is required in influencing goblet cell biology, upregulating goblet cell numbers and Muc2 production.
We recently showed a functional role for the mucus barrier in host protective immunity to T muris infection, because in the absence of Muc2, worm expulsion is delayed (12). We have shown that around the time of worm expulsion, the mucus barrier is less porous in the resistant strains of mice (BALB/c and C57BL/6 mice, which expel the parasites) than in the susceptible mice (AKR mice, which do not expel the parasites and develop chronic infection), and this alteration in physical properties of the barrier after infection may directly affect the niche of the worms. Another possibility is that the lower levels of mucins result in a network that may compromise host defense because of inappropriate presentation or concentration of other host defense proteins (such as Relm-β and Tff3) in the environment of the worms. As goblet cells are the main source of mucins in the gut, these observations suggest that smaller amounts of mucins, due to reduced numbers of goblet cells, might interfere with worm expulsion.
Immune responses associated with many intestinal nematode infections, including T. muris infection, are characterized by the activation of Th2-type immune response. Previous studies suggested that IL-4 and IL-13 cooperate in the development of Th2 responses, and although their functions overlap, they perform additive roles. Expulsion of the parasites took place in the absence of either cytokine but not in the absence of both cytokines (35). It is also suggested that that blocking of either IL-4 or IL-13 is sufficient to inhibit worm expulsion in T. muris infection (36). In this study, we observed a significant difference in intestinal IL-4 levels but not in IL-13 levels between Nod DKO and WT mice after T. muris infection despite the inhibition in worm expulsion, further supporting the redundancy in the roles of IL-4 and IL-13 in the expulsion of nematode parasites.
The GI tract is colonized by a complex, heterogenous, and dynamic microbial ecosystem containing up to 1 × 1014 CFU of bacteria (13, 37). This colonization of the mammalian GI tract occurs soon after birth. A mutually beneficial relationship exists between the gut and many of its symbionts: the gut provides nutrients to the resident bacteria, while they aid in the digestion of food and absorption of nutrients, producing biotin and vitamin K, while regulating immune system function and hindering the colonization of pathogenic microorganisms (38). Variations in gut microbial composition can result from genetic and environmental factors such as diet, living conditions, and birthplace (39). The gut microbiota is implicated in various GI disorders, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and enteric infections (40). Gut microbes can regulate mucin production by activating different signaling cascades and secretory elements. Beneficial bacteria, such as Lactobacillus plantarum 299V, were reported to induce MUC2 and MUC3 and inhibit the adherence of enteropathogenic Escherichia coli, indicating that an enhanced mucus layer and glycocalyx overlying the intestinal epithelium and the occupancy of the microbial binding sites by Lactobacillus spp. provide protection against invasion by pathogens (17). Bacterial products such as LPS and flagellin A from Gram-negative bacteria and lipoteichoic acids (LTA) from Gram-positive bacteria are the most common modulators of mucin production by affecting mainly Muc2 and Muc5AC. In addition to evidence of enhanced mucus secretion in response to intestinal microbes, studies have shown altered goblet cell responses in germfree animals (18, 19, 41). In our recent study, we observed that treatment with beneficial bacteria L. rhamnosus (JB-1) promotes T. muris expulsion in association with upregulation of goblet cells (42). It was also shown that depletion of flora with antibiotics reduced the worm burden in T. muris infection in susceptible mice (43). In this study, we observed significantly fewer PAS+ goblet cells and less Muc2 in germfree mice than in SPF mice. We also observed fewer PAS+ goblet cells in naive mice treated with a broad-spectrum antibiotic, neomycin, but not those treated with rifampin. These findings demonstrate an important role of gut microbiota in the intestinal goblet cell response. Interestingly, we observed that simultaneous treatment with Nod1 and Nod2 agonists upregulated goblet cell numbers and Muc2 production in germfree mice, indicating an important role of Nod protein-mediated stimulation in the goblet cell response. Taking into consideration the role of gut microbiota in the intestinal goblet cell response and in establishment of T. muris infection (43), the findings of this study suggest a potential relationship among T. muris, gut microbiota, and Nod proteins in goblet cell response and in host defense in this parasitic infection. However, there is extensive complexity in this relationship, and further work is required to explore how the microbiota-Nod protein axis specifically regulates intestinal goblet cell responses that can contribute to parasite expulsion.
The findings of this study provide us with novel information on the role of Nod proteins in intestinal goblet cell response and mucin production and identify Nod proteins as potential new targets in modulating mucin production in the gut. In addition, this study suggests an important contribution of Nod proteins in mediating goblet cell response and Muc2 production in the context of intestinal innate defense in T. muris infection.
ACKNOWLEDGMENTS
This work is supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to Waliul I. Khan and from the Wellcome Trust, UK to David J. Thornton and Richard K. Grencis. Waliul I. Khan is a recipient of a CIHR New Investigator Award.
We thank Elena Verdu of the Farncombe Axenic Gnotobiotic Unit (AGU), McMaster University.
Funding Statement
The Wellcome Trust Centre for Cell-Matrix Research, University of Manchester, is supported by core funding from the Wellcome Trust, UK under grant number 088785/Z/09/Z.
REFERENCES
- 1.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. 2000. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Specian RD, Oliver MG. 1991. Functional biology of intestinal goblet cells. Am J Physiol 260:C183–C193. [DOI] [PubMed] [Google Scholar]
- 3.Khan WI, Blennerhasset P, Ma C, Matthaei KI, Collins SM. 2001. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol 23:39–42. doi: 10.1046/j.1365-3024.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 4.Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K. 2009. Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1:123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan WI. 2008. Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitology 135:671–682. doi: 10.1017/S0031182008004381. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima H. 2012. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol Pharm Bull 35:1637–1641. doi: 10.1248/bpb.b12-00412. [DOI] [PubMed] [Google Scholar]
- 7.Hasnain SZ, Gallagher AL, Grencis RK, Thornton DJ. 2013. A new role for mucins in immunity: insights from gastrointestinal nematode infection. Int J Biochem Cell Biol 45:364–374. doi: 10.1016/j.biocel.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 8.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. 2007. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JJ, Khan WI. 2013. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens 2:55–70. doi: 10.3390/pathogens2010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay DM, Khan WI. 2003. STAT-6 is an absolute requirement for murine rejection of Hymenolepis diminuta. J Parasitol 89:188–189. doi: 10.1645/0022-3395(2003)089[0188:SIAARF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Miller HR. 1987. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology 94(Suppl):S77–S100. doi: 10.1017/S0031182000085838. [DOI] [PubMed] [Google Scholar]
- 12.Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. 2010. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao WL, Lee YK. 2004. Microflora of the gastrointestinal tract: a review. Methods Mol Biol 268:491–502. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. 2014. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 16.Moreira LO, Zamboni DS. 2012. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol 3:328. doi: 10.3389/fimmu.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276:G941–G950. [DOI] [PubMed] [Google Scholar]
- 18.Szentkuti L, Riedesel H, Enss ML, Gaertner K, Von Engelhardt W. 1990. Pre-epithelial mucus layer in the colon of conventional and germ-free rats. Histochem J 22:491–497. doi: 10.1007/BF01007234. [DOI] [PubMed] [Google Scholar]
- 19.Enss ML, Grosse-Siestrup H, Schmidt-Wittig U, Gärtner K. 1992. Changes in colonic mucins of germfree rats in response to the introduction of a “normal” rat microbial flora. Rat colonic mucin. J Exp Anim Sci 35:110–119. [PubMed] [Google Scholar]
- 20.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. 2011. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 21.Wakelin D. 1967. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 57:515–524. doi: 10.1017/S0031182000072395. [DOI] [PubMed] [Google Scholar]
- 22.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 24.Bowcutt R, Bramhall M, Logunova L, Wilson J, Booth C, Carding SR, Grencis R, Cruickshank S. 2014. A role for the pattern recognition receptor Nod2 in promoting recruitment of CD103+ dendritic cells to the colon in response to Trichuris muris infection. Mucosal Immunol 7:1094–1105. doi: 10.1038/mi.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa N, Wakelin D, Mahida YR. 1997. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542–549. doi: 10.1053/gast.1997.v113.pm9247474. [DOI] [PubMed] [Google Scholar]
- 26.Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. 1995. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis infection in mice. Parasite Immunol 17:485–491. doi: 10.1111/j.1365-3024.1995.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 27.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. 2011. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. 2003. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun 71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan WI, Motomura Y, Blennerhassett PA, Kanbayashi H, Varghese AK, El-Sharkawy RT, Gauldie J, Collins SM. 2005. Disruption of CD40-CD40 ligand pathway inhibits the development of intestinal muscle hypercontractility and protective immunity in nematode infection. Am J Physiol Gastrointest Liver Physiol 288:G15-22. doi: 10.1152/ajpgi.00159.2004. [DOI] [PubMed] [Google Scholar]
- 30.Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during C. rodentium infection in mice. Infect Immun 74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. 2012. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143:708–718. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, Kudo T, Togayachi A, Ishizuka Y, Nakanishi H, Narimatsu H. 2002. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem 277:12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 33.Geddes K, Rubino S, Streutker C, Cho JH, Magalhaes JG, Le Bourhis L, Selvanantham T, Girardin SE, Philpott DJ. 2010. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect Immun 78:5107–5115. doi: 10.1128/IAI.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loving CL, Osorio M, Kim YG, Nuñez G, Hughes MA, Merkel TJ. 2009. Nod1/Nod2-mediated recognition plays a critical role in induction of adaptive immunity to anthrax after aerosol exposure. Infect Immun 77:4529–4537. doi: 10.1128/IAI.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. 1999. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med 1189:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. 1999. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infection. Curr Opin Immunol 11:420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 37.Lupp C, Finlay BB. 2005. Intestinal microbiota. Curr Biol 15:R235–R236. doi: 10.1016/j.cub.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 39.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekirov I, Russell SL, Antunes LCM, Finlay BB. 2010. Gut microbiota in health and disease. Physiol Rev 90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 41.Kandori H, Hirayama K, Takeda M, Doi K. 1996. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim 45:155–160. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- 42.McClemens J, Kim JJ, Wang H, Mao YK, Collins M, Kunze W, Bienenstock J, Forsythe P, Khan WI. 2013. Lactobacillus rhamnosus (JB-1) ingestion promotes innate host defense in an enteric parasitic infection. Clin Vaccine Immunol 20:818–826. doi: 10.1128/CVI.00047-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. 2010. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]