Abstract
Johne's disease (paratuberculosis) is a chronic enteritis in cattle that is caused by intracellular infection with Mycobacterium avium subsp. paratuberculosis. This infection is characterized by the functional exhaustion of T-cell responses to M. avium subsp. paratuberculosis antigens during late subclinical and clinical stages, presumably facilitating the persistence of this bacterium and the formation of clinical lesions. However, the mechanisms underlying T-cell exhaustion in Johne's disease are poorly understood. Thus, we performed expression and functional analyses of the immunoinhibitory molecules programmed death-1 (PD-1)/PD-ligand 1 (PD-L1) and lymphocyte activation gene 3 (LAG-3)/major histocompatibility complex class II (MHC-II) in M. avium subsp. paratuberculosis-infected cattle during the late subclinical stage. Flow cytometric analyses revealed the upregulation of PD-1 and LAG-3 in T cells in infected animals, which suffered progressive suppression of interferon gamma (IFN-γ) responses to the M. avium subsp. paratuberculosis antigen. In addition, PD-L1 and MHC-II were expressed on macrophages from infected animals, consistent with PD-1 and LAG-3 pathways contributing to the suppression of IFN-γ responses during the subclinical stages of M. avium subsp. paratuberculosis infection. Furthermore, dual blockade of PD-L1 and LAG-3 enhanced M. avium subsp. paratuberculosis-specific IFN-γ responses in blood from infected animals, and in vitro LAG-3 blockade enhanced IFN-γ production from M. avium subsp. paratuberculosis-specific CD4+ and CD8+ T cells. Taken together, the present data indicate that M. avium subsp. paratuberculosis-specific T-cell exhaustion is in part mediated by PD-1/PD-L1 and LAG-3/MHC-II interactions and that LAG-3 is a molecular target for the control of M. avium subsp. paratuberculosis-specific T-cell responses.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis is the causative bacterium of Johne's disease (paratuberculosis), which is a chronic granulomatous enteritis that leads to diarrhea and severe weight loss in cattle. Similar to other mycobacterial infections, M. avium subsp. paratuberculosis elicits strong Th1-mediated responses that are dominated by interferon gamma (IFN-γ)-secreting CD4+ T cells at the early stages of infection (1–3). IFN-γ contributes to the activation of macrophages, which eradicate intracellular mycobacteria during this phase (2, 3). The M. avium subsp. paratuberculosis-specific Th1 response is gradually suppressed during the late subclinical stage and is severely suppressed during the clinical stage (4–6), which facilitates bacterial growth, the formation of pathological lesions in the intestine, and the progression of clinical disease (2, 3). Thus, Th1-mediated immunity is considered central to the control of infection and disease progression. Therefore, to enhance Th1 responses to M. avium subsp. paratuberculosis in animals with Johne's disease, further characterization of the mechanisms responsible for dysfunctions of M. avium subsp. paratuberculosis-specific T cells is required.
Dysfunction of antigen-specific T cells is well documented as “T-cell exhaustion” and has been defined by the loss of effector functions during chronic infections (7–9). Exhausted T cells are characterized phenotypically by the surface expression of immunoinhibitory receptors such as programmed death 1 (PD-1) and lymphocyte activation gene 3 (LAG-3) (7). PD-1 and LAG-3 inhibit T-cell receptor signaling and the subsequent effector functions of T cells after binding of their ligands PD-ligand 1 (PD-L1) and major histocompatibility complex class II (MHC-II), respectively (10, 11). During various chronic infections, PD-1/PD-L1 and LAG-3/MHC-II interactions have been closely associated with the inhibition of chronically activated pathogen-specific T cells and the induction of exhausted T cells (8, 9, 12). Accordingly, blockade of PD-1/PD-L1 and LAG-3/MHC-II binding with antagonist antibodies inhibits these negative signals and reactivates T-cell responses such as proliferation, cytokine production, and cytotoxic activity of exhausted T cells (10, 11).
Our previous studies revealed that the upregulation of bovine PD-1 and LAG-3 in T cells was closely associated with disease progression in cattle infected with bovine leukemia virus (BLV) (13–15). Moreover, blockade of these inhibitory pathways restores exhausted T-cell function and induces antiviral responses in vitro (13, 15, 16). However, it remains unclear whether PD-1 and LAG-3 pathways are involved in the development of T-cell exhaustion in other chronic infections of cattle.
In a previous report, a polyclonal anti-human LAG-3 antibody was used in expression and functional analyses of bovine LAG-3 but showed only weak blockade activity toward T cells (15). In the present study, we established an anti-bovine LAG-3 monoclonal antibody (MAb) and determined whether PD-1/PD-L1 and LAG-3/MHC-II pathways downregulate M. avium subsp. paratuberculosis-specific T-cell responses during late-stage Johne's disease. In these analyses, increased expression of PD-1 and LAG-3 in T cells was associated with suppression of M. avium subsp. paratuberculosis-specific Th1 responses during the late subclinical stage. In addition, PD-L1 and MHC-II were expressed on macrophages, presumably interacting with PD-1 and LAG-3 on T cells in M. avium subsp. paratuberculosis-infected cattle. Furthermore, in vitro blockade using a novel anti-LAG-3 MAb restored IFN-γ production from M. avium subsp. paratuberculosis-specific CD4+ and CD8+ T cells. The present data indicate mechanisms that lead to exhaustion of M. avium subsp. paratuberculosis-specific T-cell responses and suggest that LAG-3 is a molecular target for the control of M. avium subsp. paratuberculosis-specific T-cell responses.
MATERIALS AND METHODS
Generation of anti-bovine LAG-3 MAb.
Two antigen peptides were designed from an extracellular region of bovine LAG-3 and included peptides 71-99 (GSAAPTPRGPGPRRYTVLRLAPGGLRIGK) and 104-132 (PRVQLEEMGLQRGDFSLWLRPARRADAGE). These peptides were synthesized and coupled to a keyhole limpet hemocyanin carrier protein at their NH2 termini. Two rats were immunized by injecting about 250 μg of each peptide emulsion in TiterMax Gold Adjuvant (Sigma-Aldrich, St. Louis, MO) into the footpad. After 21 days, lymphocytes were isolated from iliac lymph nodes (LNs), fused with SP2 myeloma cells, and grown into clonal colonies in methylcellulose-based semisolid medium. Rat immunization and hybridoma cultivation were performed at Cell Engineering Corporation (Osaka, Japan). Supernatants from hybridomas were screened using enzyme-linked immunosorbent assay (ELISA) with each immunized peptide and flow cytometry with Cos-7 cells transfected with bovine LAG-3 coding pEGFP-N2 (Clontech, Palo Alto, CA). Subsequently, anti-LAG-3 monoclonal antibodies (MAbs) were purified from culture supernatants of hybridomas. Purified MAbs were then tested using flow cytometry, and reactivity was determined with LAG-3-enhanced green fluorescent protein (EGFP)-expressing Cos-7 cells and bovine peripheral blood mononuclear cells (PBMCs) that were isolated freshly or were stimulated with phorbol myristate acetate (PMA)/ionomycin (Sigma-Aldrich) for 48 h.
Western blotting.
To confirm the reactivity of the anti-LAG-3 MAb, Western blot analyses were performed using LAG-3-EGFP-expressing Cos-7 cells. Cells (∼106) were lysed in 2× sodium dodecyl sulfate (SDS) buffer containing 125 mM Tris-HCl (pH 6.8), 4% SDS, 10% 2-mercaptoethanol, and 20% glycerol and boiled for 5 min. Samples were then separated on 12% SDS–polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA). After blocking with 3% skim milk in phosphate-buffered saline (PBS; pH 7.2) containing 0.05% Tween 20 (PBS-T), membranes were incubated at room temperature for 1 h with anti-LAG-3 MAb (71-2D8; 2 μg/ml) and then washed and incubated with horseradish peroxidase (HRP)-conjugated anti-rat IgG (1:5,000 dilution; MP Biomedicals, Irvine, CA). Membranes were also probed with anti-EGFP (0.5 μg/ml; Clontech) and antiactin antibodies (1:1,000 dilution; Merck Millipore) as positive and loading controls, respectively. After washing, membranes were incubated with Immobilon Western chemiluminescent HRP substrate (Merck Millipore) to visualize the signals, which were analyzed using a Fluor-S multiimager (Bio-Rad, Hercules, CA).
Animals and experimental infection.
Eight male Holstein calves between 1 and 6 weeks of age were orally inoculated with intestinal tissue homogenate from a clinically infected cow as described previously (17, 18). The total number of M. avium subsp. paratuberculosis organisms used in this experimental infection was 1.03 × 108 to 6.27 × 108 viable organisms per calf. Three age-matched male Holstein calves were maintained separate from inoculated cattle as uninfected control animals. All calves were kept in a biosafety level II animal facility at the National Institute of Animal Health (Tsukuba, Ibaraki, Japan). Peripheral blood and fecal samples were collected prior to infection and every 2 to 4 weeks postinoculation (wpi). All animal experiments carried out for this study were approved by the National Institute of Animal Health Ethics Committee. In addition, ileum tissues were collected from two cows in Japan which were naturally infected with M. avium subsp. paratuberculosis. These cattle showed clinical symptoms, such as chronic weight loss and intermittent diarrhea, and were confirmed to be infected with M. avium subsp. paratuberculosis by quantitative real-time PCR using fecal samples.
IFN-γ assays and detection of M. avium subsp. paratuberculosis in feces.
To examine the development of Th1 responses to M. avium subsp. paratuberculosis, whole blood cells were incubated with 5 μg of Johnin purified protein derivative (J-PPD)/ml at 37°C under 5% CO2 for 24 h. Collected culture supernatants were assayed for IFN-γ using ELISA as described previously (17, 18). The levels of fecal shedding of M. avium subsp. paratuberculosis were determined by using quantitative real-time PCR assay targeting the M. avium subsp. paratuberculosis-specific gene IS900 as described previously (19).
Cell preparation from blood and lymphoid tissues.
Seven infected cattle, excluding animal 65, and age-matched uninfected controls were euthanized at 135 to 148 wpi, and heparinized peripheral blood, spleens, ileocecal LNs, ileal and jejunal mesenteric LNs (mLNs), ilea, and jejuna were collected from all cattle. PBMCs were purified from blood samples using density gradient centrifugation on Percoll (GE Healthcare, Buckinghamshire, United Kingdom). Peyer's patches (PPs) from the ileum and jejunum were determined by gross morphological examinations of the tissues. Spleens, LNs, and PPs of ileum and jejunum tissues were minced in PBS and passed through a 40-μm-pore-size cell strainer (BD Biosciences, San Jose, CA), and then the lymphocytes collected from the tissues were washed twice with PBS. Animal 65 was separately euthanized due to its severe diarrhea at 103 wpi, and the ileum tissue sample was used only for immunohistological analysis.
Flow cytometric analysis of PD-1 and LAG-3.
PBMCs, splenocytes, and lymphocytes were isolated from tissues and incubated in PBS containing 10% goat serum (Sigma-Aldrich) at room temperature for 15 min to prevent nonspecific reactions. Cells were then washed and stained with MAbs against PD-1:5D2 (rat IgG2a [13]) or LAG-3:71-2D8 (rat IgG1; the present study) for 30 min at room temperature. After being washed with PBS containing 10% goat serum, the cells were stained with CD4-FITC:CC8 (AbD Serotec, Oxford, United Kingdom), CD8-PerCp/Cy5.5:CC63 (AbD Serotec), IgM-PE/Cy7:IL-A30 (AbD Serotec), CD3:MM1A (VMRD, Pullman, WA) prelabeled with a Zenon R-PE mouse IgG1 labeling kit (Life Technologies, Carlsbad, CA), and allophycocyanin (APC)-conjugated anti-rat immunoglobulin (Beckman Coulter, Fullerton, CA) antibody conjugates for 30 min at room temperature. CC63 and IL-A30 were conjugated with PerCp/Cy5.5 and PE/Cy7, respectively, using Lightning-Link conjugation kits (Innova Biosciences, Cambridge, United Kingdom). The cells were then washed and analyzed immediately using FACSAria (BD Biosciences) and FCS Express 4 (De Novo Software, Glendale, CA). The primary antibodies used in this study are shown in Table 1. No fewer than 20,000 lymphocytes and no more than 200,000 lymphocytes were analyzed among the samples.
TABLE 1.
Primary antibodies used in flow cytometric analysis of this study
| Target | Isotype | Clone | Company (reference) | Fluorochromea | Conjugation or labeling |
|---|---|---|---|---|---|
| Flow cytometric analysis of PD-1 and LAG-3 | |||||
| CD4 | Mouse IgG2a | CC8 | AbD Serotec | FITC | Conjugated primary antibody product |
| CD8 | Mouse IgG2a | CC63 | AbD Serotec | PerCp-Cy5.5 | Lightning-Link PerCp/Cy5.5 conjugation kit (Innova Biosciences) |
| CD3 | Mouse IgG1 | MM1A | VMRD | PE | Zenon R-PE mouse IgG1 labeling kit (Life Technologies) |
| IgM | Mouse IgG1 | IL-A30 | AbD Serotec | PE-Cy7 | Lightning-Link PE/Cy7 conjugation kit (Innova Biosciences) |
| PD-1 | Rat IgG2a | 5D2 | In house (13) | APC | APC-conjugated anti-rat immunoglobulin antibody (Beckman Coulter) |
| LAG-3 | Rat IgG1 | 71-2D8 | In house (this study) | APC | APC-conjugated anti-rat immunoglobulin antibody (Beckman Coulter) |
| Flow cytometric analysis of PD-L1 and MHC-II | |||||
| CD14 | Mouse IgG1 | CAM36A | VMRD | APC-Cy7 | Lightning-Link APC/Cy7 conjugation kit (Innova Biosciences) |
| CD11b | Mouse IgG2b | CC126 | AbD Serotec | FITC | FITC-conjugated anti-mouse IgG2b antibody (Beckman Coulter) |
| MHC-II | Mouse IgG1 | CAT82A | VMRD | PE | Zenon R-PE mouse IgG1 labeling kit (Life Technologies) |
| PD-L1 | Rat IgG2a | 4G12 | In house (20) | APC | APC-conjugated anti-rat immunoglobulin antibody (Beckman Coulter) |
| Flow cytometric analysis of IFN-γ-producing T cells (intracellular staining) | |||||
| CD4 | Mouse IgG2a | CC8 | AbD Serotec | FITC | Conjugated primary antibody product |
| CD8 | Mouse IgG2a | CC63 | AbD Serotec | PerCp-Cy5.5 | Lightning-Link PerCp/Cy5.5 conjugation kit (Innova Biosciences) |
| CD69 | Mouse IgG1 | KTSN7A | Kingfisher Biotech | Alexa Fluor 647 | Zenon Alexa Fluor 647 mouse IgG1 labeling kit (Life Technologies) |
| IFN-γ | Mouse IgG1 | CC302 | AbD Serotec | PE | Conjugated primary antibody product |
APC, allophycocyanin; Cy, cyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCp, peridinin-chlorophyll-protein complex.
Flow cytometric analysis of PD-L1 and MHC-II.
PBMCs, splenocytes, and lymphocytes were isolated from tissues and were then blocked with PBS containing 10% goat serum and washed and stained with MAbs against PD-L1:4G12 (rat IgG2a [20]) and CD11b:CC126 (mouse IgG2b; AbD Serotec) for 30 min at room temperature. After a washing step with PBS containing 10% goat serum, the cells were stained with CD14-APC/Cy7:CAM36A (VMRD), MHC-II:CAT82A (VMRD) prelabeled with Zenon R-PE mouse IgG1 labeling kit (Life Technologies, Carlsbad, CA), APC-conjugated anti-rat immunoglobulin (Beckman Coulter), and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG2b (Beckman Coulter) antibody conjugates for 30 min at room temperature. CAM36A was conjugated using Lightning-Link APC-Cy7 tandem conjugation kit (Innova Biosciences). Cells were then washed and immediately analyzed using FACSAria and FCS Express 4. The primary antibodies used in this study are shown in Table 1. More than 5,000 monocytes were analyzed among the samples.
Immunohistochemical analysis of PD-L1 and Ziehl-Neelsen staining.
Tissue sections of ileum from cattle that were experimentally or naturally infected with M. avium subsp. paratuberculosis were subjected to immunohistochemical study. The tissue samples of the experimentally infected animals were collected from animals 58 and 65, both of which showed M. avium subsp. paratuberculosis shedding in feces and clinical onset of the disease such as diarrhea. All of the naturally infected animals used in this study also showed clinical signs of Johne's disease. The ileum tissues were fixed by formalin, embedded into paraffin wax, and then cut into 4-mm-thick sections. The dried sections were deparaffinized in xylene on the slide glass. Antigen retrieval was performed in citrate buffer (0.37 g/ml citric acid and 2.4 g/ml trisodium citrate dihydrate) by microwave heating for 10 min. Endogenous peroxidase activity was blocked by incubating the sections in methanol containing 0.3% hydrogen peroxide for 15 min. Primary antibody incubation was performed at room temperature for 30 min using anti-bovine PD-L1 MAb:6C11 (rat IgG2a, 5 μg/ml; see Fig. S2 in the supplemental material and reference 20). The sections that were washed twice with PBS were incubated with Histofine simple stain MAX PO (rat) (Nichirei, Tokyo, Japan) at room temperature for 30 min, and positive staining was visualized with 3-diaminobenzidine tetrahydrochloride (DAB). The sections were observed under an optical microscope. In addition, Ziehl-Neelsen staining was performed to detect acid-fast bacilli in ileum tissues.
Blockade assays in whole blood culture and IFN-γ ELISA.
To investigate whether immunoinhibitory receptors influence M. avium subsp. paratuberculosis-specific IFN-γ responses, heparinized whole blood cells were incubated with the blocking MAb anti-PD-1:5D2 (13), anti-PD-L1:5A2 (20), or anti-LAG-3:71-2D8 (the present study) at 10 μg/ml, and rat IgG (Sigma-Aldrich) was used as a negative-control antibody in the presence of 5 μg of J-PPD/ml. For negative-control antigen, PPD was purified from the Mycobacterium bovis BCG strain (B-PPD; 5 μg/ml), or sterile PBS was used. All blood cultures were grown in 24-well plates (Corning, Inc., Corning, NY) at 37°C with 5% CO2 for 24 h. Subsequently, plasma was harvested, and IFN-γ was determined using ELISA for bovine IFN-γ (Mabtech, Nacka Strand, Sweden) according to the manufacturer's protocol. The results were calculated based on a standard curve ranging from 7.8 to 500 pg/ml. The data are presented as means of duplicate samples.
PBMC blockade assays and flow cytometric analysis of IFN-γ-producing T cells.
To examine the effects of blocking MAbs on M. avium subsp. paratuberculosis-specific T-cell responses, in vitro blockade assays were performed using PBMCs cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Cansera International, Inc., Ontario, Canada), 200 IU/ml penicillin, 200 μg/ml streptomycin, and 0.01% (vol/vol) l-glutamine (Life Technologies). All cell cultures were grown in 24-well plates (Corning, Inc.) containing 2.5 × 106 PBMCs in 500 μl of medium with 10 μg of anti-PD-1, anti-PD-L1, anti-LAG-3 MAbs, or rat IgG (Sigma-Aldrich) per ml and J-PPD (10 μg/ml) at 37°C with 5% CO2 for 19 h. For a positive control, the cells were stimulated with 10 μg of concanavalin A (ConA; Sigma-Aldrich)/ml. Brefeldin A (Sigma-Aldrich) was added at 10 μg/ml for the final 6 h.
Cultured PBMCs were collected and incubated in PBS containing 10% goat serum as described above. Subsequently, cells were washed and stained with CD4-FITC:CC8 (AbD Serotec)-, CD8-PerCp/Cy5.5:CC63 (AbD Serotec)-, and CD69-Alexa Fluor 647:KTSN7A (Kingfisher Biotech, Saint Paul, MN)-conjugated antibodies for 30 min at 4°C. KTSN7A was prelabeled using Zenon Alexa Fluor 647 mouse IgG1 labeling kit (Life Technologies). After surface staining, the cells were fixed and permeabilized using FOXP3 Fix/Perm kit (BioLegend, Cambridge, United Kingdom) according to the manufacturer's protocol. Subsequently, cells were stained with anti-IFN-γ-PE:CC302 (AbD Serotec), washed, and analyzed immediately using FACSAria (BD Biosciences) and FCS Express 4 (De Novo Software). The primary antibodies used in this study are shown in Table 1. More than 10,000 lymphocytes were analyzed among the samples.
Statistics.
Differences were identified using Welch's t test and Tukey's test with the MEPHAS program (http://www.gen-info.osaka-u.ac.jp/MEPHAS/) and were considered significant when the P value was <0.05.
RESULTS
Establishment of anti-bovine LAG-3 MAbs.
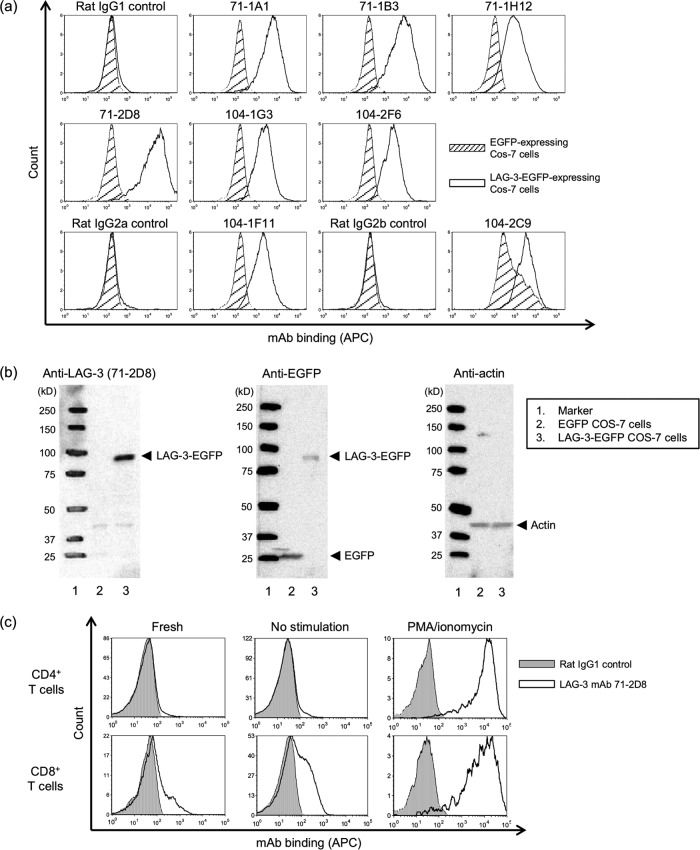
Supernatants containing antibody from 415 hybridoma clones were screened for binding activity with the LAG-3 peptides 71-99 and 104-132 using ELISA. In these experiments, 54 hybridomas produced MAbs that reacted with the immunized peptide but not with the other peptide (Table 2). To examine binding of MAbs with LAG-3 on cell membranes, Cos-7 cells expressing bovine LAG-3-EGFP were stained with supernatants or purified MAbs. Subsequently, 14 supernatants were found to bind LAG-3-EGFP-expressing Cos-7 cells (Table 2), and the strongest fluorescence intensity among eight purified anti-LAG-3 MAbs was observed in cells stained with 71-2D8 (Fig. 1a). Therefore, subsequent expression analyses of bovine LAG-3 were performed using anti-LAG-3 MAb 71-2D8. In addition, the 71-2D8 MAb recognized a heat-denatured LAG-3-EGFP protein of ∼93 kDa in Western blotting analyses (Fig. 1b).
TABLE 2.
Hybridomas generated during the establishment of the anti-bovine LAG-3 MAb
| Epitope (peptide) | No. of tested hybridomas | No. positive for hybridoma |
|
|---|---|---|---|
| LAG-3 peptide ELISA | FACS using LAG-3-EGFP COS-7 |
||
| Bovine LAG-3 (71-99) | 223 | 25 | 8 |
| Bovine LAG-3 (104-132) | 192 | 29 | 6 |
FIG 1.
Reactivity of the anti-LAG-3 MAb with LAG-3-expressing cells and bovine lymphocytes. (a) Flow cytometric analysis of bovine LAG-3. Cos-7 cells expressing LAG-3-EGFP (white area) and EGFP (shaded area) were stained with eight anti-LAG-3 MAb clones. Rat IgG1 (for 71-1A1, 71-1B3, 71-1H12, 71-2D8, 104-1G3, and 104-2F6), rat IgG2a (for 104-1F11), and rat IgG2b (for 104-2C9) were used as negative controls. (b) Western blotting of bovine LAG-3 protein from Cos-7 cells. Anti-LAG-3 MAb:71-2D8 recognized a LAG-3-EGFP protein band of ∼93 kDa. Anti-EGFP and antiactin antibodies were used as positive and loading controls, respectively. (c) Flow cytometric analysis of LAG-3 expression in CD4+ and CD8+ T cells. Freshly isolated bovine PBMCs were stained with anti-LAG-3:71-2D8 (white area), CD4, and CD8 MAbs. Rat IgG1 (gray area) was used as a negative-control stain. PBMCs were cultured with PBS (no stimulation) or PMA/ionomycin for 48 h and analyzed as described above.
To confirm the reactivity of anti-LAG-3 MAb 71-2D8 with naturally expressed LAG-3 on bovine lymphocytes, the surface expression of LAG-3 was evaluated in freshly isolated CD4+ and CD8+ T cells or in cells stimulated with PMA/ionomycin in vitro. The 71-2D8 MAb recognized LAG-3 expressed on the surfaces of CD4+ and CD8+ T cells (Fig. 1c). LAG-3 was expressed mainly on CD8+ T cells from freshly isolated PBMCs, whereas CD4+ T cells expressed low levels of LAG-3 (Fig. 1c). PMA/ionomycin stimulation strongly induced LAG-3 expression on both CD4+ and CD8+ T cells (Fig. 1c).
Suppression of T-cell responses to J-PPD and fecal shedding of M. avium subsp. paratuberculosis in M. avium subsp. paratuberculosis-infected cattle.
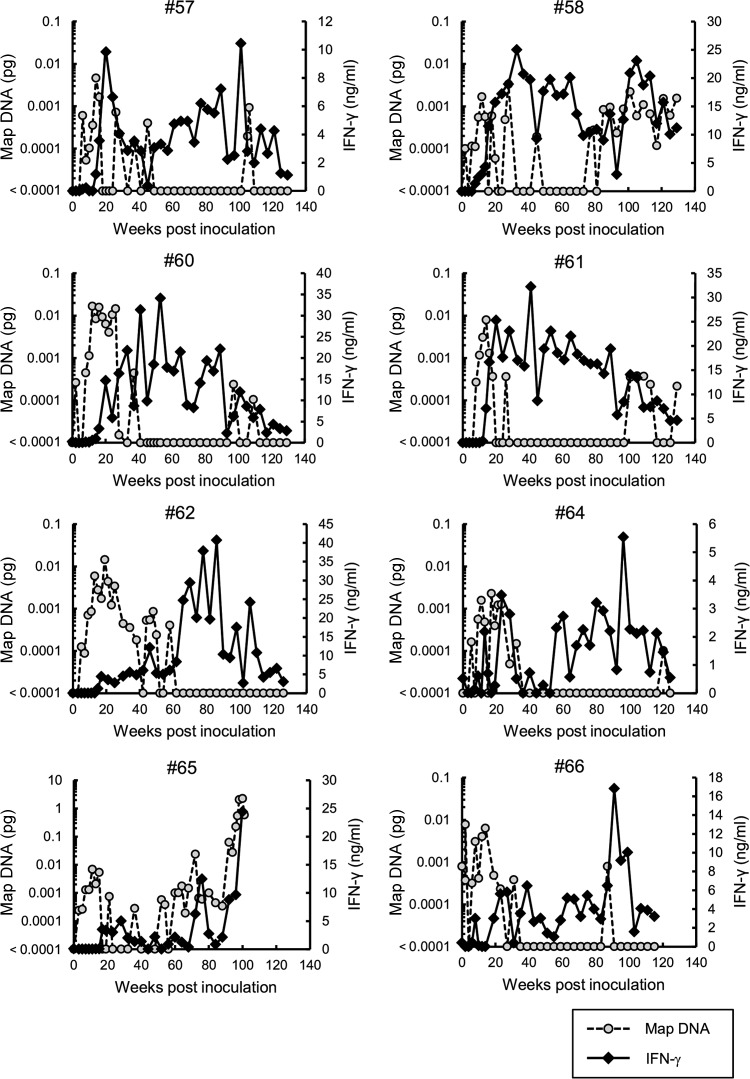
Previous studies of Johne's disease suggest that M. avium subsp. paratuberculosis-infected cattle develop strong Th1 responses, and IFN-γ production is particularly pronounced during early stages of infection. However, this response is gradually suppressed during late subclinical stages of infection (4–6). Hence, to characterize Th1 responses and disease progression in the present M. avium subsp. paratuberculosis-infected cattle, we determined IFN-γ responses from blood cells stimulated with J-PPD and evaluated M. avium subsp. paratuberculosis shedding in feces every 2 to 4 weeks. M. avium subsp. paratuberculosis DNA was detected in feces of all seven infected calves from 10 to 30 wpi (Fig. 2), and with the exception of animals 58 and 65, shedding of M. avium subsp. paratuberculosis subsequently declined and was intermittent for up to 2 years, when the study was terminated. Animals 58 and 65 resumed and sustained high levels of bacterial shedding and showed signs of the clinical onset of the disease, such as diarrhea. In particular, animal 65 showed the highest level of M. avium subsp. paratuberculosis shedding (0.027 to 2.27 pg of M. avium subsp. paratuberculosis DNA) in feces from 92 to 100 wpi just before the necropsy (Fig. 2). In infected animals 57, 58, 60, and 61, J-PPD-specific IFN-γ responses peaked during the acute phase of infection but peaked during the subclinical stage in the other three animals (i.e., animals 62, 64, and 66; Fig. 2). With the exception of animals 58 and 65, IFN-γ production was strongly suppressed by 120 wpi. The IFN-γ response of animal 58 declined until 93 wpi and increased again following bacterial growth in the intestine. In addition, unspecific production of IFN-γ to J-PPD stimulation and fecal detection of M. avium subsp. paratuberculosis in uninfected cattle is shown as negative controls in Fig. S1 in the supplemental material.
FIG 2.
IFN-γ responses to J-PPD in blood and fecal shedding of M. avium subsp. paratuberculosis in cattle experimentally infected with M. avium subsp. paratuberculosis. The dates of sample collection are indicated on the horizontal axis. The left vertical axis (gray circle) indicates the M. avium subsp. paratuberculosis (Map) DNA quantity in fecal samples determined using IS900 real-time PCR. The detection limit of this assay was 0.0001 pg/2.5 μl (19). Thus, the sample in which Map DNA was not detected is shown as “<0.0001 pg” on the graph. The right vertical axis (black diamond) shows IFN-γ production in whole blood cultures stimulated with J-PPD.
Upregulation of PD-1 and LAG-3 on T cells in M. avium subsp. paratuberculosis-infected cattle at the late subclinical stage.
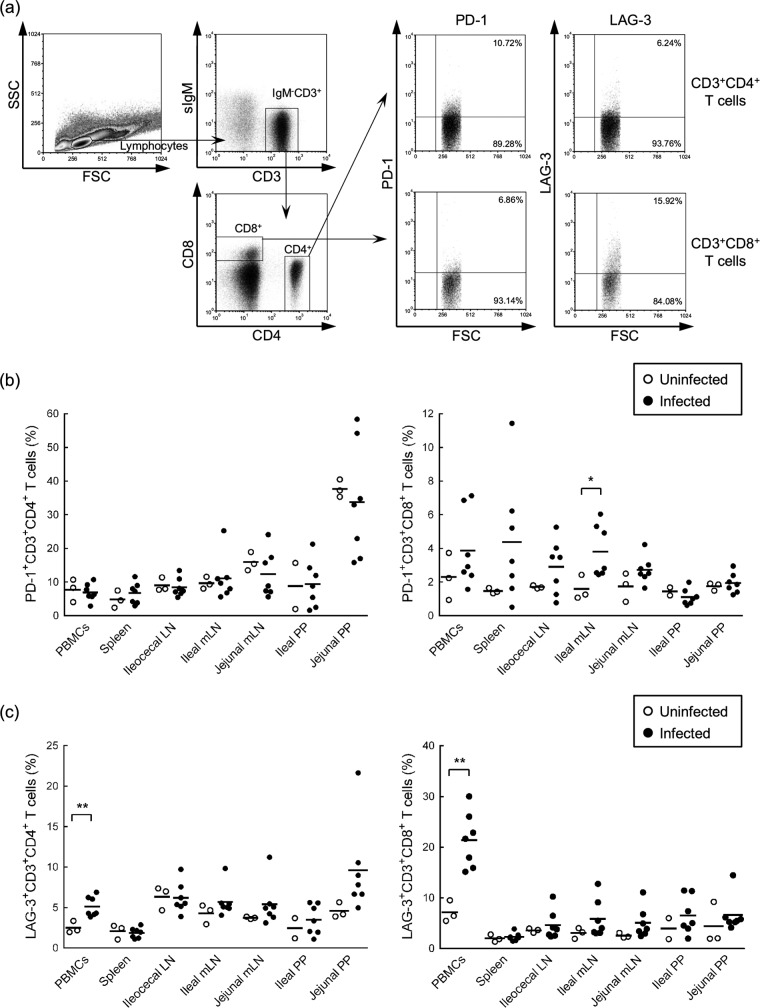
Th1 responses to the J-PPD antigen were suppressed at the subclinical stage in the present infected cattle. Thus, we hypothesized that exhaustion of M. avium subsp. paratuberculosis antigen-specific T-cell responses was caused by upregulation of immunoinhibitory receptors on T cells. Thus, to investigate the expression levels of the immunoinhibitory receptors PD-1 and LAG-3 on CD4+ and CD8+ T cells, flow cytometric analyses were performed with PBMCs and lymphocytes of spleens, LNs, and PPs that were isolated from M. avium subsp. paratuberculosis-infected cattle at 135 to 148 wpi. As shown in Fig. 3a, CD4+ and CD8+ T cells were gated in IgM− CD3+ lymphocytes and were then further analyzed for PD-1 and LAG-3 expression. No significant differences in numbers of PD-1+ CD4+ T cells in tested tissues were observed between infected and uninfected animals. However, PD-1+ CD8+ T cells were significantly more numerous in the ileal mLNs of infected animals (Fig. 3b; see Table S2a and b in the supplemental material). In contrast, LAG-3 expression was upregulated on CD4+ and CD8+ T cells in peripheral blood from infected animals (Fig. 3c; see Table S2c and d in the supplemental material), suggesting that upregulation of PD-1 and LAG-3 on T cells contributes to T-cell exhaustion in M. avium subsp. paratuberculosis-infected cattle.
FIG 3.
Expression levels of PD-1 and LAG-3 on T cells in M. avium subsp. paratuberculosis-infected cattle. (a) Gating strategy and representative dot plots for expression analyses of PD-1 and LAG-3 on IgM− CD3+ CD4+ and IgM− CD3+ CD8+ T cells from the blood of M. avium subsp. paratuberculosis-infected cattle. Values in the quadrant indicate the percentages of cells. (b and c) Percentages of PD-1-expressing (b) and LAG-3-expressing (c) cells in IgM− CD3+ CD4+ and IgM− CD3+ CD8+ T cells of peripheral blood, spleen, ileocecal LN, ileal and jejunal mLNs, and ileal and jejunal PPs isolated from uninfected cattle (n = 3) and M. avium subsp. paratuberculosis-infected cattle (n = 7). Statistical comparisons between groups were performed using Welch's t test. Differences were considered significant when P < 0.05 (*, P < 0.05; **, P < 0.01). See also Table S1 in the supplemental material for individual expression data.
High-level expression of PD-L1 and MHC-II on macrophages in M. avium subsp. paratuberculosis-infected cattle during the late subclinical stage.
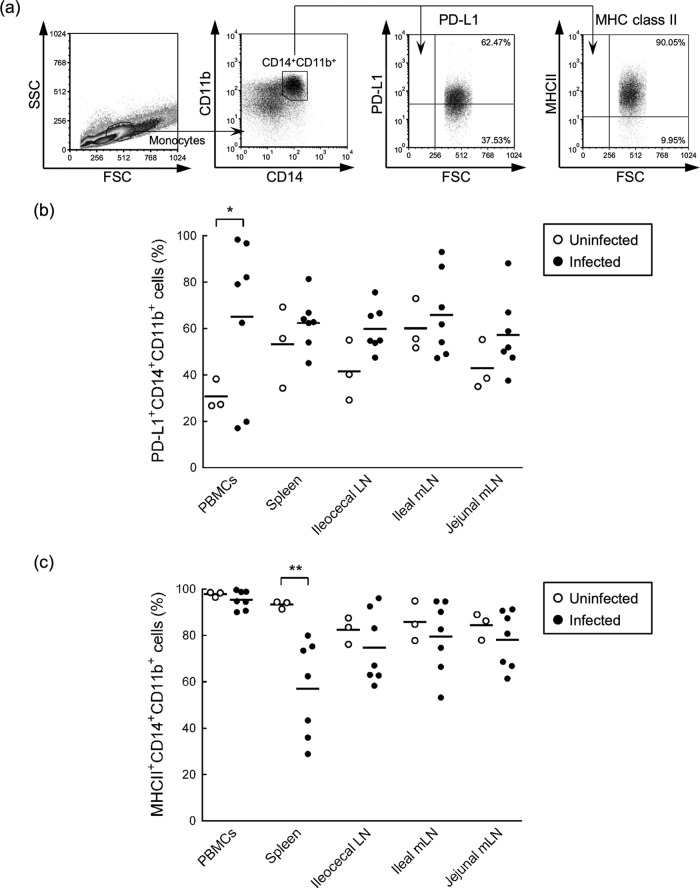
Inhibitory signals of exhausted T cells are induced by cross-linking between immunoinhibitory receptors on T cells and their ligands on antigen-presenting cells (10, 11). M. avium subsp. paratuberculosis persistently infects macrophages of the ileum and of draining LNs and escapes host immune responses through various mechanisms (2, 3). To confirm the potential of macrophages from infected cattle to interact with T cells through immunoinhibitory ligands, the expression status of PD-L1 and MHC-II on macrophages from blood, spleens, LNs and PPs was analyzed using flow cytometry. As shown in Fig. 4a, CD14+ CD11b+ macrophages were gated in monocytes from blood and were further analyzed for expression of PD-L1 and MHC-II. Large populations of CD14+ CD11b+ macrophages were found in PBMCs and LNs, whereas those in ileal and jejunal PPs were few, and expression analyses were therefore not performed using monocytes of PPs (data not shown). The mean percentages of PD-L1-expressing CD14+ CD11b+ macrophages were significantly higher in PBMCs of the M. avium subsp. paratuberculosis-infected group (Fig. 4b), although two animals, 57 and 62, showed lower levels of PD-L1 expression than uninfected animals (see Table S2a in the supplemental material). PD-L1 was expressed on the majority of CD14+ CD11b+ macrophages from spleen and LN tissues in both groups (Fig. 4b; see also Table S2a in the supplemental material). Moreover, the mean frequencies of MHC-II-expressing CD14+ CD11b+ macrophages were 95.3% in PBMCs, 57.0% in spleens, and 74.6 to 78.1% in gut-associated LNs of the infected group (see Table S2b in the supplemental material. Although the numbers of splenic MHC-II+ CD14+ CD11b+ macrophages from infected animals were significantly fewer than in uninfected animals, no significant differences in MHC-II+ CD14+ CD11b+ macrophages from PBMCs and LNs were observed between uninfected and infected animals (Fig. 4c). Taken together, macrophages from infected cattle express PD-L1 and MHC-II and have the potential to interact with PD-1- and LAG-3-expressing T cells.
FIG 4.
Expression levels of PD-L1 and MHC-II on macrophages in M. avium subsp. paratuberculosis-infected cattle. (a) Gating strategy and representative dot plots for expression analysis of PD-L1 and MHC-II on CD14+ CD11b+ macrophages from the blood of M. avium subsp. paratuberculosis-infected cattle. Values in the quadrant indicate the percentages of the cells. (b and c) Percentages of PD-L1-expressing (b) and MHC-II-expressing (c) cells among CD14+ CD11b+ cells of peripheral blood, spleen, ileocecal LNs, and ileal and jejunal mLNs from uninfected cattle (n = 3) and M. avium subsp. paratuberculosis-infected cattle (n = 7). Comparisons of groups were performed using Welch's t test. Differences were considered significant when P < 0.05 (*, P < 0.05; **, P < 0.01). See also Table S2 in the supplemental material for individual expression analyses.
Expression of PD-L1 in M. avium subsp. paratuberculosis-infected macrophages of the ileum.
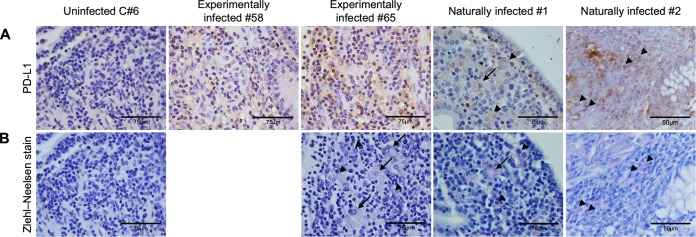
Previous reports have clarified the expression status of MHC-II in Johne's disease (21, 22), and MHC class II molecules were reportedly expressed in the small intestinal mucosa of a goat with subclinical infection (21) and in CD11c+ dendritic cells from M. avium subsp. paratuberculosis-induced cattle granulomas (22). Therefore, we examined the expression status of PD-L1 in intestinal macrophages infected with M. avium subsp. paratuberculosis using immunohistological staining with PD-L1 and Ziehl-Neelsen stain. PD-L1+ cells were detected in the lamina propria of the ileum in experimentally infected cattle (58 and 65) with M. avium subsp. paratuberculosis shedding in feces (Fig. 5A). M. avium subsp. paratuberculosis-infected cells were observed in the same lesion of animal 65 (Fig. 5B), but not in those of animal 58 (data not shown), which is consistent with the lower level of bacterial shedding in animal 58 than animal 65 (Fig. 2). Furthermore, M. avium subsp. paratuberculosis-infected macrophages and epithelioid cells expressed PD-L1 in the ileum of naturally infected cattle with clinical diarrhea (Fig. 5A and B).
FIG 5.
PD-L1 expression and localization of M. avium subsp. paratuberculosis in ileal mucosa of M. avium subsp. paratuberculosis-infected cattle. (A and B) Immunohistochemical staining of PD-L1 (A) and Ziehl-Neelsen staining for acid-fast bacilli (B) in ileum tissues. Immunohistochemical staining was performed using anti-PD-L1 MAb (6C11). Arrowheads and arrows indicate M. avium subsp. paratuberculosis-infected macrophages and epithelioid cells, respectively, both of which express PD-L1.
Dual blockade of the PD-1 and LAG-3 pathways reactivates M. avium subsp. paratuberculosis-specific IFN-γ production in blood from M. avium subsp. paratuberculosis-infected cattle.
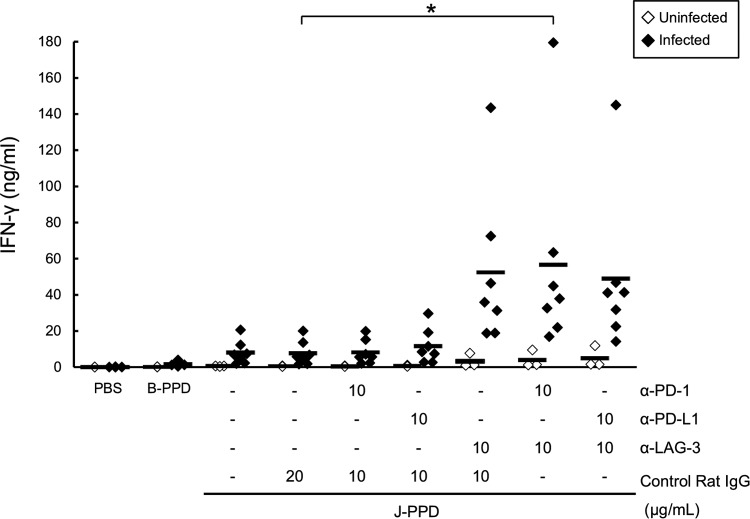
In previous studies, we showed that inhibition of PD-1/PD-L1 and LAG-3/MHC-II pathways by blocking antibodies reactivates IFN-γ responses in PBMCs of BLV-infected cattle (13, 15, 16). Thus, in the present study, we determined whether IFN-γ responses to M. avium subsp. paratuberculosis antigens are restored by blockade of immunoinhibitory pathways. To address this question, IFN-γ responses to J-PPD in whole blood were assessed in the presence of the blocking MAbs anti-PD-1, anti-PD-L1, and anti-LAG-3. Although IFN-γ production in the blood of infected animals tended to be more strongly induced by J-PPD than B-PPD, this difference (4.4-fold) was not significant (Fig. 6). Nonetheless, LAG-3 blockade tended to elevate IFN-γ production in the presence of J-PPD compared to the control IgG (Fig. 6). Furthermore, dual blockade using anti-PD-1 and anti-LAG-3 MAbs significantly enhanced the IFN-γ response to J-PPD relative to that in the presence of control IgG or anti-PD-1 MAb alone (Fig. 6). These results suggest that combined blockade of PD-1 and LAG-3 pathways reactivates M. avium subsp. paratuberculosis-specific IFN-γ responses in M. avium subsp. paratuberculosis-infected cattle.
FIG 6.
Reactivation of J-PPD-specific IFN-γ responses by LAG-3 blockade. Whole blood cells were cultured with blocking MAbs (anti-PD-1, anti-PD-L1, and anti-LAG-3 MAbs; 10 μg/ml) or rat IgG control in the presence of J-PPD (5 μg/ml). IFN-γ production in plasma was measured using ELISA (uninfected, n = 3; infected, n = 7). Comparisons of blocking MAbs in animal groups were performed using Tukey's test. Differences were considered significant when P < 0.05, indicated by an asterisk.
LAG-3 blockade restores IFN-γ production from M. avium subsp. paratuberculosis-specific T cells.
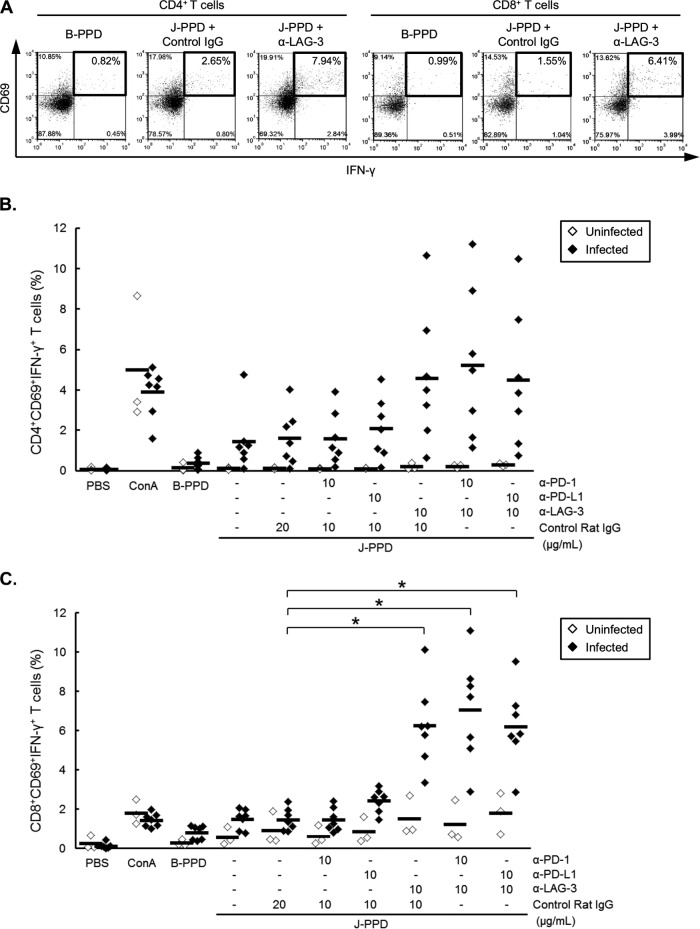
It is broadly recognized that J-PPD induces IFN-γ-producing CD4+ and CD8+ T cells in M. avium subsp. paratuberculosis-infected cattle (23, 24). Thus, we determined whether blockade of PD-1 and LAG-3 pathways activates J-PPD-specific IFN-γ production in CD4+ and CD8+ T cells. As expected, CD4+ CD69+ and CD8+ CD69+ T cells from M. avium subsp. paratuberculosis-infected cattle produced IFN-γ in response to J-PPD (Fig. 7A). Accordingly, CD4+ CD69+ IFN-γ+ and CD8+ CD69+ IFN-γ+ T cells that are induced by J-PPD were defined as J-PPD-specific T cells (Fig. 7A). Interestingly, percentages of J-PPD-specific T cells in total CD4+ and CD8+ T-cell preparations were dramatically increased by LAG-3 blockade in infected animals (Fig. 7). Moreover, compared to control IgG treatments, LAG-3 blockade significantly enhanced IFN-γ production in J-PPD-specific CD8+ T cells (Fig. 7C), indicating that IFN-γ responses of J-PPD-specific CD4+ and CD8+ T cells can be reactivated by LAG-3 blockade in M. avium subsp. paratuberculosis-infected cattle.
FIG 7.
Reactivation of J-PPD-specific T cells by LAG-3 blockade. PBMCs from M. avium subsp. paratuberculosis-infected cattle (n = 7) and uninfected cattle (n = 3) were cultured with blocking MAbs (anti-PD-1, anti-PD-L1, and anti-LAG-3 MAbs; 10 μg/ml) or rat IgG control in the presence of J-PPD (10 μg/ml). (A) Representative dot plots for CD4+ CD69+ IFN-γ+ and CD8+ CD69+ IFN-γ+ T cells of an infected animal after treatment with B-PPD (left panels) or J-PPD with or without LAG-3 blockade (right and central panels, respectively). Values in the quadrant indicate the percentages of cells evaluated by flow cytometry. (B and C) Percentages of CD69+ IFN-γ+ cells among CD4+ (B) and CD8+ (C) T cells after treatment with J-PPD and blocking MAbs. Comparisons of blocking MAbs in animal groups were performed using Tukey's test. Differences were considered significant when P < 0.05, indicated by an asterisk.
DISCUSSION
During chronic infection, pathogens evade host immune responses and persist after the effector phase (25, 26), leading to persistent antigen stimulation and a progressive T-cell dysfunction known as T-cell exhaustion (7, 26). Exhausted T cells are controlled by the immunoinhibitory receptors PD-1 and LAG-3 on the cell surface via T-cell inhibitory signals that follow cross-linking to the ligands PD-L1 and MHC-II, respectively (10, 11).
In cattle with Johne's disease, intracellular M. avium subsp. paratuberculosis infection of macrophages leads to presentation of bacterial antigens to M. avium subsp. paratuberculosis-specific T cells, which elicit strong Th1 responses during the acute phase of infection (1–3). When antigen presentation is extended beyond the T-cell expansion phase, M. avium subsp. paratuberculosis-specific T cells are gradually exhausted and become unresponsive to stimulation with M. avium subsp. paratuberculosis antigen (4–6). Thus, to elucidate the associated molecular mechanisms, we determined whether immunoinhibitory receptors mediate the development of exhausted T cells during subclinical M. avium subsp. paratuberculosis infection in cattle.
In the present study, we demonstrated that PD-1 was upregulated on CD8+ T cells of ileal mLNs in M. avium subsp. paratuberculosis-infected cattle with long-term suppression of Th1 responses at the late subclinical stage. In addition, PD-L1 was expressed in macrophages of mLNs and in M. avium subsp. paratuberculosis-infected cells of the ileum, indicating that the interaction of PD-1/PD-L1 inhibits T-cell function in infected lesions. In agreement, PD-1 is reportedly upregulated on CD4+ T cells in Mycobacterium bovis-infected mice and in Mycobacterium tuberculosis-infected patients, leading to CD4+ T-cell exhaustion and bacterial persistence (27, 28). Thus, T-cell exhaustion via the PD-1/PD-L1 pathway appears to be common among mycobacterial infections. Besides that, large populations of PD-1+ CD4+ T cells (15 to 58% of total CD4+ T cells) were found in jejunal PPs from both uninfected and infected animals, and no significant differences were observed between these groups (Fig. 3b; see also Table S1a in the supplemental material). Potentially, these cells act as follicular helper T cells, which reportedly play key roles in the development and maintenance of B cells in PPs (29, 30) but differ from the present PD-1+ CD4+ exhausted T cells. In the present study, PD-L1 was significantly upregulated on CD14+ CD11b+ macrophages in the PBMCs of M. avium subsp. paratuberculosis-infected cattle. In addition, the percentages of PD-L1-expressing CD14+ CD11b+ macrophages tended to be elevated in the spleens and LNs of the infected animals, although there was no significant difference because of the limited number of samples tested in the present study.
LAG-3 was upregulated on CD4+ and CD8+ T cells in peripheral blood of M. avium subsp. paratuberculosis-infected cattle, indicating that LAG-3 plays a more dominant immunomodulatory role than PD-1 in circulating CD4+ and CD8+ T cells. In contrast, LAG-3 expression was not changed on CD4+ and CD8+ T cells in mLNs and PPs from the infected animals (Fig. 3c; see also Table S2c and d in the supplemental material), where T cells encountered the pathogen. In PBMCs, there are a number of J-PPD-responding T cells, including “M. avium subsp. paratuberculosis-specific T cells” (Fig. 7). For the significance of LAG-3 expression in PBMCs, we hypothesize that LAG-3 would be upregulated on M. avium subsp. paratuberculosis-specific T cells in the peripheral blood and lose their effector functions. Further studies are required to clarify the mechanisms of LAG-3 upregulation in peripheral blood, but not in the lymphoid tissues. The present data are the first to show LAG-3 expression on T cells during mycobacterial infection, and further studies are required to define the roles of LAG-3 in T-cell exhaustion during other mycobacterial infections. Nonetheless, a previous report showed that in vitro M. avium subsp. paratuberculosis infection of bovine macrophages resulted in downregulation of MHC-II (31), and MHC-II was downregulated on splenic CD14+ CD11b+ macrophages in the current study. However, these molecules were not downregulated in blood and LNs, despite prevalent expression (95% and 74 to 79% of macrophages in blood and LNs, respectively; see also Table S2b in the supplemental material).
CD8+ cytotoxic T cells play a central role in killing intracellular bacteria, including M. avium subsp. paratuberculosis, in macrophages. In the present study, CD8+ T cells upregulated PD-1 and LAG-3 in the infected animals, suggesting the functional exhaustion of CD8+ cytotoxic T cells during subclinical M. avium subsp. paratuberculosis infection. This exhaustion then might facilitate the bacterial persistence and disease progression.
In chronic infections, the expression of PD-1 and LAG-3 is induced by continuous antigen presentation and T-cell receptor (TCR) stimulation (7, 10, 11). Thus, we hypothesized that PD-1 and LAG-3 were upregulated on M. avium subsp. paratuberculosis antigen-specific T cells and lost their effector functions. PD-1 and LAG-3 were upregulated in total CD4+ and CD8+ T-cell populations from M. avium subsp. paratuberculosis-infected cattle. However, we did not perform flow cytometric analyses of PD-1 and LAG-3 on M. avium subsp. paratuberculosis antigen-specific T cells because of lack of an MHC-peptide tetramer. The construction of an M. avium subsp. paratuberculosis-specific MHC-peptide tetramer would enable further analysis of M. avium subsp. paratuberculosis-specific PD-1+ and LAG-3+ exhausted T cells. However, the present broad expression analysis revealed upregulation of PD-1 and LAG-3 on T cells from different sites of M. avium subsp. paratuberculosis-infected cattle, suggesting that PD-1 and LAG-3 are expressed and play immunomodulatory roles in various populations of T cells. These observations warrant further studies to determine whether PD-1 and LAG-3 are coexpressed to synergistically depress the function of exhausted T cells in Johne's disease. Furthermore, other immunoinhibitory receptors T-cell immunoglobulin domain and mucin domain-3 (Tim-3 [32]) and cytotoxic T-lymphocyte antigen 4 (CTLA-4 [33]) are also likely involved in the development of exhausted T cells in Johne's disease. Accordingly, a recent study showed that CD4+ T cells upregulated CTLA-4 in cattle during the subclinical stage of M. avium subsp. paratuberculosis infection (34).
Previous studies show that M. avium subsp. paratuberculosis infection inhibits phagocytic maturation of macrophages (35), downregulates MHC class I and II molecules (31), and induces interleukin-10 (IL-10) production (36), resulting in immune evasion and bacterial persistence. Accordingly, PD-L1 was upregulated on circulating macrophages and localized to M. avium subsp. paratuberculosis-infected cells of the ileum in M. avium subsp. paratuberculosis-infected cattle. These observations suggest that M. avium subsp. paratuberculosis evades the immune system by upregulating PD-L1 in infected macrophages.
In vitro blockade assays in whole blood cells and PBMCs showed that blockade with anti-LAG-3 MAb efficiently reactivated M. avium subsp. paratuberculosis-specific T-cell responses, and this was consistent with the high-level expression of LAG-3 on T cells in PBMCs of the present infected animals. In addition, CD8+ T cells in PBMCs strongly expressed LAG-3 and responded to LAG-3 blockade with significant restoration of M. avium subsp. paratuberculosis-specific IFN-γ production. Moreover, PD-1 was upregulated on CD8+ T cells in ileal mLN tissues, indicating that the reactivation effect of PD-1/PD-L1 blockade may also occur in blockade assays of lymphocytes from mLN tissues. From the diagnostic point of view, enhancing M. avium subsp. paratuberculosis-specific IFN-γ responses by LAG-3 blockade could be helpful to the diagnosis of M. avium subsp. paratuberculosis using the IFN-γ test (37) in the infected cattle with T-cell exhaustion.
We examined here M. avium subsp. paratuberculosis-specific IFN-γ production as a key response of Th1-mediated immunity and described novel mechanisms of T-cell exhaustion mediated by immunoinhibitory receptors in Johne's disease. However, additional studies are required to determine multiple effects of blocking MAbs during rejuvenation of exhausted T cells. Specifically, measurements of other Th1 cytokines, such as tumor necrosis factor alpha, IL-2, and IL-12, and T-cell proliferation and cytotoxic activity may reveal further aspects of T-cell exhaustion in Johne's disease. The present findings may contribute to the development of novel strategies for manipulating M. avium subsp. paratuberculosis-specific T-cell responses to prevent disease progression.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Hideyuki Takahashi and Yoshiyuki Goto, National Agriculture and Food Research Organization, BRAIN, for valuable advice and discussion. We thank Enago for the English language review.
Funding Statement
This study was supported by grants-in-aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and by special grants for the Promotion of Basic Research Activities for Innovative Biosciences from the Bio-oriented Technology Research Advancement Institution (BRAIN) and Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01014-15.
REFERENCES
- 1.Stabel JR. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim Health Res Rev 7:61–70. doi: 10.1017/S1466252307001168. [DOI] [PubMed] [Google Scholar]
- 2.Coussens PM. 2004. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect Immun 72:3089–3096. doi: 10.1128/IAI.72.6.3089-3096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohal JS, Singh SV, Tyagi P, Subhodh S, Singh PK, Singh AV, Narayanasamy K, Sheoran N, Singh Sandhu K. 2008. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology 213:585–598. doi: 10.1016/j.imbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bassey EOE, Collins MT. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun 65:4869–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrells C, Clarke C, Colston A, Kay J, Porter J, Little D, Sharp J. 1998. A study of immunological responses of sheep clinically affected with paratuberculosis (Johne's disease): the relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet Immunol Immunopathol 66:343–358. doi: 10.1016/S0165-2427(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 6.Weiss DJ, Evanson OA, Souza CD. 2006. Mucosal immune response in cattle with subclinical Johne's disease. Vet Pathol 43:127–135. doi: 10.1354/vp.43-2-127. [DOI] [PubMed] [Google Scholar]
- 7.Wherry EJ. 2011. T cell exhaustion. Nat Immunol 12:492–499. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaitan A, Unutmaz D. 2011. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep 8:4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamphorst A, Ahmed R. 2013. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol 25:381–388. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sierro S, Romero P, Speiser DE. 2011. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets 15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 12.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2012. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. 2013. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet Res 44:59. doi: 10.1186/1297-9716-44-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirai T, Konnai S, Ikebuchi R, Okagawa T, Suzuki S, Sunden Y, Onuma M, Murata S, Ohashi K. 2011. Molecular cloning of bovine lymphocyte activation gene-3 and its expression characteristics in bovine leukemia virus-infected cattle. Vet Immunol Immunopathol 144:462–467. doi: 10.1016/j.vetimm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Konnai S, Suzuki S, Shirai T, Ikebuchi R, Okagawa T, Sunden Y, Mingala CN, Onuma M, Murata S, Ohashi K. 2013. Enhanced expression of LAG-3 on lymphocyte subpopulations from persistently lymphocytotic cattle infected with bovine leukemia virus. Comp Immunol Microbiol Infect Dis 36:63–69. doi: 10.1016/j.cimid.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Ikebuchi R, Konnai S, Shirai T, Sunden Y, Murata S, Onuma M, Ohashi K. 2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of antiviral immune responses in vitro via PD-L1 blockade. Vet Res 42:103. doi: 10.1186/1297-9716-42-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata R, Muneta Y, Yoshihara K, Yokomizo Y, Mori Y. 2005. Expression cloning of gamma interferon-inducing antigens of Mycobacterium avium subsp. paratuberculosis. Infect Immun 73:3778–3782. doi: 10.1128/IAI.73.6.3778-3782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata R, Kawaji S, Minakawa Y, Wang X, Yanaka T, Mori Y. 2010. A specific induction of interleukin-10 by the Map41 recombinant PPE antigen of Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol 135:71–78. doi: 10.1016/j.vetimm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Kawaji S, Nagata R, Mori Y. 2014. Detection and confirmation of Mycobacterium avium subsp. paratuberculosis in direct quantitative PCR positive fecal samples by the manual fluorescent MGIT culture system. J Vet Med Sci 76:65–72. doi: 10.1292/jvms.13-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. 2014. Influence of PD-L1 cross-linking on cell death in PD-L1-expressing cell lines and bovine lymphocytes. Immunology 142:551–561. doi: 10.1111/imm.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valheim M, Siguroardóttir ÓG, Storset AK, Aune LG, Press CM. 2004. Characterization of macrophages and occurrence of T cells in intestinal lesions of subclinical paratuberculosis in goats. J Comp Pathol 131:221–232. doi: 10.1016/j.jcpa.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Lei L, Plattner BL, Hostetter JM. 2008. Live Mycobacterium avium subsp. paratuberculosis and a killed-bacterium vaccine induce distinct subcutaneous granulomas, with unique cellular and cytokine profiles. Clin Vaccine Immunol 15:783–793. doi: 10.1128/CVI.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabel JR, Kimura K, Robbe-Austerman S. 2007. Augmentation of secreted and intracellular gamma interferon following johnin purified protein derivative sensitization of cows naturally infected with Mycobacterium avium subsp. paratuberculosis. J Vet Diagn Invest 19:43–51. doi: 10.1177/104063870701900107. [DOI] [PubMed] [Google Scholar]
- 24.Plattner BL, Chiang YW, Roth JA, Platt R, Huffman E, Zylstra J, Hostetter JM. 2011. Direct inoculation of Mycobacterium avium subspecies paratuberculosis into ileocecal Peyer's patches results in colonization of the intestine in a calf model. Vet Pathol 48:584–592. doi: 10.1177/0300985810383874. [DOI] [PubMed] [Google Scholar]
- 25.Finlay BB, McFadden G. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Klenerman P, Hill A. 2005. T cells and viral persistence: lessons from diverse infections. Nat Immunol 6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 27.Sakai S, Kawamura I, Okazaki T, Tsuchiya K, Uchiyama R, Mitsuyama M. 2010. PD-1–PD-L1 pathway impairs Th1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Guérin. Int Immunol 22:915–925. doi: 10.1093/intimm/dxq446. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, Mohan A, Dey AB, Mitra DK. 2013. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis 208:603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 29.Kawamoto S, Tran T, Maruya M, Suzuki K, Doi Y, Tsutui Y, Kato L, Fagarasan S. 2012. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 336:485. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 30.Maruya M, Kawamoto S, Kato LM, Fagarasan S. 2013. Impaired selection of IgA and intestinal dysbiosis associated with PD-1-deficiency. Gut Microbes 4:165–171. doi: 10.4161/gmic.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss DJ, Evanson OA, McClenahan DJ, Abrahamsen MS, Walcheck BK. 2001. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect Immun 69:1002–1008. doi: 10.1128/IAI.69.2.1002-1008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okagawa T, Konnai S, Ikebuchi R, Suzuki S, Shirai T, Sunden Y, Onuma M, Murata S, Ohashi K. 2012. Increased bovine Tim-3 and its ligand expressions during bovine leukemia virus infection. Vet Res 43:45. doi: 10.1186/1297-9716-43-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S, Konnai S, Okagawa T, Ikebuchi R, Nishimori A, Kohara J, Mingala CN, Murata S, Ohashi K. 2015. Increased expression of the regulatory T cell-associated marker CTLA-4 in bovine leukemia virus infection. Vet Immunol Immunopathol 163:115–124. doi: 10.1016/j.vetimm.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Leite FL, Eslabão LB, Pesch B, Bannantine JP, Reinhardt TA, Stabel JR. 2015. ZAP-70, CTLA-4 and proximal T cell receptor signaling in cows infected with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol 167:15–21. doi: 10.1016/j.vetimm.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Frehel C, Canonne-Hergaux F, Gros P, de Chastellier C. 2002. Effect of Nramp1 on bacterial replication and on maturation of Mycobacterium avium-containing phagosomes in bone marrow-derived mouse macrophages. Cell Microbiol 4:541–556. doi: 10.1046/j.1462-5822.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 36.Balcewicz-Sablinska MK, Gan H, Remold HG. 1999. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J Infect Dis 180:1230–1237. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 37.Jungersen G, Huda A, Hansen JJ, Lind P. 2002. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin Diagn Lab Immunol 9:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.