Abstract
Importance
Staphylococcus aureus is a frequent cause of infection in hospitalized infants. These infections are associated with increased mortality and morbidity, and longer hospital stays, but data on the burden of S. aureus disease in hospitalized infants are limited.
Objective
To compare demographics and mortality of infants with invasive methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA), determine the annual proportion of S. aureus infections that were MRSA, and compare the risk of death following an invasive MRSA infection to the risk following an invasive MSSA infection.
Design
Multicenter retrospective study of a large, nationally representative cohort.
Setting
348 neonatal intensive care units managed by the Pediatrix Medical Group.
Participants
3888 infants with an invasive S. aureus infection who were discharged between 1997 and 2012.
Exposure
Invasive S. aureus infection.
Main Outcomes and Measures
Incidence of invasive S. aureus infections. Infant characteristics and mortality following MRSA or MSSA infection.
Results
The 3888 infants had 3978 invasive S. aureus infections (2868 MSSA, 1110 MRSA). The incidence of invasive S. aureus infection was 44.8 infections/10,000 infants. The yearly proportion of invasive infections caused by MRSA increased from 1997 to 2006 and has remained relatively stable since then. Infants with invasive MRSA or MSSA infections had similar gestational ages and birth weights. Invasive MRSA infections occurred more often at a younger postnatal age. For infants with available mortality data, more infants with invasive MSSA infections died at hospital discharge (N=237) than those with invasive MRSA infections (N=110). The proportion of infants who died following invasive MSSA or MRSA infection were similar: 237/2474 (9.6%) and 110/926 (11.9%), P=.05, respectively. Adjusted risk of death at hospital discharge was similar after invasive MSSA and MRSA infections overall (risk ratio, 1.19; 95% CI, 0.96-1.49). Risks of death at 7 and 30 days after invasive infection were similar between infants with invasive MSSA and MRSA infection.
Conclusion
Infant mortality following invasive MRSA and MSSA infections is similar. MSSA causes more infections and more deaths in infants than MRSA. Measures to prevent S. aureus infection should include MSSA in addition to MRSA.
INTRODUCTION
Staphylococcus aureus (S. aureus) is a frequent cause of infection in hospitalized infants.1 Infections due to S. aureus are associated with increased mortality2, morbidity,3 and length of hospital stay.4 S. aureus is the second most frequent cause of late-onset sepsis in very low birth weight (<1500 g; VLBW) infants1 and is resistant to methicillin in 8-28% of cases.5-7
Antibiotic-resistant S. aureus strains, specifically methicillin-resistant S. aureus (MRSA), have emerged and become prevalent in neonatal intensive care units (NICUs). Because MRSA-colonized infants often serve as a reservoir for spread to other infants, many NICUs have developed procedures to detect and isolate colonized infants.8,9 Identification of colonization is often followed by efforts to eradicate MRSA.8,10 Although infection due to methicillin-susceptible S. aureus (MSSA) also occurs, few centers have screening protocols for MSSA.11
The proportion of nosocomial S. aureus infections caused by MRSA has decreased in some settings, including NICUs.12,13 However, most studies evaluating S. aureus and MRSA in NICU patients have included small numbers of patients or culture sites, and have been conducted in single institutions, at tertiary care centers, or in outbreak settings.5,6,14 Our objective was to describe the epidemiology of S. aureus invasive infections using a large, nationally representative cohort of hospitalized infants.
METHODS
We identified all infants with an invasive S. aureus infection who were discharged between 1997 and 2012 from 348 NICUs managed by the Pediatrix Medical Group. The Pediatrix Medical Group cares for >20% of infants admitted to NICUs in the US and is composed of both academic and community sites in 34 states. Data were obtained from the electronic medical record generated prospectively by clinicians on all infants. Data were extracted, de-identified, and stored in the Pediatrix Clinical Data Warehouse.13 Information stored for infants includes maternal history, birth information, and demographics. Medications, laboratory results, microbiology results, diagnoses, and procedures are recorded on a daily basis. Microbiology results are reported as documented by the treating physician in the medical record.
Definitions
Positive S. aureus cultures obtained within 21 days of each other were considered to be a single infection.15 Infections in which any positive culture was obtained from cerebrospinal fluid (CSF), blood, sterile fluid, or an abscess were considered to be invasive. Infections in which all positive cultures were obtained from the trachea, urine, conjunctiva, or a wound were considered to be non-invasive. Abscess cultures included all cultures labeled “abscess” and may have included both cutaneous and deep tissue abscesses. Because the method of collection was missing for most urine samples, positive urine cultures were considered to be non-invasive. Cultures obtained from the skin surface, umbilicus, rectum, nasopharynx, or gastric aspirate were considered to represent surveillance cultures. Non-invasive and surveillance cultures were excluded from the analysis. Cultures for which the specimen type was “unknown” or “other” were also excluded. Infections that had growth of both MSSA and MRSA were considered to be MRSA infections.
Inotropic support was defined as exposure to dopamine, dobutamine, epinephrine, norepinephrine, or milrinone. Mechanical ventilation was defined as exposure to any invasive mechanical ventilation. Oxygen supplementation was defined as the administration of any fraction of inspired oxygen >21%. Antibiotic exposure was defined as exposure to any antibiotic. Anti-MRSA antibiotic exposure was defined as exposure to vancomycin, rifampin, linezolid, daptomycin, clindamycin, trimethoprim-sulfamethoxazole, or mupirocin. Small-for-gestational-age (SGA) status was defined as previously described.16
Statistical Analysis
We determined the proportion of S. aureus infections that were methicillin-resistant by year of discharge and by NICU site. We calculated the incidence of positive MSSA and MRSA infections per 10,000 admitted infants for each year.
Infant demographics were assigned to S. aureus type (MRSA vs MSSA) by the type of first invasive infection and were compared using Wilcoxon rank sum tests for continuous variables and chi-square tests for categorical variables. We compared the median number of days of exposure to inotropic support, oxygen support, ventilator support, antibiotic use, and MRSA-active antibiotic use prior to the first positive invasive culture for infants with MRSA to those parameters for infants with MSSA using Wilcoxon rank sum tests.
We determined the number of deaths that occurred before hospital discharge and within 7 and 30 days after the first positive culture of an invasive infection, both overall and separately for infants <1500 g birth weight and for infants ≥1500 g birth weight. For infants with more than one invasive infection, we considered the risk of death following the last infection. We compared the risk of death following an invasive MRSA infection to the risk of death following an invasive MSSA infection using modified Poisson regression adjusted for gestational age, SGA status, male sex, and race/ethnicity with random effects for site.
Statistical analyses were performed using Stata 13 (College Station, TX). Statistical significance was set at P<.05. This study was approved by the Duke University Institutional Review Board.
RESULTS
Burden of Invasive MSSA and MRSA Disease
We identified 3888/887,910 (0.4%) infants with 3978 invasive S. aureus infections. Infections were caused more commonly by MSSA (2868/3978, 72%) than MRSA (1110/3978, 28%).
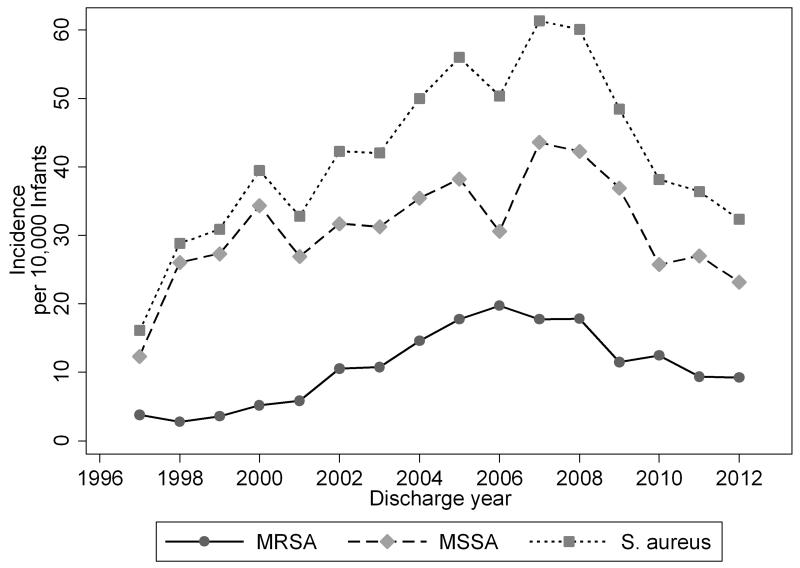
Overall, invasive S. aureus infections occurred at an incidence of 44.8 infections/10,000 infants. The annual incidence of invasive S aureus infection increased from 1997 to 2007 and declined from 2007 to 2012 (Figure 1). Invasive S. aureus infections were more common in infants born at <1500 g than those ≥1500 g (3061/136,797 [223.8/10,000 infants] vs 917/751,113 [12.2/10,000 infants], respectively, P<.01) (Table 1). MSSA was more common than MRSA for all birth weight categories.
Figure 1.
Annual Incidence of Invasive Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus, and Methicillin-Sensitive Staphylococcus aureus Infections per 10,000 Discharged Infants
Table 1.
Incidence of Invasive Staphylococcus aureus Infections for Hospitalized Infants, Number of Infections/10,000 Infants
| MSSA | MRSA | S. aureus | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Culture Type | N | Infections/10,000 | N | Infections/10,000 | N | Infections/10,000 |
| <1500 g birth weight (N=136,797) |
||||||
| All sites | 2208 | 161.4 | 853 | 62.4 | 3061 | 223.8 |
| ≥1500 g birth weight (N=751,113) |
||||||
| All sites | 660 | 8.8 | 257 | 3.4 | 917 | 12.2 |
Abbreviations: MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Characteristics of Infants With MSSA and MRSA Disease
Most characteristics of infants with invasive MSSA and MRSA infections were similar, including gestational age, birth weight, and the proportion that were male, small for gestational age or born by Cesarean-section (Table 2). MSSA was more common than MRSA for all races except for African American infants. MRSA infections occurred at a younger median postnatal age than MSSA infections (15 days [25th, 75th percentiles: 9, 26] vs 18 days [10, 32], respectively, P<.001). When considering exposure to potential risk factors before the first invasive S. aureus infection, infants whose first invasive S. aureus infection was MRSA had a shorter median duration of oxygen support before the infection than those with MSSA (5 days [1, 15] vs 8 days [1, 20], P<.001). Previous exposure to a surgical procedure and the number of days of antibiotic exposure, MRSA-active antibiotic exposure, ventilator support, and inotropic support before the first invasive S. aureus infection were similar for infants with MSSA and those with MRSA.
Table 2.
Characteristics of All Infants and Infants With An Invasive Culture for Staphylococcus aureusa
| Characteristic | MSSA (N=2825) |
MRSA (N=1063) |
P Value |
|---|---|---|---|
| Gestational age, weeks | .72 | ||
| <25 | 723 (26) | 270 (25) | |
| 26–28 | 966 (34) | 345 (32) | |
| 29–32 | 660 (23) | 2659 (24) | |
| 33–36 | 253 (9) | 107 (10) | |
| ≥37 | 219 (8) | 82 (8) | |
| Birth weight, g | .42 | ||
| <1000 | 1480 (52) | 528 (50) | |
| 1000–1499 | 689 (24) | 284 (27) | |
| 1500–2499 | 387 (14) | 145 (14) | |
| 2500–3499 | 194 (7) | 82 (8) | |
| >3500 | 73 (3) | 24 (2) | |
| Apgar score | .24 | ||
| 0-3 | 147 (5) | 49 (5) | |
| 4-6 | 512 (19) | 215 (21) | |
| 7-10 | 2087 (76) | 762 (74) | |
| Race/ethnicity | <.001 | ||
| White | 1329 (49) | 467 (45) | |
| African American | 681 (25) | 330 (32) | |
| Hispanic | 564 (21) | 201 (19) | |
| Other | 151 (6) | 37 (4) | |
| Male | 1555 (55) | 575 (54) | .60 |
| Inborn | 2236 (80) | 783 (74) | <.001 |
| Cesarean section | 2033 (73) | 741 (71) | .16 |
| Small for gestational age | 541 (19) | 207 (20) | .84 |
| Congenital anomaly | 363 (13) | 150 (14) | .30 |
| Age at 1st positive culture, days |
.01 | ||
| <7 | 324 (11) | 123 (12) | |
| 7–14 | 659 (23) | 292 (27) | |
| 15–28 | 905 (32) | 348 (33) | |
| >28 | 937 (33) | 300 (28) | |
| Previous surgical procedure |
476 (17) | 186 (18) | .63 |
| Inotropic supportb | 0 (0, 2) | 0 (0, 2) | .61 |
| Oxygen supportb | 8 (1, 20) | 5 (1, 15) | <.001 |
| Ventilator supportb | 5 (0, 16) | 5 (1, 13) | .06 |
| Antibiotic exposureb | 4 (1, 11) | 4 (1, 10) | .62 |
| Anti-MRSA antibiotic exposureb |
0 (0, 4) | 0 (0, 3) | .52 |
Abbreviations: MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Except where indicated, data are presented as no. (%). Invasive cultures = blood, cerebrospinal fluid, abscess and sterile sites. For infants with more than one infection, only the first invasive infection was evaluated.
Median (25th, 75th percentiles) number of days with exposure prior to first invasive Staphylococcus aureus infection.
Mortality of Infants With MSSA and MRSA Disease
Among the 2474 infants with invasive MSSA infections for whom mortality information was available, 237 (9.6%) had died at hospital discharge. Mortality was higher among infants with invasive MRSA infection (110/926 [11.9%], P=.049). Infants < 1500 g birth weight with invasive S. aureus infection died at hospital discharge more often than infants ≥ 1500 g (302/2596 [11.6%] vs 45/804 [5.6%], P<.01). When the risk of death was adjusted for birth weight, gender, and race with random effects for site, there was no statistically significant difference in the risk of death following an invasive MSSA infection compared to an invasive MRSA infection overall, or separately for infants < 1500 g and ≥ 1500 g (Table 3). The risk of death was also similar at 7 and 30 days after the start day of the invasive infection.
Table 3.
Mortality Following Invasive Staphylococcus aureus Disease by Last Infection
| No./No. (%) |
|||
|---|---|---|---|
| Death | MSSA | MRSA | Adjusted Risk Ratio,a MRSA vs MSSA (95% CI) |
| By hospital discharge | |||
| All infants | 237/2474 (10) | 110/926 (12) | 1.19 (0.96-1.49) |
| <1000 g birth weight | 180/1262 (14) | 82/457 (18) | 1.23 (0.93, 1.62) |
| <1500 g birth weight | 205/1883 (11) | 97/713 (14) | 1.24 (0.98-1.57) |
| ≥1500 g birth weight | 32/591 (5) | 13/213 (6) | 0.98 (0.60-1.61) |
| Within 7 days of culture | |||
| All infants | 95/2474 (4) | 35/926 (4) | 0.90 (0.65-1.24) |
| <1000 g birth weight | 64/1262 (5) | 23/457 (5) | 0.91 (0.55, 1.51) |
| <1500 g birth weight | 78/1883 (4) | 30/713 (4) | 0.96 (0.66-1.41) |
| ≥1500 g birth weight | 17/591 (3) | 5/213 (2) | 0.58 (0.18-1.82)b |
| Within 30 days of culture | |||
| All infants | 178/2474 (7) | 81/926 (9) | 1.15 (0.90-1.46) |
| <1000 g birth weight | 129/1262 (10) | 59/457 (13) | 1.20 (0.87, 1.65) |
| <1500 g birth weight | 151/1883 (8) | 70/713 (10) | 1.18 (0.91-1.54) |
| ≥1500 g birth weight | 27/591 (5) | 11/213 (5) | 0.97 (0.56-1.68) |
Abbreviations: MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Risk ratio calculated using Poisson regression adjusted for gestational age, small for gestational age status, gender, and race with random effects for site.
Poisson regression will not converge, so results are reported from random effects logistic regression model controlling for the same covariates.
Trends in Methicillin Resistance Over Time
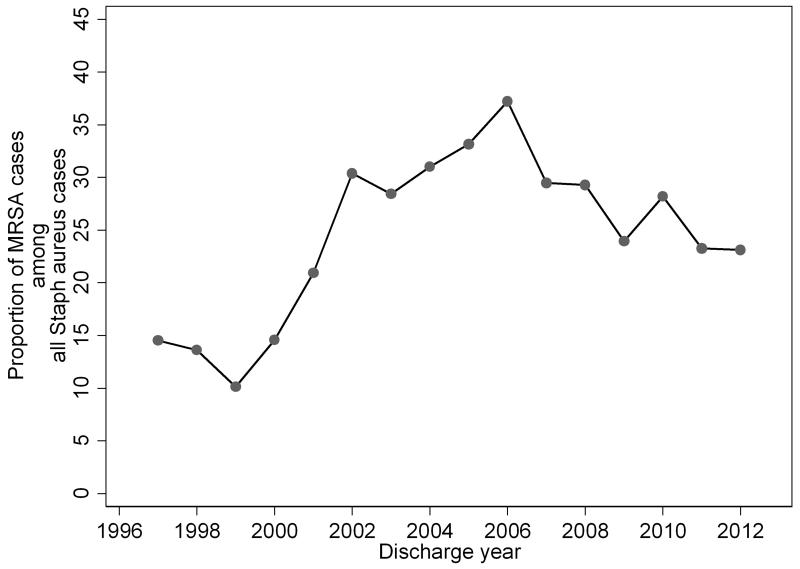
The proportion of S. aureus infections resistant to methicillin increased from 1999 to 2006 (Figure 2). A slight decrease in the proportion of S. aureus cultures that were MRSA occurred in 2007, but the annual proportion of MRSA cultures has overall remained increased. For the 100 sites with ≥10 episodes of invasive S. aureus infection, the proportion of invasive S. aureus infections caused by MRSA varied widely by site (median 26.3% [16.7, 36.8]). The proportion of invasive infections caused by MRSA ranged from 0 at 5 sites to 86.7% at 1 site.
Figure 2.
Proportion of Staphylococcus aureus Positive Cultures That Were Methicillin-Resistant by Year of Discharge
DISCUSSION
In this large representative cohort of hospitalized infants, invasive S. aureus infections occurred in 0.4% of all infants and in 2.2% of VLBW infants. Overall, MSSA caused 2.5 times more invasive S. aureus infections than MRSA. The adjusted risk of death was similar for MSSA and MRSA invasive infections overall and for VLBW infants.
Our findings confirm results of earlier studies demonstrating that infections due to MSSA are more common than MRSA infections. A single-center retrospective study of 172 S. aureus infections found that 72% of S. aureus infections were caused by MSSA, with most of the infections occurring in infants <1000 g birth weight.6 A study of VLBW infants in the National Institute of Child Health and Human Development’s Neonatal Research Network study similarly found that 72% of bacteremia and meningitis cases due to S. aureus were caused by MSSA.5 Infection prevention strategies should broadly target both MSSA and MRSA in this population.
In our cohort, invasive S. aureus infections occurred at an incidence of 2.2% in VLBW infants. Previous studies found that the incidence of S. aureus infections in this population was approximately 3%.5,6 Our observed disease incidence of 2.2% may better reflect the national burden of disease but may not be applicable to all high-acuity centers. If current birth trends continue, an incidence of invasive S. aureus disease of 2.2% in VLBW infants and 0.1% in non-VLBW infants will result in more than 5000 invasive S. aureus infections each year.17
Studies of older patients have found that prolonged hospital stay, presence of a central line, recent surgical procedures and recent antibiotic therapy may predispose patients to infection with MRSA.18-20 In infants, some studies have noted that a low birth weight and gestational age, as well as recent surgical procedure and prior treatment with systemic antibiotics, are associated with a higher incidence of MRSA colonization and subsequent infection.6,21,22 We found that infants with invasive MRSA infection were more often of African American race, outborn status, and younger postnatal age. Other studies have also noted that infants with MRSA infection are younger in postnatal age than those with MSSA.6,23,24 This is intuitively unexpected because, as a hospital-associated infection, it would be expected that more days in the hospital and more days of antibiotic exposure would increase risk of MRSA infections. MRSA infections have been noted to be more common in African American patients.25-27 The reasons for this difference are not clear but may be due to differences in immune function, maternal colonization rates or other unknown factors.28
Worse outcomes following MRSA infection compared to MSSA infections have been noted in older patients with bloodstream infections,29,30 endocarditis,31 and osteomyelitis.32 Differences in outcomes following MRSA infection compared to MSSA infection in infants, however, have been less clear, and our findings are consistent with several previous studies. We found no difference in adjusted mortality following MRSA or MSSA invasive infection at 7 days, 30 days, or hospital discharge. A single-center study of 172 S. aureus infections in hospitalized infants found that mortality was similar between infants with MRSA and MSSA (16% vs 17%, respectively, P=1.00).6 Hospital length of stay was also similar (64 days for MRSA and 64 days for MSSA, P=.80).6 Another single-center study found no differences in the duration of hospital length of stay (P=.70), death (P=.20), or the incidence of neurodevelopmental impairment at 12-36 months of age (P=.28) for 53 infants with S. aureus bacteremia.24 A multicenter retrospective study of 316 infants found no difference in survival for infants ≤ 1500 g with MRSA versus MSSA bacteremia or meningitis.5
Given similar outcomes in infants with MRSA and MSSA disease, prevention strategies should focus broadly on S. aureus prevention and not focus solely on prevention of MRSA. Single-center studies have suggested that MRSA screening and decolonization may reduce MRSA infection. However, a lack of controlled trials has led to a large variation in approaches in MRSA identification and treatment in the NICU.8 Even though MSSA causes more infections and more deaths per year in infants, most centers only consider MRSA in their screening and decolonization protocols.8 As we have previously described, a broader approach to S. aureus prevention should go beyond MRSA to also include MSSA.10 When MSSA has been included in screening and decolonization efforts, subsequent MSSA infections have decreased.33,34 One NICU was able to dramatically reduce both MRSA and MSSA infections with a universal S. aureus screening and decolonization program.33 Screening and decolonization protocols have been demonstrated to reduce infections due to both MRSA and MSSA in adult orthopedic and cardiac surgery patients.35,36
The annual proportion of MRSA cultures in our population increased from 1996 to 2006 when an initial decrease occurred followed by a plateau. The early increase has been seen in other studies. A study including data from 40 freestanding children’s hospitals found that the incidence of MRSA increased from 2002 to 2007 with MSSA incidence remaining stable.37 A 10-year single-center study that included 156 infants with S. aureus bloodstream infections found a peak in the incidence of infections due to MRSA in 2005 with a steadily decreasing incidence from 2006-2009.23 Further studies are needed to identify differences between centers with high and low proportions of isolates with methicillin-resistance.
The stabilization in MRSA incidence that we observed starting in 2007 is also consistent with the findings of other investigators. A study including 1751 infants found that surveillance cultures positive for MRSA peaked in 2006 at 5.8% and then plateaued with approximately 3% of cultures being positive.12 Using national surveillance data, the Centers for Disease Control found that the incidence of MRSA decreased nearly 10% per year from 2005-2010 in infants <90 days postnatal age.38 A large study that included infants, children, and adults found that S. aureus bacteremia decreased by 41% from 2006-2010.13 This stabilization in MRSA rates may be due to improved infection control practices leading to fewer hospital-acquired infections.38
We opted to exclude surveillance and non-invasive cultures from analysis because of varying surveillance practices (i.e., centers with no surveillance, surveillance for MRSA only, varying surveillance frequency and methods). Specimens from invasive infections may have been classified as surveillance cultures or non-invasive infections, but this number was likely small and should have been similar for MRSA and MSSA. Our analysis evaluated all-cause mortality not S. aureus-attributable mortality; however, the relative risk of death following invasive MRSA compared to invasive MSSA is not likely to be influenced by this distinction.
CONCLUSIONS
The absolute numbers of infections and deaths due to MSSA exceed those due to MRSA. Consideration should be given to expanding hospital infection control efforts targeting MRSA to include MSSA as well.10,39,40 Future studies to better define the relationship between MSSA colonization and subsequent infection will help to clarify the importance of such interventions for preventing MSSA disease.
Acknowledgments
Drs Smith and Benjamin Jr. receive research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr Fowler receives grant/research support from Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, and Theravance; has been a paid consultant to Affinium, Basilea, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, The Medicines Company, MedImmune, Pfizer, Theravance, and Trius; has received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, and Vertex Pharmaceuticals; and has been a member of the Merck V710 Vaccine Scientific Advisory Board. Dr Milstone receives grant support from Sage Products LLC.
Funding/Support: Dr Ericson receives support from the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) (5T32HD060558). Dr Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NICHD (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01). Dr Benjamin Jr. receives support from the NIH (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, NICHD contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases [NIAID] contract HHSN272201500006I). Dr Fowler receives support from NIH K24-AI093969, R01-AI068804, and the NIH Antibiotic Resistant Leadership Group under award number UM1AI104681. Dr. Milstone receives support from the NIAID (award R03AI117169) and the Agency for Healthcare and Research Quality (award R01HS022872).
Role of the Funding Sources: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosures: The other authors have no financial relationships to disclose.
Authors’ Contributions: Drs. Ericson and Benjamin had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drs. Milstone, Popoola, Smith, and Ericson.
Drafting of the manuscript: Dr Ericson.
Critical revision of the manuscript for important intellectual content: Drs Smith, Popoola, Benjamin, Benjamin Jr., and Milstone.
Statistical analysis: Drs Ericson, Benjamin
Approval of the final manuscript as submitted: All authors.
REFERENCES
- 1.Boghossian NS, Page GP, Bell EF, et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162(6):1120–1124. 1124–e1. doi: 10.1016/j.jpeds.2012.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstraete E, Boelens J, De Coen K, et al. Healthcare-associated bloodstream infections in a neonatal intensive care unit over a 20-year period (1992-2011): trends in incidence, pathogens, and mortality. Infect Control Hosp Epidemiol. 2014;35(5):511–518. doi: 10.1086/675836. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 4.Song X, Perencevich E, Campos J, Short BL, Singh N. Clinical and economic impact of methicillin-resistant Staphylococcus aureus colonization or infection on neonates in intensive care units. Infect Control Hosp Epidemiol. 2010;31(2):177–182. doi: 10.1086/649797. [DOI] [PubMed] [Google Scholar]
- 5.Shane AL, Hansen NI, Stoll BJ, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129(4):e914–e922. doi: 10.1542/peds.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010;30(2):135–139. doi: 10.1038/jp.2009.119. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs D, Fraser S, Hogg G, Li HY. Staphylococcus aureus infections in Australasian neonatal nurseries. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F331–F335. doi: 10.1136/adc.2002.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milstone AM, Song X, Coffin S, Elward A. Identification and eradication of methicillin-resistant Staphylococcus aureus colonization in the neonatal intensive care unit: results of a national survey. Infect Control Hosp Epidemiol. 2010;31(7):766–768. doi: 10.1086/653615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popoola VO, Budd A, Wittig SM, et al. Methicillin-resistant Staphylococcus aureus transmission and infections in a neonatal intensive care unit despite active surveillance cultures and decolonization: challenges for infection prevention. Infect Control Hosp Epidemiol. 2014;35(4):412–418. doi: 10.1086/675594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popoola VO, Milstone AM. Decolonization to prevent Staphylococcus aureus transmission and infections in the neonatal intensive care unit. J Perinatol. 2014;34(11):805–810. doi: 10.1038/jp.2014.128. [DOI] [PubMed] [Google Scholar]
- 11.Hacek DM, Robb WJ, Paule SM, Kudrna JC, Stamos VP, Peterson LR. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466(6):1349–1355. doi: 10.1007/s11999-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macnow T, O’Toole D, DeLaMora P, et al. Utility of surveillance cultures for antimicrobial resistant organisms in infants transferred to the neonatal intensive care unit. Pediatr Infect Dis J. 2013;32(12):e443–e450. doi: 10.1097/INF.0b013e3182a1d77f. [DOI] [PubMed] [Google Scholar]
- 13.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 14.Haley RW, Cushion NB, Tenover FC, et al. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J Infect Dis. 1995;171(3):614–624. doi: 10.1093/infdis/171.3.614. [DOI] [PubMed] [Google Scholar]
- 15.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–S74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 17.Martin J, Hamilton BE, Osterman MJK, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 18.Reighard A, Diekema D, Wibbenmeyer L, Ward M, Herwaldt L. Staphylococcus aureus nasal colonization and colonization or infection at other body sites in patients on a burn trauma unit. Infect Control Hosp Epidemiol. 2009;30(8):721–726. doi: 10.1086/598681. [DOI] [PubMed] [Google Scholar]
- 19.Jennings A, Bennett M, Fisher T, Cook A. Impact of a surveillance screening program on rates of methicillin-resistant Staphylococcus aureus infections with a comparison of surgical versus nonsurgical patients. Proc (Bayl Univ Med Cent) 2014;27(2):83–87. doi: 10.1080/08998280.2014.11929064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez JN, Ocampo AM, Vanegas JM, et al. A comparison of methicillin-resistant and methicillin-susceptible Staphylococcus aureus reveals no clinical and epidemiological but molecular differences. Int J Med Microbiol. 2013;303(2):76–83. doi: 10.1016/j.ijmm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Geraci DM, Giuffre M, Bonura C, et al. Methicillin-resistant Staphylococcus aureus colonization: a three-year prospective study in a neonatal intensive care unit in Italy. PLoS One. 2014;9(2):e87760. doi: 10.1371/journal.pone.0087760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaki H, Nishioka M, Kanda K, Takahashi Y. An investigation of the risk factors for infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37(7):580–586. doi: 10.1016/j.ajic.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Dolapo O, Dhanireddy R, Talati AJ. Trends of Staphylococcus aureus bloodstream infections in a neonatal intensive care unit from 2000-2009. BMC Pediatr. 2014;14:121. doi: 10.1186/1471-2431-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen-Wolkowiez M, Benjamin DK, Jr, Fowler VG, Jr>, et al. Mortality and neurodevelopmental outcome after Staphylococcus aureus bacteremia in infants. Pediatr Infect Dis J. 2007;26(12):1159–1161. doi: 10.1097/INF.0b013e31814620a6. [DOI] [PubMed] [Google Scholar]
- 25.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus in three communities. N Engl J Med. 2005;352(14):1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 26.McCormick ME, Chun RH, Lander L, Shah RK. Socioeconomic implications of pediatric cervical methicillin-resistant Staphylococcus aureus infections. JAMA Otolaryngol Head Neck Surg. 2013;139(2):124–128. doi: 10.1001/jamaoto.2013.1234. [DOI] [PubMed] [Google Scholar]
- 27.Schrag SJ, Zywicki S, Farley MM, et al. Group B Streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342(1):15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto M, Mu Y, Lynfield R, et al. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics. 2013;132(4):e817–e824. doi: 10.1542/peds.2013-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaw LK, Robinson JO, Ho KM. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis. 2014;14(10):967–975. doi: 10.1016/S1473-3099(14)70876-X. [DOI] [PubMed] [Google Scholar]
- 30.Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273–279. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 31.Hill EE, Peetermans WE, Vanderschueren S, et al. Methicillin-resistant versus methicillin-sensitive Staphylococcus aureus infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27(6):445–50. doi: 10.1007/s10096-007-0458-2. [DOI] [PubMed] [Google Scholar]
- 32.Saavedra-Lozano J, Mejias A, Ahmad N, et al. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop. 2008;28(5):569–575. doi: 10.1097/BPO.0b013e31817bb816. [DOI] [PubMed] [Google Scholar]
- 33.Delaney HM, Wang E, Melish M. Comprehensive strategy including prophylactic mupirocin to reduce Staphylococcus aureus colonization and infection in high-risk neonates. J Perinatol. 2013;33(4):313–318. doi: 10.1038/jp.2012.102. [DOI] [PubMed] [Google Scholar]
- 34.Lepelletier D, Lucet J-C. Controlling meticillin-susceptible Staphylococcus aureus: not simply methicillin-resistant S. aureus revisited. J Hosp Infect. 2013;84(1):13–21. doi: 10.1016/j.jhin.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Schweizer ML, Chiange HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip or knee surgery. JAMA. 2015;313(21):2162–2171. doi: 10.1001/jama.2015.5387. [DOI] [PubMed] [Google Scholar]
- 36.Chen AF, Wessel CB, Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res. 2013;471(7):2383–2399. doi: 10.1007/s11999-013-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49(1):65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwamoto M, Mu Y, Lynfield R, et al. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics. 2013;132(4):e817–e824. doi: 10.1542/peds.2013-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray J, Patel M, Turner H, Reynolds F. MRSA screening on a paediatric intensive care unit. Arch Dis Child. 2012;97(3):243–244. doi: 10.1136/adc.2010.185785. [DOI] [PubMed] [Google Scholar]
- 40.Romano-Bertrand S, Filleron A, Mesnage R, et al. Staphylococcus aureus in a neonatal care center: methicillin-susceptible strains should be a main concern. Antimicrob Resist Infect Control. 2014;3:21. doi: 10.1186/2047-2994-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]