Abstract
Background:
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive procedure originally performed using a 22-gauge (22G) needle. A recently introduced 21-gauge (21G) needle may improve the diagnostic yield and sample adequacy of EBUS-TBNA, but prior smaller studies have shown conflicting results. To our knowledge, this is the largest study undertaken to date to determine whether the 21G needle adds diagnostic benefit.
Methods:
We retrospectively evaluated the results of 1,299 patients from the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation (AQuIRE) Diagnostic Registry who underwent EBUS-TBNA between February 2009 and September 2010 at six centers throughout the United States. Data collection included patient demographics, sample adequacy, and diagnostic yield. Analysis consisted of univariate and multivariate hierarchical logistic regression comparing diagnostic yield and sample adequacy of EBUS-TBNA specimens by needle gauge.
Results:
A total of 1,235 patients met inclusion criteria. Sample adequacy was obtained in 94.9% of the 22G needle group and in 94.6% of the 21G needle group (P = .81). A diagnosis was made in 51.4% of the 22G and 51.3% of the 21G groups (P = .98). Multivariate hierarchical logistic regression showed no statistical difference in sample adequacy or diagnostic yield between the two groups. The presence of rapid onsite cytologic evaluation was associated with significantly fewer needle passes per procedure when using the 21G needle (P < .001).
Conclusions:
There is no difference in specimen adequacy or diagnostic yield between the 21G and 22G needle groups. EBUS-TBNA in conjunction with rapid onsite cytologic evaluation and a 21G needle is associated with fewer needle passes compared with a 22G needle.
Lung cancer is the third most commonly diagnosed cancer and accounts for the greatest number of cancer-related deaths in the United States.1 The majority of patients with lung cancer present with advanced stage disease and will not benefit from surgical resection. It is imperative to obtain mediastinal lymphatic tissue sampling in order to stage the mediastinum to prevent patients with advanced disease from undergoing unnecessary surgery or to proceed with surgery in patients who could be cured. Several techniques are available to evaluate mediastinal and hilar lymph nodes, including mediastinoscopy, video-assisted thoracic surgery, transbronchial needle aspiration; esophageal ultrasound-guided fine needle aspiration (EUS-FNA), and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Not all of these procedures allow access to all lymph node stations, and each is associated with its own risk/benefit profile.2,3
EBUS-TBNA is a minimally invasive diagnostic procedure shown to be equivalent if not superior to mediastinoscopy in the evaluation of mediastinal and hilar lymph nodes.4,5 EBUS-TBNA was originally performed with a dedicated 22-gauge (22G) aspiration needle; however, a larger 21-gauge (21G) needle has been introduced. In a small investigation involving 33 patients, Nakajima et al6 studied the differences in the diagnostic yield and clinical utility of the 21G and 22G needles and found no difference in diagnostic yield. A study of 56 patients by Saji et al,7 however, found superiority of the 21G needle regarding cytologic and combined cytologic/histologic diagnostic yield. Finally, Oki et al,8 showed no difference in diagnostic sampling yield between 21G and 22G needles in a prospective trial involving 60 patients. These conflicting results have led to the present study, which aims to compare the utility of the 22G vs the 21G needle for sample adequacy and diagnostic yield using a significantly larger sample size than previous studies and with data obtained from multiple centers throughout the United States.
Materials and Methods
Between February 2009 and August 2010, 1,299 patients with hilar and mediastinal lymphadenopathy were referred for EBUS-TBNA. The study was approved by the Johns Hopkins University Institutional Review Board for all institutions participating in this study (NA_00049578). Data were prospectively compiled from six US academic health-care centers into the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation (AQuIRE) and retrospectively reviewed for this study. Participating institutions were Beth Israel Deaconess Medical Center (Boston, Massachusetts), Chicago Chest Center (Elk Grove Village, Illinois), Henry Ford Medical Center (Detroit, Michigan), Johns Hopkins University (Baltimore, Maryland), Kaiser Permanente (Portland, Oregon), and the University of Texas MD Anderson Cancer Center (Houston, Texas). All patients in the AQuIRE Registry who underwent sampling of hilar and mediastinal lymph nodes using EBUS-TBNA were included. Exclusion criteria was the use of more than one needle gauge during the procedure. All procedures were performed using Olympus EBUS bronchoscopes and EBUS-TBNA needles (Olympus America Inc). The needle gauge used during the procedure was selected at the discretion of the bronchoscopist, with all centers using both 21G and 22G needles. Rapid onsite cytologic evaluation (ROSE), when available at the institution, was also used at the discretion of the bronchoscopist.
Samples were considered adequate if cytologic analysis determined the presence of lymphocytes or a definitive diagnosis was established. Diagnostic yield was defined as a specific diagnosis made based on EBUS-TBNA samples (eg, malignancy, granuloma). Comorbid conditions included the presence of any of the following: COPD, congestive heart failure, history of stroke, coronary artery disease, diabetes mellitus, renal failure while on dialysis, renal failure while not on dialysis, and hematologic malignancy.
Demographics and differences in sample adequacy and diagnostic yield were summarized using mean ± SD for continuous variables and proportions for categorical variables. Unadjusted 21G vs 22G group comparisons were performed using χ2 test for categorical variables and Student t test for continuous variables. The primary outcome was diagnostic yield (previously defined) as analyzed on a per-patient and per-lymph node basis. The secondary outcome was sample adequacy (previously defined) as analyzed on a per-lymph node basis. Univariate analysis of diagnostic yield and sample adequacy was performed using logistic regression. It was decided a priori that all variables with P < .2 on univariate analysis would be included in multivariate hierarchical models. The hierarchical model for diagnostic yield on a per-patient basis was specified as patients nested within hospitals. Hierarchical modeling for diagnostic yield and sample adequacy on a per-lymph node basis was specified as lymph nodes nested in patients nested within hospitals. We defined statistical significance as a two-sided P < .05. All analyses were performed using STATA version 11.2 (StataCorp LP) statistical software.
Results
Patient Characteristics
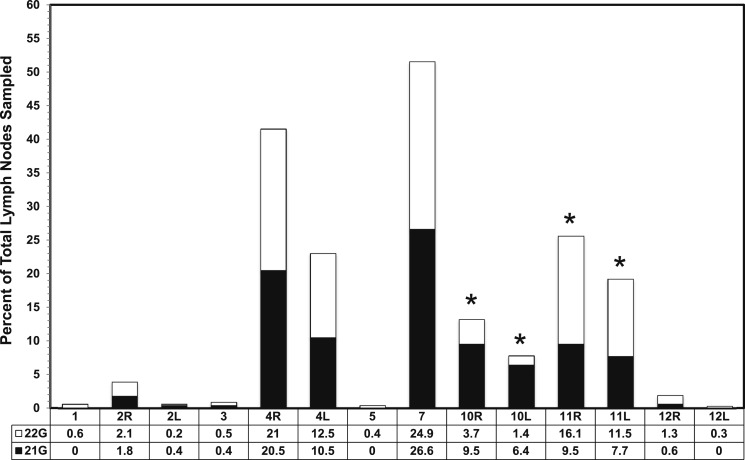
During the 19 months of data collection, 1,299 patients met the inclusion criteria. Of the 1,299, 64 procedures included the use of more than one TBNA needle and were excluded from the final analysis. Of the remaining 1,235 patients, 249 and 995 underwent biopsy with the 21G and 22G needles, respectively. Patient distribution and demographics are shown in Table 1. Mean age, sex, ethnicity, smoking status, and prevalence of comorbid conditions were not significantly different. Significantly more lymph nodes were sampled in the 22G group. The mean age of patients was 62.8 years. There were 591 (48%) women and 644 (52%) men, with 1,001 (81%) identifying as white and 234 (19%) identifying as nonwhite (black/African American, Asian, Hispanic/Latino, or Native Alaskan). A total of 2,768 lymph node sites were sampled, including 497 with a 21G needle and 2,271 with a 22G needle. There was no correlation of needle used and lymph node station sampled aside from the 22G needle being used more frequently at station 11 and the 21G needle being used more frequently at station 10 (Fig 1).
Table 1.
—Patient Characteristics
| Characteristic | 21 Gauge (n = 240) | 22 Gauge (n = 995) | Total (N = 1,235) | P Valuea |
| Age, y | 63 ± 15 | 63 ± 13 | 63 ± 13 | .85 |
| Female sex | 113 (47) | 478 (48) | 591 (48) | .79 |
| White race | 185 (77) | 816 (82) | 1,001 (81) | .08 |
| Current/prior smoker | 174 (73) | 736 (74) | 910 (74) | .64 |
| Presence of at least one comorbid conditionb | 107 (45) | 459 (46) | 566 (46) | .67 |
| Lymph nodes sampled per patient | 2.1 ± 0.9 | 2.3 ± 1.2 | 2.2 ± 1.2 | .01 |
Data are presented as mean ± SD or No. (%).
Calculated using χ tests for categorical variables and Student t tests for continuous variables.
Comorbities include COPD, congestive heart failure, history of stroke, coronary artery disease, diabetes mellitus, renal failure while on or off dialysis, and hematologic malignancy.
Figure 1.
Percentage of total lymph nodes sampled comparing 21G and 22G endobronchial ultrasound needles by lymph node station. *P < .05. 21G = 21 gauge; 22G = 22 gauge; L = left; R = right.
21G vs 22G EBUS-TBNA Needles
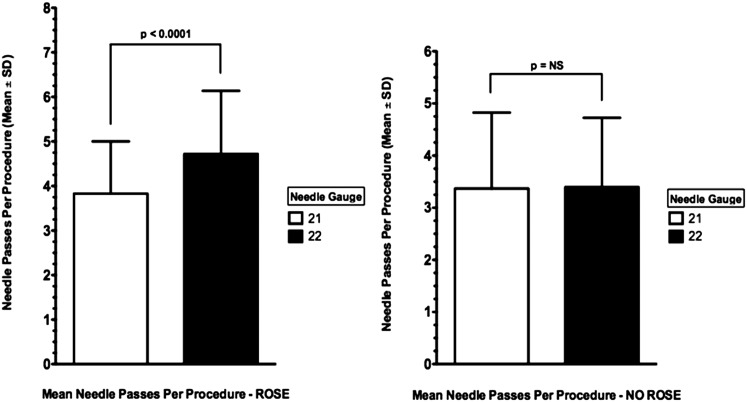
With ROSE, the mean number of needle passes per lymph node station sampled was greater in the 22G group than in the 21G group (4.6 vs 3.7, P < .001); however, this was not seen in the absence of ROSE (Fig 2, Table 2). Univariate analysis of EBUS-TBNA characteristics with diagnostic yield on a per-patient and per-lymph node basis and sample adequacy on a per-lymph node basis are shown in Tables 3–5, respectively. Sample adequacy and final diagnosis based on EBUS-TBNA findings were obtained in 1,172 (94.9%) and 634 (51.3%) patients, respectively. Multivariate hierarchical logistic regression was used to account for heterogeneity between hospital centers and patients. The multivariate hierarchical logistic regression models were adjusted for needle size, age, sex, prevalence of comorbid conditions, average number of needle passes per lymph node, number of stations sampled, ethnicity, CT scan short-axis lymph node size, anatomic site sampled, ROSE, and smoking history. Results of the multivariate hierarchical logistic regression analysis of diagnostic yield on a per-patient and per-lymph node basis and sample adequacy on a per-lymph node basis showed no significant difference between the two needles (Table 6).
Figure 2.
The effect of ROSE on the number of needle passes per lymph node sampled during endobronchial ultrasound-guided transbronchial needle aspiration compared by needle type. P significant at < .05. NS = not statistically significant; ROSE = rapid onsite cytologic evaluation.
Table 2.
—EBUS-TBNA Average Number of Needle Passes per Lymph Node by Use of ROSE and Needle Gauge
| Procedure | 21 Gauge (n = 497 lymph nodes) | 22 Gauge (n = 2,271 lymph nodes) | P Valuea |
| Total (N = 2,768) | 3.5 ± 1.2 | 4.2 ± 1.3 | < .01 |
| ROSE yes (n = 2,064) | 3.7 ± 1.1 | 4.6 ± 1.2 | < .01 |
| ROSE no (n = 704) | 3.2 ± 1.3 | 3.2 ± 1.1 | .69 |
Data are presented as mean ± SD. EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; ROSE = rapid onsite cytologic evaluation.
P value calculated using the Student t test.
Table 3.
—Univariate Analysis of EBUS-TBNA Characteristics by Diagnostic Yield per Patient Procedure
| EBUS-TBNA Characteristic | OR (95% CI) | P Value |
| Needle gauge (21 vs 22) | 1.1 (0.9-1.3) | .56 |
| Age (< 63 vs ≥ 63 y) | 1.1 (1.0-1.3) | .17 |
| Number of sites sampled | ||
| 1 (reference) | … | |
| 2 | 0.7 (0.5-0.9) | .002 |
| 3 | 0.7 (0.5-0.9) | .001 |
| 4 | 0.3 (0.2-0.4) | < .001 |
| ≥ 5 | 0.5 (0.4-0.7) | < .001 |
| Sex (male or female) | 1.3 (1.1-1.5) | .002 |
| Ethnicity (white or nonwhite) | 0.7 (0.6-0.9) | .002 |
| CT scan short-axis node size, cm | ||
| < 1 (reference) | … | |
| 1-2 | 1.5 (1.3-1.8) | < .001 |
| 2-3 | 3.1 (2.3-4.1) | < .001 |
| ≥ 3 | 3.7 (2.6-5.3) | < .001 |
| Lymph nodes sampled | ||
| Subcarinal (reference) | … | |
| Left hilar | 0.9 (0.7-1.2) | .39 |
| Right hilar | 0.8 (0.7-1.0) | .10 |
| Superior mediastinum/aortic (not including station 4) | 1.7 (1.3-2.3) | .001 |
| Low right paratracheal | 1.1 (0.9-1.4) | .26 |
| Low left paratracheal | 1.1 (0.9-1.4) | .45 |
| ROSE (no vs yes) | 1.0 (0.9-1.2) | .80 |
| Smoking status (ever vs never) | 1.3 (1.1-1.5) | .003 |
| Comorbid conditions present (no vs yes) | 0.6 (0.6-0.7) | < .001 |
See Table 2 legend for expansion of abbreviations.
Table 5.
—Univariate Analysis of EBUS-TBNA Characteristics by Sample Adequacy per Lymph Node Sampled
| EBUS-TBNA Characteristic | OR (95% CI) | P Value |
| Needle gauge (21 vs 22) | 0.9 (0.7-1.3) | .63 |
| Age (< 63 vs ≥ 63 y) | 1.0 (0.8-1.3) | .96 |
| Number of sites sampled | ||
| 1 (reference) | … | |
| 2 | 0.8 (0.5-1.3) | .39 |
| 3 | 0.7 (0.4-1.0) | .05 |
| 4 | 1.0 (0.6-1.6) | .96 |
| ≥ 5 | 1.5 (0.8-2.6) | .17 |
| Sex (female or male) | 1.0 (0.8-1.3) | .73 |
| Ethnicity (white or nonwhite) | 0.8 (0.6-1.1) | .15 |
| CT scan short-axis node size, cm | ||
| < 1 (reference) | … | |
| 1-2 | 1.5 (1.1-2.0) | .007 |
| 2-3 | 1.5 (0.9-2.5) | .08 |
| ≥ 3 | 0.8 (0.5-1.2) | .27 |
| Lymph nodes sampled | ||
| Subcarinal (reference) | … | |
| Left hilar | 0.5 (0.3-0.8) | .002 |
| Right hilar | 1.2 (0.8-1.8) | .48 |
| Superior mediastinum/aortic (not including station 4) | 0.4 (0.2-0.6) | < .001 |
| Low right paratracheal | 1.1 (0.7-1.7) | .70 |
| Low left paratracheal | 0.4 (0.3-0.6) | < .001 |
| ROSE (no vs yes) | 0.6 (0.5-0.9) | .01 |
| Smoking status (ever vs never) | 1.2 (0.9-1.6) | .20 |
| Comorbid conditions present (no vs yes) | 0.9 (0.7-1.2) | .67 |
| Average number of passes per node | 0.9 (0.9-1.0) | .19 |
See Table 2 legend for expansion of abbreviations.
Table 6.
—Multivariate Hierarchical Logistic Regression of EBUS-TBNA Diagnostic Yield and Sample Adequacy by Needle Gauge
| Variable | 21 Gauge (n = 240 Patients, n = 497 Lymph Nodes) | 22 Gauge (n = 995 Patients, n = 2,271 Lymph Nodes) | Adjusted OR (95% CI) | P Value |
| By patient | ||||
| Diagnostic yield | 123 (51) | 511 (51) | 1.2 (0.9-1.8) | .26a |
| By lymph node | ||||
| Diagnostic yield | 197 (40) | 746 (33) | 1.5 (0.7-3.1) | .27b |
| Sample adequacy | 446 (90) | 2,054 (90) | 1.4 (0.7-2.8) | .37c |
Data are presented as No. (%) unless otherwise indicated. See Table 2 legend for expansion of abbreviation.
Adjusted for needle size, age, sex, anatomic site sampled, number of sites sampled, ethnicity, CT scan short-axis lymph node diameter, smoking status, and comorbid conditions.
Adjusted for needle size, sex, anatomic site sampled, number of sites sampled, ROSE, CT scan short-axis lymph node diameter, comorbidity, and average number of needle passes.
Adjusted for needle size, anatomic site sampled, number of sites sampled, ethnicity, ROSE, smoking status, CT scan short-axis lymph node diameter, and average number of needle passes.
Table 4.
—Univariate Analysis of EBUS-TBNA Characteristics by Diagnostic Yield per Lymph Node Sampled
| EBUS-TBNA Characteristic | OR (95% CI) | P Value |
| Needle gauge (21 vs 22) | 1.3 (1.1-1.6) | .004 |
| Age (< 63 vs ≥ 63 y) | 0.9 (0.8-1.1) | .42 |
| Number of sites sampled | ||
| 1 (reference) | … | |
| 2 | 0.4 (0.3-0.5) | < .001 |
| 3 | 0.3 (0.2-0.4) | < .001 |
| 4 | 0.2 (0.1-0.2) | < .001 |
| ≥ 5 | 0.2 (0.1-0.2) | < .001 |
| Sex (female or male) | 1.2 (1.0-1.4) | .031 |
| Ethnicity (white or nonwhite) | 1 (0.8-1.2) | .90 |
| CT scan short-axis node size, cm | ||
| < 1 (reference) | … | |
| 1-2 | 3.3 (2.8-4.0) | < .001 |
| 2-3 | 7.6 (5.7-10.2) | < .001 |
| ≥ 3 | 9.3 (6.4-13.3) | < .001 |
| Lymph nodes sampled | ||
| Subcarinal (reference) | … | |
| Left hilar | 0.8 (0.6-1.0) | .05 |
| Right hilar | 0.8 (0.7-1.1) | .18 |
| Superior mediastinum/aortic (not including station 4) | 2.2 (1.6-3.0) | < .001 |
| Low right paratracheal | 1.2 (0.9-1.5) | .14 |
| Low left paratracheal | 0.8 (0.6-1.0) | .07 |
| ROSE (no vs yes) | 0.7 (0.5-0.8) | < .001 |
| Smoking status (ever vs never) | 1.1 (0.9-1.3) | .59 |
| Comorbid conditions present (no vs yes) | 0.7 (0.6-0.8) | < .001 |
| Average number of passes per node | 0.9 (0.9-1.0) | .07 |
See Table 2 legend for expansion of abbreviations.
Discussion
The present study, the largest to date to our knowledge, confirms no difference in specimen adequacy or diagnostic yield between the 21G and 22G EBUS-TBNA needles. This study was performed in response to previous smaller studies reporting conflicting results regarding differences in sample adequacy and diagnostic yield between the different-sized needles.6‐8 EBUS-TBNA is known to have a high diagnostic yield and few postprocedure complications and is credited with reducing the number of invasive surgical diagnostic procedures.5,9‐13
Studies of EUS-FNA (a procedure reported to have complementary diagnostic capabilities to EBUS-TBNA6) evaluating differences in diagnostic yield and sample adequacy have found no differences when comparing different needle gauges but suggested that use of a larger needle may improve histologic diagnostics and structural preservation.14,15 In addition, studies have suggested that a smaller needle gauge may hold some utility in EUS-FNA in that the smaller needle would be easier to handle and more maneuverable, result in fewer procedural complications, and cause less blood contamination of the specimen.6,15,16 Based on the present findings and compared with previous reports in the gastroenterology literature, it would appear that the experience documented in EUS-FNA is applicable to EBUS-TBNA with regard to differences in diagnostic yield and sample adequacy by needle size.
Saji et al7 suggested that the 21G needle may provide superior sample quality. The present analysis using ROSE is based on a larger patient sample, and we found that the greater number of needle passes per patient does not support this claim. We assessed the effect of ROSE on specimen adequacy and diagnostic yield through multivariate hierarchical regression and the number of needle passes per lymph node station. The results show no significant improvement in sample adequacy or diagnostic yield when adjusting for multiple covariates, including ROSE; however, a trend toward improved diagnostic yield and sample adequacy when using the 21G needle was noted. Of note, the internal diameters of the 21G and 22G EBUS-TBNA needles are equivalent to 20-gauge and 21G conventional TBNA needles, respectively, possibly accounting for the lack of difference seen between the two needle sizes. These findings appear to confirm the results of two previous prospective randomized trials investigating the effect of ROSE on conventional TBNA, but prospective trials on the effect of ROSE on EBUS-TBNA are needed.17,18 When comparing the effect of ROSE on the number of needle passes per nodal station for each needle individually, we found that the ROSE group had more passes per node than the non-ROSE group. This can potentially be explained in two ways: The nodal sampling ended after the standard practice of three needle passes in EBUS-TBNA19,20 when ROSE was not available, or when ROSE was available and a diagnosis was made at the bedside, sampling was continued in an effort to obtain adequate tissue for immunohistochemistry and molecular subtyping. Additionally, comparisons of the number of passes between needle gauges within the ROSE group showed that fewer passes were performed with the 21G needle, which seems to confirm the hypothesis that the 21G needle may be superior regarding the quantity of tissue obtained as well as the preservation of histologic structure. In a related study, Levy et al14 reported a difference in the number of needle passes between a 19-gauge and a 22G needle during EUS, favoring the larger needle size; however, this analysis was performed without ROSE. This study and the present findings suggest that the clinical significance of fewer needle passes both in total and when substratified to the presence of ROSE is that the 21G needle may afford a more adequate tissue sample in fewer passes, thus limiting the potential for trauma and adverse events from TBNA. In addition, it raises the question of whether the use of a larger needle than those evaluated in this study, such as a 19-gauge needle, would improve diagnostic yield, sample adequacy, and the histopathologic evaluation of EBUS-TBNA samples, specifically in the assessment of lymphoma.
When assessing lymph node station sampling, significant differences in frequencies of lymph node stations sampled were seen, with a higher percentage of station 10 nodes being sampled with the 21G needle in contrast to station 11, where the 22G needle was used more often. This was a surprising finding without clear cause. One possibility may be operator or bronchoscopist comfort with a smaller needle being deployed in a more distal airway. The only caveat to this is that station 10 lymph nodes are potentially more difficult to access using a 21G needle because of and increased acuity in angle of nodal access and decreased scope flexibility.
There are some limitations to this study. Although multivariate logistic regression methods were used to account for hospital and patient heterogeneity and to adjust for needle size, age, sex, prevalence of comorbid conditions, average number of needle passes per lymph node, number of stations sampled, ethnicity, CT scan short-axis lymph node size, anatomic site sampled, ROSE, and smoking history, it is possible that there were still some residual confounders that affected the results. Despite the large sample size and prospective data collection, the retrospective nature of the study is also a limitation. This is particularly true because there were no prespecified criteria for which needle gauge to use and likely reflects real-world clinical practice. In addition, positive diagnostic yield could not include true-negative nodal sampling because the AQuIRE Registry data collection ended at 30 days. Furthermore, the procedure was not performed on the same individuals using both needle gauges studied; therefore, even though patient characteristics were adjusted for, the results may be confounded.
Another limitation of the study is the lack of analysis of the differences in diagnostic yield and sample adequacy between benign and malignant disease when comparing needle sizes. This was due to a very small number of patients in the data set with benign disease, thus potentially skewing the results and invalidating that portion of the analysis. Finally, although the multicentric nature of this study is a recognized strength, it could be considered a potential weakness because bias may be introduced due to technical differences in lymph node sampling and specimen processing during EBUS-TBNA at the different institutions. The only way these limitations could be avoided would be with a randomized trial.
Conclusions
The results show no significant difference in conventional sample adequacy or diagnostic yield between 21G and 22G EBUS-TBNA needles. Future studies should examine whether there is a difference between the needle sizes in obtaining adequate tissue for ancillary tests, such as immunohistochemistry, molecular analysis, and gene typing.
Acknowledgments
Author contributions: Dr Yarmus had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Yarmus: contributed as the principal investigator for this study and to project oversight, organization, data collection, statistical analysis, and writing of the manuscript.
Dr Akulian: contributed to the project organization, statistical analysis, and writing of the manuscript.
Dr Lechtzin: contributed to the statistical analysis and writing of the manuscript.
Ms Yasin: contributed to the statistical analysis and writing of the manuscript.
Dr Kamdar: contributed to data collection, cleaning, statistical analysis, and editing the manuscript.
Dr Ernst: contributed to project oversight, data collection, and writing of the manuscript.
Dr Ost: contributed to the registry design and organization, data collection and auditing, statistical analyses, and writing of the manuscript.
Dr Ray: contributed to the data collection and writing of the manuscript.
Dr Greenhill: contributed to the data collection and writing of the manuscript.
Dr Jimenez: contributed to the data collection, study design, and writing of the manuscript.
Dr Filner: contributed to the data collection and writing of the manuscript.
Dr Feller-Kopman: contributed to the data collection, study design, and writing of the manuscript.
Other contributions: The data used for this article was provided through the ACCP AQuIRE Registry. We thank staff members Joyce Bruno, Priyal Patel, Jeff Maitland, and Danielle Jungst. Rodolfo C. Morice, MD, FCCP, and Archan M. Shah, DO, FCCP, contributed to the data collection, study design, and writing of the manuscript. Gaetane Michaud, MD, contributed to the data collection and writing of the manuscript. David C. Rice, MD, contributed to the writing of the manuscript. Biren Kamdar, MD, MHS, contributed to the statistical analysis and writing of the manuscript. The AQuIRE Registry participants in this study (listed alphabetically by center) were Beth Israel Deaconess Medical Center, Boston, Massachusetts (Adnan Majid, MD, and Gaetane Michaud, MD); Chicago Chest Center, Elk Grove Village, Illinois (Sarah R. Greenhill, MD, and Kevin L. Kovitz, MD, FCCP [principal investigator (PI)]); Cleveland Clinic, Cleveland, Ohio (Thomas R. Gildea, MD [PI], and Michael S. Machuzak, MD); Henry Ford Hospital, Detroit, Michigan (Javier Diaz, MD; Cynthia Ray, MD; and Michael J. Simoff, MD, FCCP [PI]); The Johns Hopkins Hospital, Baltimore, Maryland (Jason Akulian, MD; David Feller-Kopman, MD, FCCP [PI]; Biren Kamdar, MD, MHS; and Lonny B. Yarmus, DO, FCCP); Kaiser Permanente, Portland, Oregon (Joshua Filner, MD, FCCP [PI]); St Elizabeth’s Medical Center, Brighton, Massachusetts (Armin Ernst, MD, FCCP); University of Texas MD Anderson Cancer Center, Houston, Texas (George A. Eapen, MD, FCCP; Carlos A. Jimenez, MD, FCCP; Rodolfo C. Morice, MD, FCCP; and David E. Ost, MD, FCCP [PI]).
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Ernst is a consultant for Olympus America Inc. Dr Jimenez is an investigator for the Intrapleural Catheter Daily Versus Three Times a Week Drainage study (NCT00761618) funded by CareFusion Corporation. Drs Yarmus, Akulian, Lechtzin, Kamdar, Ost, Ray, Greenhill, Filner, and Feller-Kopman and Ms Yasin have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: Although the American College of Chest Physicians has reviewed and approved the proposal for this project, the researchers are solely responsible for the analysis and any conclusions drawn from the data as presented in this article.
Abbreviations
- 21G
21 gauge
- 22G
22 gauge
- AQuIRE
American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation
- EBUS-TBNA
endobronchial ultrasound-guided transbronchial needle aspiration
- EUS-FNA
esophageal ultrasound-guided fine needle aspiration
- ROSE
rapid onsite cytologic evaluation
Footnotes
Funding/Support: The American College of Chest Physicians (ACCP) funded database construction for the AQuIRE program.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.National Program of Cancer Registries. United States cancer statistics: 2008 incidence and mortality data. Department of Health and Human Services website. http://www.cdc.gov/uscs. Accessed October 25, 2012.
- 2.De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32(1):1-8. [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA; American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):202S-220S. [DOI] [PubMed]
- 4.Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142(6):1393-1400. [DOI] [PubMed]
- 5.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3(6):577-582. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology. 2011;16(1):90-94. [DOI] [PubMed] [Google Scholar]
- 7.Saji J, Kurimoto N, Morita K, et al. Comparison of 21-gauge and 22-gauge needles for endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. J Bronchol Intervent Pulmonology.. 2011;18(3):239-246. [DOI] [PubMed] [Google Scholar]
- 8.Oki M, Saka H, Kitagawa C, et al. Randomized study of 21-gauge versus 22-gauge endobronchial ultrasound-guided transbronchial needle aspiration needles for sampling histology specimens. J Bronchol Intervent Pulmonology. 2011;18(4):306-310. [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61(9):795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cetinkaya E, Seyhan EC, Ozgul A, et al. Efficacy of convex probe endobronchial ultrasound (CP-EBUS) assisted transbronchial needle aspiration for mediastinal staging in non-small cell lung cancer cases with mediastinal lymphadenopathy. Ann Thorac Cardiovasc Surg. 2011;17(3):236-242. [DOI] [PubMed] [Google Scholar]
- 11.Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164. [DOI] [PubMed] [Google Scholar]
- 12.Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50(3):347-354. [DOI] [PubMed] [Google Scholar]
- 13.Yasufuku K, Nakajima T, Fujiwara T, et al. Role of endobronchial ultrasound-guided transbronchial needle aspiration in the management of lung cancer. Gen Thorac Cardiovasc Surg. 2008;56(6):268-276. [DOI] [PubMed] [Google Scholar]
- 14.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57(1):101-106. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24(3):384-390. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41(5):445-448. [DOI] [PubMed] [Google Scholar]
- 17.Trisolini R, Cancellieri A, Tinelli C, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest. 2011;139(2):395-401. [DOI] [PubMed] [Google Scholar]
- 18.Yarmus L, Van der Kloot T, Lechtzin N, Napier M, Dressel D, Feller-Kopman D. A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens. J Bronchol Intervent Pulmonol. 2011;18(2):121-127. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station?. Chest. 2008;134(2):368-374. [DOI] [PubMed] [Google Scholar]
- 20.Diacon AH, Schuurmans MM, Theron J, et al. Transbronchial needle aspirates: how many passes per target site?. Eur Respir J. 2007;29(1):112-116. [DOI] [PubMed] [Google Scholar]