Abstract
Advancing age is associated with reductions in nitric oxide bioavailability and changes in metabolic activity, which are implicated in declines in motor and cognitive function. In preclinical models, sodium nitrite supplementation (SN) increases plasma nitrite and improves motor function, whereas other nitric oxide-boosting agents improve cognitive function. This pilot study was designed to translate these findings to middle-aged and older (MA/O) humans to provide proof-of-concept support for larger trials. SN (10 weeks, 80 or 160 mg/day capsules, TheraVasc, Inc.) acutely and chronically increased plasma nitrite and improved performance on measures of motor and cognitive outcomes (all p<0.05 or better) in healthy MA/O adults (62 ± 7 years). Untargeted metabolomics analysis revealed that SN significantly altered 33 (160 mg/day) to 45 (80 mg/day) different metabolites, 13 of which were related to changes in functional outcomes; baseline concentrations of 99 different metabolites predicted functional improvements with SN. This pilot study provides the first evidence that SN improves aspects of motor and cognitive function in healthy MA/O adults, and that these improvements are associated with, and predicted by, the plasma metabolome. Our findings provide the necessary support for larger clinical trials on this promising pharmacological strategy for preserving physiological function with aging.
Keywords: aging, metabolomics, neuromuscular, precision medicine, nitrates/nitrites
INTRODUCTION
Advancing age is associated with declines in several domains of motor function including muscle strength/power, endurance, balance, dexterity and locomotor performance [1-3]. These changes can lead to functional limitations and increased risk of disability, falls, and loss of independence [4-7]. Given the marked increases in the number of older adults expected in the coming decades, establishing the efficacy of interventions that enhance motor function in the late middle-aged and older (MA/O) population is a high biomedical research priority [8-10].
There is strong evidence that regular exercise is an effective strategy for optimizing physical function with aging [11, 12], however, physical activity decreases with age and most MA/O adults fall well short of the recommended weekly guidelines [13-15]. As such, there is growing interest in pharmacological compounds, including dietary supplements and nutra-ceuticals, that may exert at least some of the benefits of physical activity and other healthy lifestyle practices on physical function with aging [8].
Recently, the potential health benefits of nitrate and nitrite supplementation have received considerable attention. Nitrate and nitrite are precursors of nitric oxide (NO), a ubiquitous gaseous signaling molecule that plays a critical role in systemic physiological function. Studies assessing the effects of dietary and/or pharmacological supplementation with nitrates or nitrites on motor function in healthy young adults have yielded mixed results [16-20]. However, NO bioavailability declines with aging and, therefore, NO-boosting treatments may have greater effects on physiological function in MA/O adults. Consistent with this possibility, we recently showed that supplementation with sodium nitrite in the drinking water for 8 weeks improved grip strength, locomotor activity and endurance performance in old mice [21]. Presently, it is unknown if sodium nitrite supplementation can improve motor function in MA/O humans.
To obtain preliminary support for this possibility, we performed a small-scale pilot (feasibility) trial to assess the potential efficacy of sodium nitrite supplementation for improving multiple domains of motor function in healthy MA/O adults. Two different doses of sodium nitrite (80 and 160 mg/day, TheraVasc, Inc.) were assessed over a 10-week treatment period using a randomized, placebo controlled, double-blind design. Because cognitive function is closely linked to motor performance [22, 23], and treatment with sodium nitrite or NO donors improves learning and memory in rodents [24], we took the opportunity to also assess effects on two simple, time-efficient measures of processing speed and executive function. Finally, to gain initial insight into the molecular signaling mechanisms underlying any improvements in motor or cognitive function observed, we assessed the plasma metabolome before and after sodium nitrite supplementation using an untargeted approach. In an extensive post-hoc analysis, we determined changes in small metabolite signatures with treatment, the relation between these changes and improvements in function, and identified plasma metabolites at baseline that predicted responsiveness to treatment.
RESULTS
Subject characteristics and safety
Characteristics of the groups are shown in Table 1. At baseline and following 10 weeks supplementation with 80 mg or 160 mg sodium nitrite per day or placebo, no group differences in body composition characteristics (total body mass, lean body mass and regional lean mass), basic blood panel (fasting glucose, insulin and cholesterol) or habitual daily activity were observed (p>0.05, all). Sodium nitrite was well tolerated. No severe adverse events occurred in any group (for details, see [25]). Methemoglobin did not exceed peak levels of 1.5% in any group (12% safety cut-off). Two subjects were withdrawn due to moderate adverse events (placebo: dizziness; high dose: headache).
Table 1. Subject characteristics before and after 10 weeks of placebo and sodium nitrite supplementation.
| Placebo | Nitrite (80 mg/day) | Nitrite (160 mg/day) | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| n (women) | 10 (4) | 10 (5) | 10(5) | |||
| Age (years) | 62 ± 8 | -- | 60 ± 6 | -- | 64 ± 6 | -- |
| BMI (kg/m2) | 25.9 ± 2.7 | 25.8 ± 2.8 | 24.5 ± 3.2 | 24.6 ± 2.8 | 24.2 ± 3.0 | 24.4 ± 3.2 |
| Body mass (kg) | 75.1 ± 10.2 | 74.8 ± 10.1 | 70.6 ± 9.3 | 71.3 ± 9.2 | 70.4 ± 10.2 | 70.9 ± 10.1 |
| Lean mass (kg) | 48.6 ± 14.3 | 50.1 ± 11.8 | 47.2 ± 7.7 | 47.9 ± 8.4 | 47.6 ± 8.2 | 48.2 ± 8.4 |
| Body fat (%) | 30.4 ± 11.1 | 30.0 ± 11.3 | 29.7 ± 7.9 | 29.4 ± 8.7 | 27.9 ± 10.0 | 28.1 ± 10.3 |
| Fasting glucose (mg/dL) | 94 ± 5 | 94 ± 6 | 90 ± 6 | 90 ± 5 | 93 ± 4 | 91 ± 5 |
| Fasting insulin (μU/mL) | 8 ± 2 | 10 ± 3 | 9 ± 3 | 8 ± 2 | 8 ± 3 | 7 ± 3 |
| Total cholesterol (mg/dL) | 164 ± 22 | 158 ± 22 | 175 ± 25 | 174 ± 28 | 180 ± 19 | 183 ± 25 |
| HDL-cholesterol (mg/dL) | 59 ± 6 | 58 ± 6 | 58 ± 4 | 60 ± 4 | 57 ± 4 | 59 ± 4 |
| LDL-cholesterol (mg/dL) | 90 ± 5 | 87 ± 4 | 100 ± 6 | 101 ± 7 | 109 ± 6 | 108 ± 8 |
| Triglycerides (mg/dL) | 79 ± 8 | 67 ± 5 | 70 ± 6 | 70 ± 13 | 75 ± 6 | 80 ± 9 |
| Activity (steps/wk) | 60.7 ± 24.3 | 55.4 ± 18.3 | 70.2 ± 36.4 | 67.4 ± 35.5 | 67.7 ± 32.0 | 66.0 ± 36.2 |
Data are mean ± SD. HDL, high-density lipoprotein; LDL, low-density lipoprotein. Activity is reported as value × 10,000 steps.
Plasma nitrite
Plasma nitrite concentrations did not differ among groups at baseline (Table 2). Levels increased 6- to 25-fold at 30 min after acute ingestion of the initial dose of sodium nitrite in the low- (80 mg/day) and high- (160 mg/day) dose sodium nitrite groups, respectively, with no change observed in the placebo group. These observations demonstrate that the treatment acutely increased plasma nitrite concentrations in our subjects.
Table 2. Subject characteristics before and after 10 weeks of placebo and sodium nitrite supplementation.
| Placebo | Nitrite (80 mg/day) | Nitrite (160 mg/day) | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Acute (baseline and 30 min after 1st dose) | ||||||
| Nitrite | 0.23 ± 0.24 | 0.20 ± 0.19 | 0.47 ± 0.47 | 3.34 ± 1.88* | 0.27 ± 0.31 | 7.13 ± 3.36* |
| Chronic (baseline and 12-18 h after final dose at week 10) | ||||||
| Nitrite | 0.20 ± 0.21 | 0.26 ± 0.16 | 0.21 ± 0.17 | 0.47 ± 0.27† | 0.15 ± 0.15 | 0.37 ± 0.21† |
p<0.05;
p<0.1
Following 10 weeks of sodium nitrite supplementation, a trend was observed for a small elevation of plasma nitrite levels 12-18 h after ingestion of the last capsule in 80 mg/day and 160 mg/day nitrite groups (0.05<p<0.1; Table 2). Consistent with its short half-life, these data suggest that the sodium nitrite supplementation produced a small chronic increase in circulating nitrite con-centrations [26].
Sodium nitrite supplementation and motor function
Rate of torque development and muscle strength
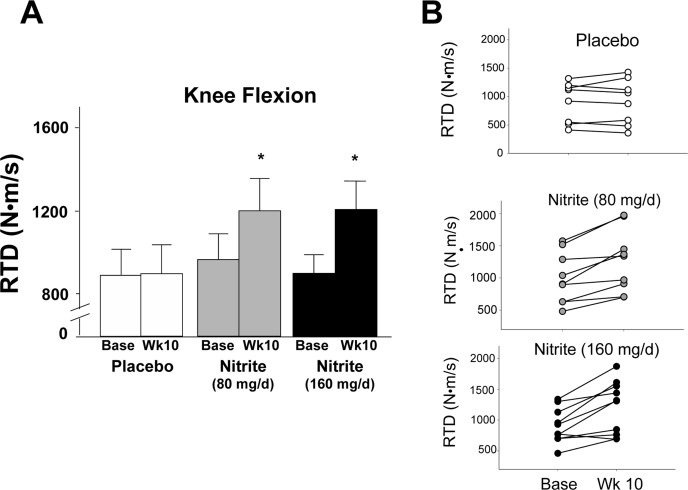
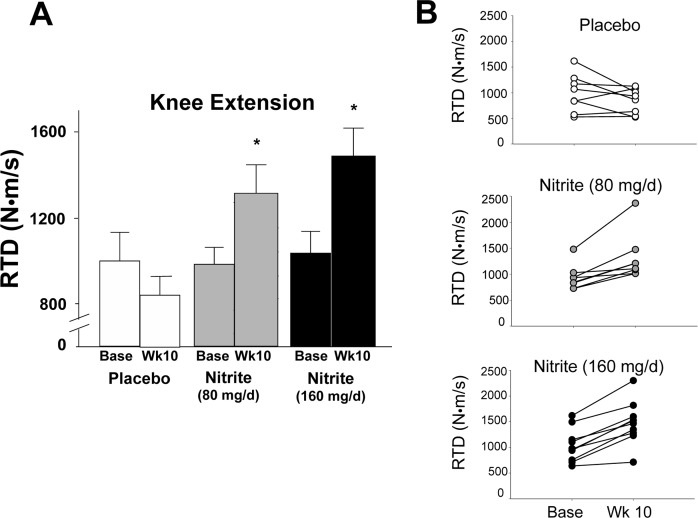
The motor outcomes demonstrating the most consistent improvements across groups (panel A) and individual participants (panel B) were knee flexor and extensor rate of torque development (RTD) (Figures 1 and 2). Specifically, knee flexor and knee extensor RTD were improved by 33 and 43% and 26 and 34%, respectively, with 80 mg/day and 160 mg/day sodium nitrite compared with placebo (p<0.01); the changes were not different between the two doses (p>0.05 between 80 and 160 mg/day). For the knee flexors, 7 of 10 individuals in the 80 mg/day group and 8 of 10 individuals in the 160 mg/day nitrite group demonstrated ≥10% improvements in RTD following sodium nitrite supplementation (Figure 1B). For the knee extensors, improvements of ≥10% were observed in 8 of 10 subjects in both the low- and high-dose groups (Figure 2B). The improvements in RTD were observed in the absence of significant changes in maximum voluntary contraction (MVC) torque for knee extensors or flexors (p>0.05, Table 3). Sodium nitrite supplementation did not alter grip strength, although a trend for improvement was observed in the 80 mg/day nitrite group (p<0.1, Table 3).
Figure 1.
Rate of torque development (RTD) for the knee flexors at baseline and following 10 weeks of placebo (white) or sodium nitrite supplementation (gray – 80 mg/day; black – 160 mg/day). Sodium nitrite supplementation improved knee flexor RTD as shown by time × treatment interaction for three groups (A); * p<0.05). Individual subject data (B) indicated that knee flexor RTD changed little across 10 weeks for the 10 subjects in the placebo group (white circles), but improved for most subjects taking sodium nitrite 80 mg/day (gray circles), and subjects supplemented with 160 mg/day (black circles).
Figure 2.
Rate of torque development (RTD) for the knee extensors at baseline and following 10 weeks of placebo (white) or sodium nitrite supplementation (gray – 80 mg/day; black – 160 mg/day). Sodium nitrite supplementation improved knee extensor RTD as shown by time × treatment interaction for three groups (A); * p<0.05). Individual subject data (B) indicated that knee extensor RTD changed little across 10 weeks for placebo (white circles), but improved for most subjects taking sodium nitrite 80 mg/day (gray circles), and subjects supplemented with 160 mg/day (black circles).
Table 3. Measures of motor function before and after 10 weeks of placebo and sodium nitrite supplementation.
| Placebo | Nitrite (80 mg/day) | Nitrite (160 mg/day) | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Balance | ||||||
| Rapid step errors | 3.2 ± 1.4 | 3.3 ± 1.0 | 2.9 ± 1.7 | 2.0 ± 1.3* | 3.5 ± 1.7 | 2.4 ± 1.5* |
| Rapid step time (s) | 40.2 ± 6.6 | 38.1 ± 6.2 | 40.4 ± 4.8 | 39.6 ± 4.0 | 41.3 ± 4.3 | 38.7 ± 4.0 |
| Fatigability | ||||||
| Heel-rise time (s) | 64 ± 32 | 56 ± 21 | 88 ± 40 | 121± 56† | 92 ± 53 | 113 ± 55 |
| Fatigue (FSS score) | 23.8 ± 9.6 | 21.9 ± 9.0 | 25.2 ± 11.3 | 22.1 ± 11.8 | 17.6 ± 8.1 | 15.8 ± 10.0 |
| Muscle Strength | ||||||
| Grip strength (kg) | 38.4 ± 9.2 | 37.6 ± 9.2 | 38.3 ± 6.9 | 41.5 ± 9.1† | 37.9 ± 7.7 | 39.3 ± 6.7 |
| MVC, Knee extensors (N·m) | 54 ± 19 | 62 ± 18 | 73 ± 33 | 78 ± 31 | 60 ± 18 | 65 ± 24 |
| MVC, Knee flexors (N·m) | 107 ± 27 | 107 ± 31 | 103 ± 29 | 107 ± 24 | 91.8 ± 27 | 103 ± 22 |
| Mobility | ||||||
| 400 m (s) | 196 ± 21 | 184 ± 24 | 206 ± 20 | 209 ± 18 | 199 ± 20 | 195 ± 20 |
| Timed up & go (s) | 5.3 ± 1.1 | 5.5 ± 1.5 | 6.2 ± 0.9 | 6.1 ± 0.5 | 5.9 ± 0.5 | 5.5 ± 0.4 |
| Manual Dexterity | ||||||
| Pegboard time (s) | 67 ± 17 | 63 ± 14 | 68 ± 10 | 64 ± 11 | 65 ± 9 | 65 ± 8 |
p<0.05;
p<0.1. FSS, fatigue severity scale; MVC, maximal voluntary contraction.
Cardiorespiratory fitness and muscle endurance
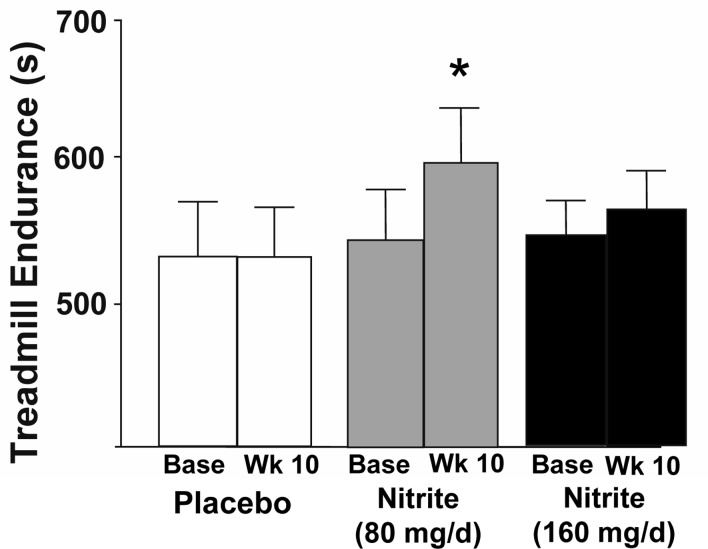
Compared with placebo, nitrite supplementation at the 80 mg/day dose improved maximal exercise capacity as assessed by treadmill time to exhaustion during the modified Balke incremental treadmill exercise protocol (Figure 3, p<0.05); no changes were observed for the 160 mg/day nitrite group. Heart rate, oxygen consumption (VO2max), ratings of perceived exertion, respiratory exchange ratio (index of hyperventilation) and minute ventilation during maximal exercise did not differ at baseline among groups nor change with nitrite supplementation (Table 4, p>0.05, all). No differences were observed at baseline among the groups or with treatment for any physiological response to submaximal levels of exercise during this treadmill protocol (data not reported).
Figure 3.
Endurance as measured by time to failure on a Balke treadmill task at baseline and following 10 weeks placebo (white) or sodium nitrite supplementation (gray – 80 mg/day; black – 160 mg/day). Sodium nitrite supplementation improved total duration subjects could sustain the treadmill test, especially at 80 mg/day supplementation (* p<0.05).
Table 4. Responses to maximal aerobic exercise before and after 10 weeks of placebo and sodium nitrite supplementation.
| Placebo | Nitrite (80 mg/day) | Nitrite (160 mg/day) | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| RPE | 18 ± 0 | 19 ± 0 | 18 ± 0 | 19 ± 0 | 18 ± 1 | 18 ± 0 |
| Heart rate (bpm) | 166 ± 5 | 163 ± 5 | 169 ± 5 | 168 ± 5 | 163 ± 3 | 163 ± 3 |
| RER | 1.17 ± 0.03 | 1.16 ± 0.02 | 1.16 ± 0.03 | 1.17 ± 0.02 | 1.19 ± 0.02 | 1.12 ± 0.02 |
| Ventilation (L/min) | 68 ± 8 | 67 ± 7 | 67 ± 4 | 68 ± 5 | 65 ± 4 | 68 ± 4 |
| VO2max (ml/(kg·min)) | 30.5 ± 6.9 | 30.0 ± 5.7 | 31.6 ± 6.7 | 31.8 ± 7.6 | 33.7 ± 9.2 | 33.6 ± 9.6 |
RPE, ratings of perceived exertion; RER, respiratory exchange ratio.
Time to failure for the heel-rise test was modestly improved with supplementation in the 80 mg/day group only (p<0.1, Table 3). There were no differences in fatigue (FSS score) at baseline or following treatment in any group (Table 3).
Balance, manual dexterity and mobility
The number of balance errors committed during the rapid step test were reduced by ∼30% after both doses of sodium nitrite treatment (p<0.05) in the absence of changes in rapid step time (p>0.1) (Table 3). The responders (i.e. > 10% improvement) for rapid step test errors were consistent with the responders for RTD. No changes were observed in pegboard times, 400-m walk time, or Timed Up & Go among groups (Table 3).
Sodium nitrite supplementation and cognitive outcomes
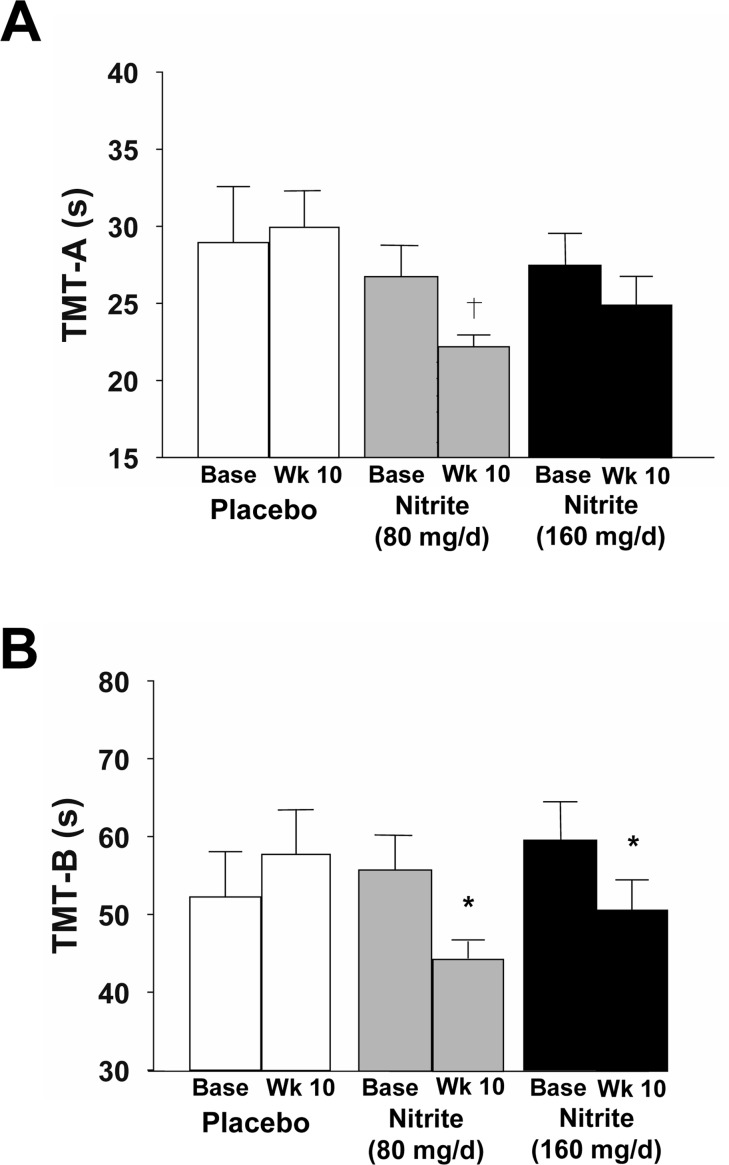
Cognitive performance was improved with 10 weeks of sodium nitrite, as indicated by reductions in time to complete the Trail Making Tests B and A (TMT-B and TMT-A) (Figure 4). Time to complete TMT-B, which is an index of executive function, was improved by 18 and 14% in response to the high- and low-doses of sodium nitrite, respectively (Figure 4A, p<0.05). Of these, 15 responded consistently for TMT-B, RTD and step errors, though one of the 5 non-responders showed a 9% improvement and was just below the 10% responder criteria. A trend was observed for an improvement in processing speed (TMT-A) in response to the 80 mg/day of sodium nitrite (14% improvement, p<0.1, Figure 4B), but not 160 mg/day. Individual performance data are shown in Supplemental Figure 1.
Figure 4.
Cognitive function as assessed by time to complete the Trail Making Test-A (A, TMT-A) and -B(B, TMT-B) at baseline and following 10 weeks placebo (white) or sodium nitrite supplementation (gray - 80 mg/day; black - 160 mg/day). Sodium nitrite supplementation tended to improve time to complete TMT-A, an index of processing speed (A; † p<0.1). A significant time × treatment interaction was observed for TMT-B(B; * p<0.05), indicating that sodium nitrite supplementation improved an index of executive function.
Sodium nitrite supplementation and the metabolome
Metabolomic signatures and sodium nitrite supplementation
In total, 3,063 metabolites were detected in the plasma of subjects in all groups and used in subsequent analyses of the plasma metabolome. We observed significantly altered metabolomic profiles following sodium nitrite supplementation compared to placebo. For some comparisons, the changes observed were specific for the 80 and 160 mg/day groups (Table 5). Of note, the changes in metabolite abundance occurred independent of any changes in subject characteristics or clinical blood markers. Overall, changes in 45 small molecules were observed in subjects administered 80 mg/day sodium nitrite (Supplemental Table 1) and 33 metabolites in subjects administered 160 mg/day (Supplemental Table 2). Both doses of sodium nitrite induced changes in abundance of compounds associated with glycerol-phospholipid, sphingolipid, carboxylic acid, peptide and pyridine metabolic pathways, whereas 80 mg/day of sodium nitrite also altered molecules involved in glycerolipid, fatty acyl and ribonucleoside metabolism.
Table 5. Metabolic pathways significantly altered by 10 weeks of sodium nitrite supplementation.
| Molecular Class | Number of Molecules Altered Within Each Pathway |
|---|---|
| Nitrite (80 mg/day) | |
| Glycerophospholipids | 13 |
| Sphingolipids | 2 |
| Glycerolipids | 1 |
| Fatty Acyls | 3 |
| Carbohydrates and Carbohydrate Conjugates | 1 |
| Carboxylic Acids and Derivatives | 2 |
| Imidazole Ribonucleosides and Ribonucleotides | 1 |
| Pyridines and Derivatives | 1 |
| Peptide Hormones | 1 |
| Unknown | 20 |
| Nitrite (160 mg/day) | |
| Glycerophospholipids | 9 |
| Sphingolipids | 2 |
| Carboxylic Acids and Derivatives | 2 |
| Polypeptide | 1 |
| Pyridines and Derivatives | 1 |
| Unknown | 18 |
Relation of functional changes to the metabolome and clinical characteristics
To investigate the relation between alterations in small molecule concentrations and changes in motor and cognitive function, metabolites shown to change with sodium nitrite supplementation were tested for an association with a subset of functional outcomes that demonstrated the most consistent change following supplementation (noted by significant time × treatment interactions; RTD, rapid step errors, TMT-B, Table 6). In subjects receiving 80 mg/day sodium nitrite, increases in knee-extensor and knee-flexor RTD were significantly related to three metabolites each; two of these metabolites (C28 H44 O4 and C26 H58 N2 O2 S2) were related to the improvements in RTD for both muscle groups.
Table 6. Significant relations between specific metabolite concentrations and prioritized measures of physical and cognitive function following 10 weeks of sodium nitrite supplementation.
| Beta | Standard Error | P-value | |
|---|---|---|---|
| Motor Outcomes | |||
| Knee extensor rate of torque development | |||
|
Nitrite (80 mg/day) Molecule | |||
| C28 H44 O4 | −0.74 | 5.999 | 0.023 |
| C26 H58 N2 O2 S2 | 0.75 | 4.474 | 0.02 |
| C35 H62 N O15 P | −0.763 | 44.736 | 0.017 |
| Knee flexor rate of torque development | |||
|
Nitrite (80 mg/day) Molecule | |||
| C28 H44 O4 | −0.704 | 8.841 | 0.034 |
| C26 H58 N2 O2 S2 | 0.752 | 7.155 | 0.019 |
| LysoPE (18:3(9Z, 12Z, 15Z)/0:0) | 0.785 | 7.028 | 0.012 |
|
Nitrite (160 mg/day) Molecule | |||
| C22 H23 F N6 | 0.692 | 9.275 | 0.039 |
| PC(38:4) | −0.709 | 4.854 | 0.032 |
| Rapid Step Test | |||
|
Nitrite (80 mg/day) Molecule | |||
| β-Methylcrotonyl coenzyme A | 0.888 | 0.014 | 0.001 |
| Nitrite (160 mg/day) | |||
| C22 H23 F N6 | 0.647 | 0.042 | 0.043 |
| C15 H43 N15 O2 S4 | 0.824 | 0.043 | 0.003 |
| Cognitive Outcome | |||
| Trail Making Test B | |||
|
Nitrite (80 mg/day) Molecule | |||
| LysoPC(18:1) | −0.768 | 0.621 | 0.009 |
| 5-Aminoimidazole-4-carboxamide-1-βD-ribofuranosyl- 5′-monophosphate | −0.792 | 1.235 | 0.006 |
|
Nitrite (160 mg/day) Molecule | |||
| C37 H58 N3 P3 | 0.649 | 0.789 | 0.042 |
| C37 H59 N P4 | −0.779 | 0.496 | 0.008 |
Improvements in the number of errors during the rapid step test were significantly related to changes in one metabolite, β-Methylcrotonyl coenzyme A, in the 80 mg/day group. Changes in TMT-B were significantly associated with changes in two known metabolites, a lysophosphatidylcholine (LysoPC 18:1) and a ribonucleoside (5-Aminoimidazole-4-carboxamide-1-βD-ribofuranosyl-5′-monophosphate). Several meta-bolites significantly altered by 160 mg/day sodium nitrite were related to improvements in knee-flexor RTD, rapid step errors, and TMT-B (Table 6). Importantly, the diverse profile of metabolites that were significantly associated with improvements in motor and cognitive function indicates the broad metabolic effects of sodium nitrite supplementation.
Baseline metabolomic predictors of functional responsiveness to treatment
Baseline metabolite concentrations were assessed for their ability to predict an individual's responsiveness to sodium nitrite supplementation. Of the 3,063 metabolites measured at baseline, at least one and, in many cases, several metabolites within a molecular class, predicted a subject's responsiveness to sodium nitrite intervention within each functional test (Table 7).
Table 7. Metabolic pathways with metabolites predicting responsiveness to 10 weeks of sodium nitrite supplementation.
| Molecular Class | Number of Predictor Molecules by Pathway |
|---|---|
| Knee Flexor Rate of Torque Development | |
| Glycerophospholipids | 14 |
| Sphingolipids | 1 |
| Carboxylic Acids | 1 |
| Indoles and Derivatives | 1 |
| Fatty Acyls | 1 |
| Unknown | 26 |
| Knee Extensor Rate of Torque Development | |
| Glycerophospholipids | 2 |
| Sphingolipids | 1 |
| Carbohydrates and Carbohydrate Conjugates | 2 |
| Unknown | 26 |
| Rapid Step | |
| Glycerophospholipids | 2 |
| Carbonyl Compounds | 1 |
| Carboxylic Acids and Derivatives | 1 |
| Unknown | 11 |
| Trail Making Test B | |
| Glycerophospholipids | 2 |
| Glycerolipids | 1 |
| Unknown | 14 |
Improvements in knee-extensor RTD with sodium nitrite were significantly related to baseline concentrations of 31 molecules belonging to three known molecular classes (specific molecules or molecular weights are listed in Supplemental Table 3), whereas gains in knee-flexor RTD was significantly related to 44 metabolites populating five identified molecular classes (Supplemental Table 4). The molecular classes predicting an individual subject's RTD response to intervention included glycerol-phospholipid, sphingolipid, carboxylic acid, indole, fatty acyl and carbohydrate metabolism. Reduced occurrence of stepping errors in the rapid step test was related to small molecules within glycerophospholipid, carbonyl and carboxylic acid metabolite classes (Supplemental Table 5). Lastly, concentrations of 17 metabolites at baseline were significantly associated with individual improvements in cognitive function (TMT-B) in response to sodium nitrite (Supplemental Table 6).
DISCUSSION
The pilot study yielded three key findings. First, 10 weeks of sodium nitrite supplementation in healthy, highly functioning MA/O adults increased plasma nitrite levels and improved several measurements of motor and cognitive function compared with placebo control. Second, sodium nitrite altered the plasma metabolome, with 75 metabolites exceeding our specified threshold for meaningful change in the two dosage groups. Importantly, the changes in 13 of these metabolites were independently related to the improvements in motor and cognitive outcomes, and the abundance of 99 metabolites detected at baseline predicted responsiveness to sodium nitrite supplementa-tion. Finally, improvements in function and changes in the plasma metabolome generally were observed in both the low- and high-dose groups, indicating that the lower dose of sodium nitrite is sufficient to obtain physiological benefits. Overall, these findings in healthy MA/O men and women provide critical preliminary evidence for conducting a larger clinical trial of sodium nitrite supplementation on motor and cognitive function and the plasma metabolome in older adults and groups with existing functional impairments.
Efficacy: Motor and cognitive function
This pilot investigation demonstrates the efficacy of sodium nitrite supplementation for enhancing motor and cognitive functions in healthy MA/O adults with normal baseline function. These observations support a possible role for sodium nitrite supplementation in maintaining physiological function with aging in not only healthy MA/O adults, but also older and other groups with baseline functional impairments.
To our knowledge, the present results are the first to show that chronic supplementation with sodium nitrite can improve RTD in MA/O adults; a previous study reported improvements in young adults in response to acute supplementation of dietary nitrate [29]. Our novel observations in MA/O adults are potentially clinically important given that aging is associated with a progressive reduction in RTD [30, 31], which is related to adverse outcomes including loss of balance and falls [32]. Though no clinical cut points exist for RTD, age-related declines of 25-40% have been reported in healthy adults for knee flexion and extension RTD [31].
The present trial demonstrated 24-43% improvements in knee flexion and extension RTD in response to sodium nitrite supplementation, suggesting that much of the presumed decline in function with age was reversed [33, 34]. The age-related declines in RTD are largely dependent on reductions in the rate of muscle activation [35-37]. As such, sodium nitrite may have improved RTD via changes in neural mechanisms; this will be a key goal of future investigations.
The improvements observed in the TMT-B, an index of executive function, and the number of errors during the rapid step test, are consistent with a positive influence of sodium nitrite on neural/cognitive actions in MA/O adults. Performance on the TMT-B decreases with advancing age [38, 39] and in early stages of cognitive decline [40]. Although the average TMT-B time at baseline of 57.6 ± 17.3 s for our subjects already was ∼10% faster than the previously published age- and education-matched normative value of 64.6 ± 18.6 s [39], sodium nitrite supplementation nevertheless reduced the time to complete the task to those reported for healthy subjects 25-34 years of age [39]. The rapid step test also represents a challenging cognitive choice-reaction task [7, 41]. These findings are in agreement with a recent study demonstrating improvements in reaction time in type 2 diabetics following dietary nitrate supplementation [42], but contrasts with the results from a study of short-term dietary nitrate on computerized cognitive test performance [43]. Collectively, the present findings and those from these previous trials underscore the importance of duration of the supplementation period and test selection in future clinical trials. Given this clustering of positive neural/cognitive results with sodium nitrite supplementation, future clinical trials will need to expand the scope of cognitive functions assessed and use fMRI to understand potential neural mechanisms in vivo.
In addition to the potential neural/cognitive effects of sodium nitrite are the findings of improved cardiorespiratory exercise capacity in the 80 mg/day sodium nitrite group. The moderate improvement in treadmill time to exhaustion in this population is in agreement with our recent observation of marked improvements in endurance rota-rod running performance in old mice following 8 weeks of supplementation with sodium nitrite [21]. Improvements in endurance and exercise tolerance during large muscle activity have also been reported in studies in young adults following ingestion of sodium nitrate or beetroot juice, even in the absence of changes in VO2max, heart rate, ventilation and other physiological responses [16-18, 44, 45], although no effects have been reported in some studies [43]. We also found a strong trend for improvement in heel-rise time in the low dose group, suggesting enhanced muscle endurance. The 33 s improvement in heel-rise in the present study with low-dose supplementation appears highly significant given observations of heel-rise performance declining by ∼30 s with aging in healthy adults [46]. In contrast, 400-m walk time did not improve, though this is likely due to the fast baseline walk times in all subjects indicative of a ceiling effect. Overall, these findings support the idea of conducting a larger trial using the lower (80 mg/day) dose.
Though a detailed description of the potential mechanisms by which motor and cognitive functions were improved is beyond the scope of this pilot investigation, some speculation may be helpful for designing future trials. One possibility is that the cognitive/neural functional improvements were mediated, at least in part, by neuromuscular effects, as age-related motor dysfunction has a neurological, not only muscular, etiology [47-49]. Although 10 weeks of supplementation is unlikely to affect age-related loss of motor neurons, enhanced neural signaling and activation of muscle are possible via NO's role as a multifunctional messenger molecule and regulator of cerebrovascular function [50-53]. There also are strong associations between vascular and cognitive function with age [54], and sodium nitrite supplementation reverses age-related vascular endothelial dysfunction in old animals [55]. Further, reduced NO bioavailability and signaling has been implicated in disrupted neurovascular coupling in which cerebrovascular blood flow is no longer matched to regional metabolic demand, thereby destabilizing neurons, synapses and neurotransmission [53, 56]. Consistent with this idea, evidence of improved coupling of blood flow to visual stimuli with co-administration of nitrate and the carbonic anhydrase inhibitor acetazolamide has been shown in young men [57]. Lastly, in preclinical studies in mice, we have demonstrated that chronic low-grade inflammation, as indicated by elevated tissue expression of pro-inflammatory cytokines, is a major mechanism contributing to vascular and motor dysfunction with aging [58, 59], and that sodium nitrite supplementation reverses age-related increases in vascular and skeletal muscle inflammation in these models [55, 59]. Moreover, increases in pro-inflammatory cytokines are associated with motor dysfunction and disability in older adults [5, 60], as well as with reduced cognitive performance with aging [61, 62]. Thus, it is possible that reductions in inflammation played a role in improved cognitive and/or motor performance with sodium nitrite supplementation in the present study.
Clinical markers and metabolomics
In the present study, sodium nitrite administration markedly raised plasma nitrite concentrations acutely, but only modest increases in plasma nitrite were observed with chronic supplementation. This was expected given the short half-life of the compound [63]. Sodium nitrite treatment did not influence general subject characteristics including body weight, body composition, or clinical blood markers (fasting glucose, insulin or lipids). The latter suggests that improvements in function with sodium nitrite supplementation were not secondary to changes in these factors.
To gain initial insight into the molecular events related to improvements in motor and cognitive function with sodium nitrite treatment, we analyzed the composition and abundance levels of metabolites in the circulating plasma [64, 65]. This approach has been used previously, albeit in a limited manner, to assess the associations between changes in circulating metabolites and improvements in physical and/or metabolic function in response to interventions such as weight loss, bariatric surgery, and resistance exercise training [66-68].
The first goal of the present analysis was to determine if the intervention altered the plasma metabolome compared with placebo control. We found that sodium nitrite supplementation induced numerous changes in the plasma metabolome at each of the two doses. Changes in several of the metabolites were common to both doses, with additional metabolites modulated primarily by the low (80 mg/day) dose. The main molecular class changed by the two sodium nitrite doses was the glycerophospholipids. Previous research has shown that glycerophospholipid pathways are altered with advancing age [69], and that some of these pathways are associated with changes in the biological processes underlying aging, such as inflammation and oxidative stress. Our analysis demonstrated that sodium nitrite supplementation altered numerous subclasses of glycerophospholipids including lysophosphatidyl-cholines (LysoPCs), a group of pro-oxidant and inflammatory metabolites known to increase with advancing age and to be associated with declines in locomotor activity [70-73]. Both doses of sodium nitrite supplementation reduced the concentrations of LysoPCs and may partially explain the improvements in motor and cognitive outcomes. Other molecular classes influenced by both dose groups included sphingo-myelins, carboxylic acids and pyridines, which are implicated in inflammatory pathways, protein synthesis, and NAD+ metabolism, respectively [74-78].
A second goal of our analysis was to determine the relation, if any, between changes in individual metabolites or categories of metabolites with treatment and corresponding improvements in motor or cognitive function. The changes in the prioritized functional outcomes (knee flexor/knee extensor RTD, executive function and rapid step test errors) were significantly related to changes in selective metabolites, although the exact identity of approximately half of these molecules remain unknown and await future classification. For example, two unknown metabolites (C28 H44 O4 and C26 H58 N2 O2 S2) were significantly related to both knee flexor and extensor RTD in the 80 mg/day nitrite group, suggesting a possible link to the rate of muscle force production. Additionally, the relation of several glycerophospholipids and, more specifically, LysoPCs, with several functional outcomes indicate that some of the benefits associated with sodium nitrite supplementation may occur through changes in inflammation, as shown recently by our group in older mice [21]. Overall, these findings suggest that changes in small molecules related to lipid metabolism, inflammation and oxidative stress may reflect modifications in metabolic pathways that are involved in mediating improvements in physiological function with sodium nitrite supplementation. Further investigations into these associations may lead to the identification of novel pathways that can be targeted to improve physiological function in MA/O adults.
The final aim of the metabolomics analysis was to identify concentrations of metabolites at baseline that predict improvements in motor or cognitive function with treatment among subjects. Utilizing metabolomics to understand variability among individuals in the physiological responses to therapeutic interventions can provide novel insight into the differing mechanisms by which function is suppressed with aging, and to identify the most effective therapeutic strategies for individuals in the context of precision (personalized) medicine. Indeed, metabolomics has been used previously as such an approach in studies of cardiovascular disease [79], insulin sensitivity [67], depression [80] and cancer [79]. Applying similar techniques, we were able to identify metabolites from numerous molecular classes that showed an ability to explain the variation in responsiveness to sodium nitrite. Although combinations of specific molecular classes created unique predictive profiles for each functional measure, glycerophospholipids remained the most common class of molecules to predict responsiveness in the various functional domains. This observation is consistent with the results of our other analyses, indicating that glycerophospholipid metabolism may be a central target of sodium nitrite supplementation.
Limitations
There are limitations in this pilot trial that should be acknowledged. The population was largely Caucasian, although the Hispanic and African American subjects who underwent sodium nitrite treatment appeared to respond similarly to the Caucasian subjects. As emphasized earlier, our subjects were healthy MA/O adults. However, it is possible that healthy older adults and/or MA/O patients with more impaired function at baseline (e.g., those with frailty/functional limitations, physical disability or mild cognitive impairment) could demonstrate even more robust responses to sodium nitrite supplementation.
Comprehensive interrogation of the mechanisms mediating the beneficial effects of sodium nitrite on function was beyond the scope of this first pilot study in human subjects, but an extensive analysis of the plasma metabolomics was performed to provide initial insight. Several of the metabolites demonstrating change with supplementation, including some that were associated with improvements in functional outcomes, were of unknown origin. Although new metabolites are continually being identified, it is not possible at present to identify all metabolites detected in an untargeted analysis [81, 82]. Moreover, our metabolomics analysis did not directly measure metabolic flux using labeled isotope techniques. Nevertheless, we believe that the use of a randomized, placebo controlled study design, and the quality control measures in our analysis allowed us to show changes in small metabolites in response to sodium nitrite supplementation.
Conclusions
This pilot study provides the first evidence that chronic sodium nitrite supplementation improves selective measures of motor and cognitive function in healthy MA/O adults with good functional status at baseline. Importantly, we also show that sodium nitrite treatment changes numerous metabolic signatures of the plasma metabolome, that improvements in function are related to specific changes in small metabolites, and that a large number of metabolites at baseline predict functional responsiveness to this intervention. Overall, these results suggest that along with healthy lifestyle behaviors such as regular physical activity, sodium nitrite holds promise as a complementary pharmacological strategy for maintaining motor and cognitive function with aging, and provides an experimental basis for conducting a larger clinical trial of sodium nitrite supplementation in this and other MA/O populations.
METHODS
Subjects
A total of 86 MA/O (age 50-79 years) adults were recruited from the community using local newspaper and radio advertisements, university email advertisements and direct mailings. After comprehensive health screening at our Clinical Translational Research Center (CTRC) at the University of Colorado Boulder, 53 candidates were excluded from the study and the remaining 33 were enrolled. The enrolled subjects were all non-diabetic, had BMI <40 kg/m2, were cognitively intact as indicated by a Mini Mental Status Exam score >25 and were healthy and free of clinical diseases as assessed by medical history, physical examination, blood chemistry and resting and maximal exercise electrocardiogram (ECG) and blood pressure (BP) responses. Subjects were not taking cardiovascular acting drugs, drugs metabolized by the same liver enzymes as sodium nitrite, CNS depressants or drugs affecting neurological health. Women were postmenopausal for at least 1 year and had not taken hormone replacement therapy for at least the previous 6 months. The minority-ethnicity status of the subjects was 28 non-Hispanic white, 3 Hispanic white and 2 non-Hispanic black/African American.
All study procedures complied with the Declaration of Helsinki and the informed consent and study documents were approved by the Institutional Review Board of the University of Colorado Boulder and the Scientific Advisory and Research Committee of the University of Colorado Denver. The nature, benefits and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation.
Study procedures
Measurements were performed at the University of Colorado Boulder CTRC and Neurophysiology of Human Movement Laboratory. The CTRC measures of subject characteristics and the plasma metabolome were obtained following 12-h overnight fast and 24-h abstention from alcohol and exercise. Measures of motor and cognitive function were obtained 2 h after ingesting a small snack, and following 24-h abstention from alcohol.
Following screening and baseline measures, subjects were randomized to low-dose (40 mg capsules, 2x/day) or high-dose (80 mg capsules, 2x/day) sodium nitrite or placebo (0 mg capsules, 2x/day) for 10 weeks. Adherence and safety were assessed at days 1 and 2, and weeks 4, 8 and 10 via safety questionnaire, monitoring of methemoglobin and blood pressure. Detailed information regarding adherence, safety and tolerability are found elsewhere [25]. Following 10 weeks of treatment, baseline study procedures were repeated. During the 10-week intervention/control period, 1 subject dropped out of the high-dose sodium nitrite group and 1 dropped out of the placebo control group. One subject in the low-dose group was excluded after positive smoking status was revealed. Therefore 30 subjects completed the study: 10 in each of the 3 groups.
Subject characteristics, blood assays and dietary analysis
BMI (body mass index) was calculated from height and weight to the nearest 0.1 kg. Total body fat, total lean mass and regional lean mass were determined using dual energy X-ray absorptiometry (DEXA, GE/Lunar) [83]. Diet composition was estimated from 3-day food intake records (The Food Processor 8.2; ESHA Research) analyzed by a CTRC bionutritionist to assess and control for nitrate and nitrite in the diet. Fasting serum concentrations of glucose, insulin and total cholesterol were determined using standard assays performed by the University of Colorado Denver Anschutz Medical Campus CTRC core laboratory. Habitual physical activity was assessed at baseline from estimates of daily energy expenditure during leisure time and occupational activities, as previously described [84, 85], and estimated total kcals and total steps were measured objectively during 7-day (5 weekday, 2 weekend day) monitoring using Actigraph monitors (wGT3X-BT).
Acute and chronic levels of plasma nitrite were assessed by HPLC [86]. Acute levels were obtained 30 min following a single (initial) dose. Chronic levels were assessed at 10 weeks of sodium nitrite or placebo, 12-18 h after the most recent dose.
Motor and cognitive function
Motor and cognitive function was assessed during a single, ∼3-h session. Motor function was assessed by measures of muscle strength and rate of force development, balance, fatigability, mobility and dexterity, and two simple tests of cognition, Trail Making Tests part A and B, also were obtained, along with questionnaires related to fatigue. Subjects were allowed ample rest time between the tests of motor function to ensure safety and adequate recovery from the previous task.
Motor function outcomes
Rate of torque development and muscle strength
Rate of torque development (RTD) and maximal voluntary contractions (MVCs) were measured during fast or slow isometric contractions for the knee extensors and flexors of each subject as described in detail previously [87]. During the RTD task, subjects were instructed to perform 5-10 isometric contractions, with each contraction performed as quickly and forcefully as possible, but without holding the contraction at maximal torque; the peak torque per unit time (N·m/s) was recorded and calculated offline as the linear slope of the torque-time curve from 10-90% of the fast contractions. MVC was measured as peak torque (N·m) during 3-5 slow isometric contractions [87, 88]. Subjects also performed grip strength MVC with a standard dynamometer (hydraulic hand dynamometer, 300 lb capacity, Baseline Evaluation Instruments).
Cardiorespiratory fitness
Subjects performed incremental treadmill exercise using a modified Balke protocol as previously described [84] during a separate visit to the CU-Boulder CTRC. Oxygen consumption was measured using on-line, computer-assisted, open circuit spirometry during submaximal and maximal (VO2max) exercise workloads. Heart rate and ratings of perceived exertion (RPE) were measured throughout exercise and treadmill endurance was recorded as total exercise time to exhaustion as determined by subject's desire to stop despite strong verbal encouragement.
Fatigability and fatigue
Performance fatigability was assessed as the time to failure during a heel-rise test. The heel-rise test is a single-leg test in which subjects perform plantar flexion contractions at a rate of one maximal height heel-rise every 2 s until task failure [89]. The test was terminated when the subject voluntarily stopped due to an inability to continue or discomfort, used more than fingertips for balance during 2 consecutive heel raises, or decreased the required plantar flexion range of motion by more than 50%. Fatigue was assessed as a trait characteristic via two questionnaires: the Fatigue Severity Scale (FSS) and a brief Fatigue Questionnaire [90].
Balance
Balance was measured as the time and number of errors committed during a rapid step test [7]. Prior to the rapid step test, the maximal step length was determined and standardized across pre- and post-intervention conditions. Subjects completed three trials of rapid stepping to a random leg-direction (right or left leg: front, side, back) to taped targets placed on the floor corresponding to 80% of the maximal step length for each leg and direction as described previously [7]. An error was defined as failure to step beyond the target, loss of balance as indicated by the need for assistance to prevent a fall, failure to return to initial position, multiple steps, or noncompliance with a leg or direction command.
Mobility
Mobility was quantified with measures of endurance walk and Timed Up and Go. Walking endurance was assessed as the time to walk 400 m [27]. Subjects walked at a brisk pace for 2.5 laps around an indoor track as described previously [87, 91]. Mobility was also quantified by a Timed Up and Go task as the time to stand unassisted, walk to a target located 3 m from the chair, and return to a seated position [92].
Manual dexterity
Manual dexterity was measured as the time to complete the Grooved Pegboard test, a standardized test of manual dexterity that is known to decline in older compared with middle-aged and young adults [2]. Subjects were instructed to place the 25 keyhole-shaped pegs into the holes of a pegboard (Lafayette Instruments) as quickly as possible. All subjects completed three trials and the average and fastest times were recorded [2].
Cognitive outcomes
Trail Making Tests A and B (TMT-A and TMT-B) were administered according to guidelines [39, 93]. Participants were instructed to complete each part of the TMT as quickly and accurately as possible. When an error was made, the participant was instructed to return to the “circle” where the error originated and continue. Time to complete each part was recorded.
Metabolomics
EDTA-treated plasma was collected before and after 10 weeks of sodium nitrite or placebo treatment, frozen and stored at −80°C until analysis. Compounds were extracted from samples using an in-house developed technique that yields molecules from 4 major classes (lipid, protein, carbohydrate and nucleic acids) [94-96]. Aqueous and lipid fractions were analyzed using Liquid Chromatography Mass Spectrometry (LCMS, Agilent 6210 ESI-TOF) and data analyzed using commercial and in-house developed software [96-98]. Quality control included duplicate samples and analysis of a pooled plasma sample containing labeled or exogenous negative or positive spike-in controls, resulting in false discovery rates <3%. Spectral data was extracted and aligned based on mass and retention time using Profinder software (Agilent). Metabolites were annotated using IDBrowser in Mass Profiler Professional (MPP, Agilent) using a combination of available databases, including METLIN, Human Metabolome Database (HMDB), LipidMaps, and Kyoto Encyclopedia of Genes and Genomes (KEGG). Metabolites identified as being significantly altered by treatment were confirmed using MS/MS (Agilent 6520 Q-TOF) and matched to the NIST 14 MSMS spectral library, as shown previously [99]. Chemical formula generation was attempted for all molecules that remained unidentified in both LC-MS and LC-MS/MS analyses.
Statistics
General statistics
Data are presented as mean ± SEM (figures) or mean ± SD (tables and text). Statistical analysis was performed with SPSS software (v21, IBM). Prior to primary analysis of the motor function outcomes, normality of each variable was assessed with the Kolmogorov-Smirnov test [87]. No outliers (>3 SD) were identified for either physical or cognitive outcomes. Homogeneity of variance was assessed by Levene's test and did not differ among groups (p>0.05). Subject characteristics and outcomes of interest were compared at baseline via one-way ANOVA. Group differences in motor and cognitive functions with sodium nitrite treatments compared with placebo was determined by mixed-model ANOVA (within factor, time; between factor, treatment group), with contrast set to changes observed in placebo controls, and followed by post-hoc Tukey's HSD means-comparison. The α-level for significance was 0.05; however, as this was a pilot investigation being used to determine outcomes of interest for a larger parent trial, trends are also reported with an α-level of p < 0.1.
Metabolomics analysis
Metabolomics data were filtered for analysis using Mass Profiler Professional (Agilent). Small molecules not present in at least 50% of any one group before or after the intervention were excluded to reduce the probability of false discovery, resulting in 3,063 detected small molecules for subsequent analyses. Molecular concentrations were transformed to a log2 scale to assess their total magnitude of change. To detect metabolites significantly altered by treatment, small molecules were required to withstand a two-fold change filter from baseline and remain significant after a paired t-test (p<0.05), while not being significantly (p<0.05) altered in the placebo group. Based upon a database search, metabolites known to be attributed to dietary consumption of foods were excluded from analysis. The analysis resulted in 45 and 33 metabolites being significantly altered in the 80 mg and 160 mg groups, respectively. These molecules were then modeled independently using linear regression within each treatment group to assess their association with functional outcomes. Only metabolites significantly associated with functional measures are reported.
To assess whether baseline metabolomic signatures can predict responsiveness to sodium nitrite intervention, log2 concentrations of all 3,063 molecules detected at baseline were tested against responsiveness to nitrite intervention using independent linear regression models. Only metabolites that were significantly associated with responsiveness to the intervention and did not show an interaction with dosage are reported. Metabolites were grouped by class using the HMDB database to assess which pathways were most affected by sodium nitrite. A subject was determined as being responsive to intervention in each functional measure if they exhibited a ≥10% improvement from baseline.
SUPPLEMENTARY DATA FIGURES AND TABLES
Acknowledgments
The authors thank Dr. Roger Enoka, Mike Pont, Dr. Rick Reisdorph, Hannah J. Beck, Melissa Taylor and Evan Lian for their technical support. J.N.J. is currently at Internal Medicine – Section on Gerontology and Geriatrics at Wake Forest School of Medicine, Winston-Salem, NC (current affiliated email: jnjustic@wakehealth.edu). C.C-Q and N.R. are now affiliated with Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO.
Footnotes
SUPPLEMENTAL DATA
Please browse Full Text version of this manuscript to see Supplemental Figure and Tables.
Funding
This work was supported by NIH R37 AG013038 (DRS), T32 AG000279 (JNJ, LCJ, AED), R21 HL107105 (DRS, AED) and CTRC Grant UL1 RR025780.
Conflict of interest statement
Tony Giordano: President and CEO of TheraVasc, Inc. Nathan S. Bryan: Co-founder and CSO of Neogenis Labs, Inc. NS Bryan receives royalties from patents from the University of Texas Health Science Center in Houston.
REFERENCES
- 1.Reuben DB, Magasi S, McCreath HE, Bohannon RW, Wang YC, Bubela DJ, Rymer WZ, Beaumont J, Rine RM, Lai JS, Gershon RC. Motor assessment using the NIH Toolbox. Neurology. 2013;80:S65–75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43:560–567. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- 3.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 6.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 7.Cho BL, Scarpace D, Alexander NB. Tests of stepping as indicators of mobility, balance, and fall risk in balance-impaired older adults. J Am Geriatr Soc. 2004;52:1168–1173. doi: 10.1111/j.1532-5415.2004.52317.x. [DOI] [PubMed] [Google Scholar]
- 8.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2015 doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. The Milbank quarterly. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manton KG, Gu XL, Ullian A, Tolley HD, Headen AE, Jr., Lowrimore G. Long-term economic growth stimulus of human capital preservation in the elderly. Proc Natl Acad Sci U S A. 2009;106:21080–21085. doi: 10.1073/pnas.0911626106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth FW, Zwetsloot KA. Basic concepts about genes, inactivity and aging. Scand J Med Sci Sports. 2010;20:1–4. doi: 10.1111/j.1600-0838.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerterp KR, Meijer EP. Physical activity and parameters of aging: a physiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56:7–12. doi: 10.1093/gerona/56.suppl_2.7. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 14.Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. The international journal of behavioral nutrition and physical activity. 2010;7:38. doi: 10.1186/1479-5868-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. 2007;98(Suppl 2):S69–108. [PubMed] [Google Scholar]
- 16.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 17.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 18.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 19.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 20.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Justice JN, Gioscia-Ryan RA, Johnson LC, Battson ML, de Picciotto NE, Beck HJ, Jiang H, Sindler AL, Bryan NS, Enoka RM, Seals DR. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. J Appl Physiol (1985) 2015;118:163–169. doi: 10.1152/japplphysiol.00608.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaRoche DP, Greenleaf BL, Croce RV, McGaughy JA. Interaction of age, cognitive function, and gait performance in 50-80-year-olds. Age (Dordr) 2014;36:9693. doi: 10.1007/s11357-014-9693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale CR, Allerhand M, Sayer AA, Cooper C, Deary IJ. The dynamic relationship between cognitive function and walking speed: the English Longitudinal Study of Ageing. Age (Dordr) 2014;36:9682. doi: 10.1007/s11357-014-9682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. Indian J Med Res. 2011;133:471–478. [PMC free article] [PubMed] [Google Scholar]
- 25.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 27.Vestergaard S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation Res. 2009;12:177–184. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bischoff HA, Stahelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, Akos R, Conzelmann M, Dick W, Theiler R. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32:315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 29.Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DP, Ramamurthy S, Spearie CA, Waller S, Farmer M, Peterson LR. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2014 doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–267. doi: 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- 31.Thompson BJ, Ryan ED, Sobolewski EJ, Conchola EC, Cramer JT. Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp Gerontol. 2013;48:277–282. doi: 10.1016/j.exger.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech (Bristol, Avon) 2010;25:450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Bojsen-Møller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. Journal of Applied Physiology. 2005;99:986–994. doi: 10.1152/japplphysiol.01305.2004. [DOI] [PubMed] [Google Scholar]
- 34.Thompson BJ, Ryan ED, Sobolewski EJ. The influence of occupation and age on maximal and rapid lower extremity strength. Applied ergonomics. 2015;50:62–67. doi: 10.1016/j.apergo.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Ng AV, Kent-Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci. 1999;54:B452–458. doi: 10.1093/gerona/54.10.b452. [DOI] [PubMed] [Google Scholar]
- 36.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol (1985) 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 38.Amodio P, Wenin H, Del Piccolo F, Mapelli D, Montagnese S, Pellegrini A, Musto C, Gatta A, Umilta C. Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age-dependent decline and sociobiological variables. Aging Clin Exp Res. 2002;14:117–131. doi: 10.1007/BF03324425. [DOI] [PubMed] [Google Scholar]
- 39.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 40.Greenlief CL, Margolis RB, Erker GJ. Application of the Trail Making Test in differentiating neuropsychological impairment of elderly persons. Percept Mot Skills. 1985;61:1283–1289. doi: 10.2466/pms.1985.61.3f.1283. [DOI] [PubMed] [Google Scholar]
- 41.Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M429–433. doi: 10.1093/gerona/55.8.m429. [DOI] [PubMed] [Google Scholar]
- 42.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304:R73–83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 44.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44(Suppl 1):S35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Jan MH, Chai HM, Lin YF, Lin JC, Tsai LY, Ou YC, Lin DH. Effects of age and sex on the results of an ankle plantar-flexor manual muscle test. Phys Ther. 2005;85:1078–1084. [PubMed] [Google Scholar]
- 47.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 48.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 49.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 50.Chiueh CC. Neuroprotective properties of nitric oxide. Ann N Y Acad Sci. 1999;890:301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- 51.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 52.Contestabile A, Monti B, Polazzi E. Neuronal-glial Interactions Define the Role of Nitric Oxide in Neural Functional Processes. Curr Neuropharmacol. 2012;10:303–310. doi: 10.2174/157015912804143522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 54.Fernando MS, Ince PG Function MRCC and Ageing Neuropathology Study. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Celsis P, Agniel A, Cardebat D, Demonet JF, Ousset PJ, Puel M. Age related cognitive decline: a clinical entity? A longitudinal study of cerebral blood flow and memory performance. J Neurol Neurosurg Psychiatry. 1997;62:601–608. doi: 10.1136/jnnp.62.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aamand R, Ho YC, Dalsgaard T, Roepstorff A, Lund TE. Dietary nitrate facilitates an acetazolamide-induced increase in cerebral blood flow during visual stimulation. J Appl Physiol (1985) 2014;116:267–273. doi: 10.1152/japplphysiol.00797.2013. [DOI] [PubMed] [Google Scholar]
- 58.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor κB and Forkhead Box O Phosphorylation. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:409–18. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Justice JN, Gioscia-Ryan RA, Johnson LC, Battson ML, de Picciotto NE, Beck HJ, Jiang H, Sindler AL, Bryan NS, Enoka RM, Seals DR. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. Journal of applied physiology. 2014 doi: 10.1152/japplphysiol.00608.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blain H, Jaussent A, Beziat S, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Picot MC. Low serum IL-6 is associated with high 6-minute walking performance in asymptomatic women aged 20 to 70years. Exp Gerontol. 2012;47:143–148. doi: 10.1016/j.exger.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Teunissen C, Van Boxtel M, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch H. Inflammation markers in relation to cognition in a healthy aging population. Journal of neuroimmunology. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 62.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 63.Bryan NS. Nitrite in nitric oxide biology: Cause or consequence?: A systems-based review. Free Radical Biology and Medicine. 2006;41:691–701. doi: 10.1016/j.freeradbiomed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 64.Barderas MG, Laborde CM, Posada M, de la Cuesta F, Zubiri I, Vivanco F, Alvarez-Llamas G. Metabolomic profiling for identification of novel potential biomarkers in cardiovascular diseases. BioMed Research International. 2011;2011:790132. doi: 10.1155/2011/790132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boccard J, Veuthey JL, Rudaz S. Knowledge discovery in metabolomics: an overview of MS data handling. Journal of separation science. 2010;33:290–304. doi: 10.1002/jssc.200900609. [DOI] [PubMed] [Google Scholar]
- 66.Lustgarten MS, Price LL, Chalé A, Fielding RA. Metabolites related to gut bacterial metabolism, peroxisome proliferator-activated receptor-alpha activation, and insulin sensitivity are associated with physical function in functionally-limited older adults. Aging cell. 2014;13:918–925. doi: 10.1111/acel.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah S, Crosslin D, Haynes C, Nelson S, Turer C, Stevens R, Muehlbauer M, Wenner B, Bain J, Laferrere B. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Science translational medicine. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim S, Cheon H-S, Song J-C, Yun S-M, Park SI, Jeon J-P. Aging-related Changes in Mouse Serum Glycerophospholipid Profiles. Osong public health and research perspectives. 2014;5:345–350. doi: 10.1016/j.phrp.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee ES, Soliman KF, Charlton CG. Lysophosphatidylcholine decreases locomotor activities and dopamine turnover rate in rats. Neurotoxicology. 2005;26:27–38. doi: 10.1016/j.neuro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Lee SH, Park S, Kim H, Jung BH. Metabolomic approaches to the normal aging process. Metabolomics. 2014;10:1268–1292. [Google Scholar]
- 72.Zou Y, Kim DH, Jung KJ, Heo HS, Kim CH, Baik HS, Yu BP, Yokozawa T, Chung HY. Lysophosphatidylcholine enhances oxidative stress via the 5-lipoxygenase pathway in rat aorta during aging. Rejuvenation Res. 2009;12:15–24. doi: 10.1089/rej.2008.0807. [DOI] [PubMed] [Google Scholar]
- 73.Sevastou I, Kaffe E, Mouratis M-A, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA 2/LPC and ATX/LPA axes. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2013;1831:42–60. doi: 10.1016/j.bbalip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 74.Ahmad S, Shahab U, Baig MH, Khan MS, Khan MS, Srivastava AK, Saeed M, Moinuddin Inhibitory effect of metformin and pyridoxamine in the formation of early, intermediate and advanced glycation end-products. PLoS One. 2013;8:e72128. doi: 10.1371/journal.pone.0072128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, et al. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29:1404–1411. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol. 2010;299:C552–560. doi: 10.1152/ajpcell.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Krauss RM, Zhu H, Kaddurah-Daouk R. Pharmacometabolomics of statin response. Clinical Pharmacology & Therapeutics. 2013;94:562–565. doi: 10.1038/clpt.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, Drews M, Fiehn O, Zeng Z, Schaid D. Glycine and a Glycine Dehydrogenase (GLDC) SNP as Citalopram/Escitalopram Response Biomarkers in Depression: Pharmacometabolomics-Informed Pharmacogenomics. Clinical Pharmacology & Therapeutics. 2011:8997–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol (1985) 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 84.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 85.Justice JN, Pierpoint LA, Mani D, Schwartz R, Enoka RM. Motor function is associated with 1,25(OH)2D and indices of insulin-glucose dynamics in non-diabetic older adults. Aging-Clinical and Experimental Research. 2014;26:249–54. doi: 10.1007/s40520-013-0166-y. [DOI] [PubMed] [Google Scholar]
- 86.Jiang H, Parthasarathy D, Torregrossa AC, Mian A, Bryan NS. Analytical techniques for assaying nitric oxide bioactivity. J Vis Exp. 2012;64:e3722. doi: 10.3791/3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol. 2014;55:92–101. doi: 10.1016/j.exger.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudroff T, Justice JN, Matthews S, Zuo R, Enoka RM. Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2010;203:307–316. doi: 10.1007/s00221-010-2233-3. [DOI] [PubMed] [Google Scholar]
- 89.Lunsford BR, Perry J. The standing heel-rise test for ankle plantar flexion: criterion for normal. Phys Ther. 1995;75:694–698. doi: 10.1093/ptj/75.8.694. [DOI] [PubMed] [Google Scholar]
- 90.Vestergaard S, Nayfield SG, Patel KV, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik JM. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009:6476–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Justice JN, Pierpoint LA, Mani D, Schwartz RS, Enoka RM. Motor function is associated with 1,25(OH)(2)D and indices of insulin-glucose dynamics in non-diabetic older adults. Aging Clin Exp Res. 2014;26:249–254. doi: 10.1007/s40520-013-0166-y. [DOI] [PubMed] [Google Scholar]
- 92.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 93.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary. New York: Oxford University Press; 2006. [Google Scholar]
- 94.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz-Gutierrez V, Perez-Camino MC. Update on solid-phase extraction for the analysis of lipid classes and related compounds. J Chromatogr A. 2000;885:321–341. doi: 10.1016/s0021-9673(00)00181-3. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y, Cruickshank C, Armstrong M, Mahaffey S, Reisdorph R, Reisdorph N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J Chromatogr A. 2013;1300C:217–226. doi: 10.1016/j.chroma.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, Schnell C, Kedl R, Laflamme DJ, Reisdorph N, Kechris KJ, Bowler RP. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2012-0230OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reisdorph N, Mahaffey S, Bowler R, Petrache I, Reisdorph R, Armstrong M, Hughes G, Kechris K. Translational metabolomics and genomics for chronic obstructive pulmonary disease (COPD): Discovering a promising role for sphingolipids. American Thoracic Society and Metabolomics Society Meetings. 2012:A6831. [Google Scholar]
- 99.Cruickshank-Quinn CI, Mahaffey S, Justice MJ, Hughes G, Armstrong M, Bowler RP, Reisdorph R, Petrache I, Reisdorph N. Transient and Persistent Metabolomic Changes in Plasma following Chronic Cigarette Smoke Exposure in a Mouse Model. PLoS ONE. 2014;9:e101855. doi: 10.1371/journal.pone.0101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.