Abstract
Background:
Long-term complications of therapeutic bronchoscopy include infections and airway restenosis due to tumor. No studies have compared the incidence rates of infection in patients with stents with those without stents. We hypothesized that patients with stents would have a higher incidence of lower respiratory tract infections than would patients without stents.
Methods:
We conducted a retrospective cohort study, covering the period September 2009 to August 2011, of patients who had therapeutic bronchoscopy for malignant airways disease. Outcomes recorded were lower respiratory tract infection and airway restenosis by tumor.
Results:
Seventy-two patients had therapeutic bronchoscopy for malignant airways disease. Twenty-four of these patients had one or more stents placed. Twenty-three of the 72 patients (32%) developed lower respiratory tract infections. Stents were associated with an increased risk of infection (hazard ratio [HR], 3.76; 95% CI, 1.57-8.99; P = .003). The incidence rate of lower respiratory tract infection was 0.0057 infections per person-day in patients with stents vs 0.0011 infections per person-day in patients without stents. The incidence rate difference, 0.0046 infections per person-day, was significant (95% CI, 0.0012-0.0081; P = .0002). Restenosis due to tumor overgrowth was associated with more severe obstruction at baseline (obstruction ≥ 50% vs < 50% preprocedure; HR, 13.71; 95% CI, 1.75-107.55; P = .013).
Conclusion:
Therapeutic bronchoscopy with stent placement is associated with a higher risk of infection than is therapeutic bronchoscopy alone. If ablative techniques reopen the airway and there is a good chance that the tumor may respond to chemotherapy and/or radiation, a strategy of initially holding off on stenting may be warranted.
Central airway obstruction in patients with cancer can be due to intraluminal disease, extrinsic compression by tumor, or a combination of both.1 Ablative techniques that destroy tissue (eg, laser therapy, electrocautery, and mechanical coring with a rigid bronchoscope) are warranted to deal with intraluminal disease. Stents are warranted when there is extrinsic compression or when a barrier against tumor overgrowth is needed. A multimodal approach is often required because mixed patterns of disease are common.
Deciding whether to place a stent in a given case can be difficult. When there is severe, purely extrinsic compression, stents are clearly warranted; however, stents are used more often because there is a mixed pattern of disease or because of their barrier effect. Physicians must balance the potential benefit of stents against the potential risks, which include lower respiratory tract infection, granulation tissue formation, stent migration, and stent fracture.2,3
Previous studies have demonstrated that stent-associated lower respiratory tract infections are common, occurring in approximately 19% of patients, and are associated with a high case fatality rate.3‐9 However, respiratory tract infections are common in patients with malignancy and are probably even more common in those with airway obstruction, many of whom receive chemotherapy and/or radiation therapy. So it may be that the infection rate observed is due not purely to the stents but also to the underlying disease. Whether there is any incremental risk associated with stent placement in this population is important to know because it is the incremental risk of lower respiratory tract infection that must be balanced against the potential benefits of stent placement. Unfortunately, to our knowledge, no randomized controlled studies have compared the incidence rates of complications in patients with and without airway stents. Therefore, the aim of this study was to test our hypothesis that patients with airway stents would have a higher incidence rate of infection than would those without stents.
Materials and Methods
Study Design
We performed a retrospective cohort study of all patients aged 18 years or older who underwent therapeutic bronchoscopy for malignant airways disease at The University of Texas MD Anderson Cancer Center from September 2009 to August 2011. The protocol (DR09-0101) was approved by the institutional review board, committee 4, and a waiver of the requirement for informed consent was given. Patients with malignancy but without malignant central airway obstruction were excluded.
All procedural data had been collected prospectively as part of an ongoing interventional bronchoscopy registry. For patients with malignant central airway obstruction, stents were placed if (1) there was pure extrinsic compression with > 50% airway occlusion, or (2) if adequate airway patency (> 50%) could not be achieved with ablative techniques alone, or (3) it was felt that airway reocclusion would occur quickly if a stent was not placed following ablation for a mixed obstruction. Follow-up of patients was performed by midlevel providers using a standardized algorithm. For the current analysis, we abstracted data from the medical records using a standardized form. All definitions were developed prior to data abstraction and entered into a code book to ensure consistency. We abstracted data on age, sex, smoking status, pulmonary comorbidities, cancer diagnosis, radiation therapy, chemotherapy, degree and type of airway obstruction, procedure indication(s), complications, treatments for complications, and consequences of complications including hospitalizations, respiratory failure, and subsequent airway interventions.
Definitions
The primary outcome for this study was the time to lower respiratory tract infection. We classified respiratory infections using a method similar to those of previously published studies.3‐8 We defined a lower respiratory tract infection as present on the basis of clinical findings of fever, purulent sputum, and worsening cough, with or without radiographic evidence of pneumonia. We also required that there be documentation by the managing physician of a clinical diagnosis of lower respiratory tract infection and that antibiotics be prescribed for it. Bronchoscopic evidence of infection was not mandated. Infections were classified as pneumonia if there was evidence of new consolidation on chest radiography or CT scan. If no imaging was performed or if no new infiltrates were evident on the chest image, the infection was classified as acute bronchitis.
Secondary outcomes included time to restenosis caused by tumor overgrowth and death. Tumor overgrowth was determined by either bronchoscopy or CT scan. The severity of obstruction was graded on the basis of a combination of bronchoscopic and CT scan findings.
Other adverse events recorded were granulation tissue causing stent obstruction, mucus impaction requiring therapeutic intervention, stent migration, and stent fracture. All adverse events required bronchoscopic verification. Stents were also considered to have migrated if they were coughed out by the patient. Mucus impaction had to be severe enough to require therapeutic bronchoscopic intervention.
Statistics
Times to infection and tumor overgrowth were measured from the date of the first therapeutic bronchoscopy to the date of the event. Patients who did not experience the relevant event were censored at last follow-up. Univariate Cox proportional hazards models were fit to determine the association between characteristics and time-to-event outcomes. Extended Cox models were used to assess time-varying covariates, such as whether a stent was in place or whether restenosis occurred; these covariates were defined as 0 (absent) but were changed to 1 (present) when the events occurred. Variables significantly associated with events at the 0.2 level in univariate analyses were considered candidate variables and were entered into a multivariate extended Cox model. Backward selection was then used to retain only those variables that had a level of significance < .05. Kaplan-Meier curves were plotted for significant covariates. P values < .05 were considered statistically significant, and all tests were two sided. Statistical analysis was performed using STATA/IC version 12.1 (StataCorp LP).
Results
Patient and Procedural Characteristics
Seventy-two patients with malignant central airway disease were included in the study. Of these, 24 had a stent placed. Seventeen of the 24 patients with stents (71%) had ablative therapies concurrent with stent placement. Patient demographics, clinical characteristics, and operative findings are summarized in Table 1. Fifty percent of the 72 patients had one or more adverse long-term outcomes or procedural complications (Table 2).
Table 1.
—Patients and Procedural Characteristics
| Characteristics | Patients |
| Age, mean (SD), y | 59.4 (13.7) |
| Sex | |
| Female | 27 (38) |
| Male | 45 (63) |
| Race | |
| White | 51(71) |
| Black | 12 (17) |
| Hispanic | 6 (8) |
| Asian | 3 (4) |
| Malignancy type | |
| Non-small cell lung cancer | 32 (44) |
| Renal cell | 10 (14) |
| Sarcoma | 6 (8) |
| Colon | 4 (6) |
| Thyroid | 4 (6) |
| Melanoma | 3 (4) |
| Breast | 2 (3) |
| Head and neck | 1 (1) |
| Small cell | 1 (1) |
| Lymphoma | 1 (1) |
| Other solid tumor metastatic to the lung | 8 (11) |
| Smoking status | |
| Active smoker | 5 (7) |
| Ex-smoker | 44 (61) |
| Never smoker | 23 (32) |
| COPD | |
| Yes | 11 (15) |
| No | 61 (85) |
| Intraoperative findings at baseline | |
| Combined extrinsic and endoluminal tumor | 36 (50) |
| Endoluminal tumor | 28 (39) |
| Extrinsic compression only | 3 (4) |
| Other | 5 (7) |
| Indication was for hemoptysisa | |
| Yes | 26 (36) |
| No | 46 (64) |
| Indication was obstruction ≥ 50% preprocedurea | |
| Yes | 44 (61) |
| No | 28 (39) |
| Indication was obstruction < 50% preprocedurea | |
| Yes | 13 (18) |
| No | 59 (82) |
| Stent was placed in this patient | |
| Yes | 24 (33.3) |
| No | 48 (66.6) |
| Type of stent placedb (n = 29) | |
| Ultraflex (Boston Scientific) | 15 (52) |
| Aero (Merit Medical Systems, Inc) | 9 (31) |
| Dumon tube stent (Novatech) | 1 (3) |
| Silicone Y-stent (Novatech) | 3 (10) |
| Polyflex | 1 (3) |
| Degree of postprocedure airway obstruction | |
| 0%-49% | 66 (92) |
| 50%-100% | 6 (8) |
| Patients had more than one interventional procedure | |
| Yes | 21 (30) |
| No | 51 (70) |
| Preprocedure chemotherapy | |
| Yes | 50 (69) |
| No | 22 (31) |
| Preprocedure radiation therapy | |
| Yes | 38 (53) |
| No | 34 (47) |
| Postprocedure chemotherapy | |
| Yes | 43 (60) |
| No | 29 (40) |
| Postprocedure radiation therapy | |
| Yes | 29 (40) |
| No | 43 (60) |
Data are presented as No. (%) unless indicated otherwise.
Patients could have more than one indication for intervention.
Patients could have more than one interventional procedure and/or more than one stent placed during a single procedure.
Table 2.
—Long-term Therapeutic Bronchoscopy Outcomes
| Outcome | Occurrences (n = 72) |
| One or more adverse outcomes | |
| Yes | 36 (50) |
| No | 36 (50) |
| Lower respiratory tract infection | |
| Yes | 23 (32) |
| No | 49 (68) |
| Granulation tissue | |
| Yes | 3 (4) |
| No | 69 (96) |
| Restenosis due to tumor overgrowth | |
| Yes | 15 (21) |
| No | 57 (79) |
| Mucous impaction | |
| Yes | 5 (7) |
| No | 67 (93) |
| Stent migration | |
| Yes | 1 (1) |
| No | 71 (99) |
| Stent fracture | |
| Yes | 3 (4) |
| No | 69 (96) |
| Death | |
| Yes | 32 (44) |
| No | 40 (56) |
Data are presented as No. (%). Patients could have more than one outcome.
No differences in age, sex, comorbid conditions, performance status, chemotherapy, or radiation therapy were found between patients with and without stents. Stenting was more common in patients who were current or former smokers than in those who had never smoked (45% vs 9%; P = .003). There was a trend toward more frequent stenting at the time of first intervention in patients with obstruction ≥ 50% before the procedure than in patients with preprocedure obstruction < 50% (34% vs 14%; P = .10). Postprocedural luminal patency was not significantly different when all patients were compared (100% of patients with stents had < 50% airway obstruction after the procedure vs 89% of patients with no stent, P = .33). However, among the 44 patients who had preprocedure obstruction ≥ 50%, postprocedure airway obstruction had fallen to < 50% among more of those who received stents (100% of patients) than those who did not (79% of patients), although the difference did not reach statistical significance (P = .08).
Infection
A total of 23 patients (32%) developed lower respiratory tract infections. The median time to infection was 64 days (range, 7-632 days). Lower respiratory tract infections led to significant morbidity and mortality, with 13 patients (56%) requiring hospital admission and six (26%) dying within 14 days of infection (Table 3). Five patients developed a second respiratory tract infection and one patient had a third respiratory tract infection. A specific microbiologic cause was identified in 14 of the cases. The most common cause was Pseudomonas aeruginosa, which was isolated in 43% of patients in whom a specific microbiologic agent could be identified.
Table 3.
—Clinical Data Related to Lower Respiratory Tract Infections
| Characteristic | Infections (n = 23) |
| Infection type | |
| Acute bronchitis | 5 (22) |
| Pneumonia, not in obstruction/stent area | 2 (9) |
| Pneumonia, distal to obstruction/stent area | 10 (43) |
| Pneumonia, multilobar | 6 (26) |
| Stent removal required | |
| Yes | 5 (22) |
| No | 18 (78) |
| Care site | |
| Outpatient | 10 (44) |
| Admitted to hospital | 13 (56) |
| Respiratory support | |
| Yes | 2 (9) |
| No | 21 (91) |
| Death within 14 d of infection | |
| Yes | 6 (26) |
| No | 17 (74) |
| Microbiologya | |
| Pseudomonas aeruginosa | 6 |
| Actinomyces | 2 |
| α-Hemolytic strep | 1 |
| Aspergillus | 1 |
| Legionella species; not pneumophilia | 1 |
| Pseudomonas putida | 1 |
| Staphylococcus aureus, methicillin sensitive | 1 |
| Cytomegalovirus | 1 |
| Klebsiella pneumoniae | 1 |
| Sphingomona (Pseudomonas) paucimobilis | 1 |
| β lactamase negative Haemophilus species | 1 |
Data are presented as No. (%).
Some patients had more than one pathogen. Microbiology includes all lower respiratory tract infections, not just first episodes.
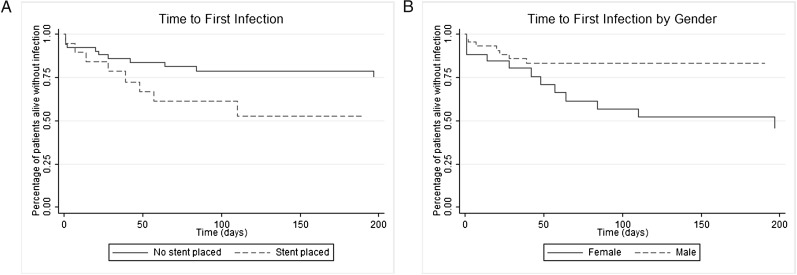
On univariate analysis, stent placement, preprocedure obstruction ≥ 50%, and male sex were associated with the development of lower respiratory tract infection (Table 4). There was a trend suggesting a possible association between poor performance status (Eastern Cooperative Oncology Group performance status score ≥ 2) and preprocedure chemotherapy with infection risk, but this failed to achieve statistical significance in the multivariate model. In multivariate analysis, only stent placement (vs no stent) (hazard ratio [HR], 3.76; 95% CI, 1.57-8.99; P = .003) and male sex (vs female) (HR = 0.40; 95% CI, 0.17-0.94; P = .035) remained associated with lower respiratory tract infection (Fig 1). The incidence rate of lower respiratory tract infection in patients with stents was 0.0057 infections per person-day. In patients without stents, the incidence rate of lower respiratory tract infection was 0.0011 events per person-day. The incidence rate risk difference, 0.0046 infections per person-day, was significant (95% CI, 0.0012-0.0081; P = .0002). This incidence rate risk difference corresponds to an additional 13% of patients developing a respiratory infection per month, with each respiratory infection carrying a 26% case fatality rate.
Table 4.
—Univariate Cox and Extended Cox Models for Time to Infection
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Age (continuous) | 0.99 | 0.97-1.02 | .750 |
| Sex: male vs female | 0.43 | 0.18-0.99 | .047 |
| ECOG performance status score ≥ 2 vs < 2 | 2.38 | 1.00-5.67 | .051 |
| Active/ex-smoker vs never smoked | 1.19 | 0.49-2.89 | .709 |
| History of COPD | 2.12 | 0.77-5.84 | .145 |
| History of CHF | 2.63 | 0.61-11.39 | .196 |
| Lung cancer vs other type of cancer | 1.10 | 0.47-2.56 | .824 |
| Preprocedure chemotherapy: yes vs no | 2.60 | 0.88-7.67 | .084 |
| Postprocedure chemotherapy: yes vs no | 1.27 | 0.49-3.32 | .620 |
| Preprocedure radiation therapy: yes vs no | 1.51 | 0.64-3.59 | .347 |
| Postprocedure radiation therapy: yes vs no | 1.43 | 0.63-3.25 | .392 |
| Obstruction ≥ 50% preprocedure | 3.27 | 1.20-8.92 | .02 |
| Obstruction immediately postprocedure ≥ 50% | 1.68 | 0.39-7.29 | .49 |
| Restenosis by tumor present (time varying) | 2.07 | 0.69-6.22 | .197 |
| Stent present (time varying) | 3.54 | 1.51-8.31 | .004 |
CHF = congestive heart failure; ECOG = Eastern Cooperative Oncology Group.
Figure 1.
Kaplan-Meier plots of time to first infection. A, Stent vs no stent. Patients with stents (dashed line) had a significantly shorter time to infection than did patients without stents (solid line). B, Male vs female patients. Female patients (solid line) had a significantly shorter time to infection than did male patients (dashed line).
Restenosis Due to Tumor Overgrowth
Fifteen patients (21%) developed restenosis due to tumor overgrowth. The median time to restenosis by tumor overgrowth was 108 days (range, 34-544 days). Tumor overgrowth was clinically significant, requiring repeat bronchoscopic intervention in 87% of cases (Table 5).
Table 5.
—Clinical Data Related to Tumor Overgrowth
| Characteristic | Tumor Overgrowth (n = 15) |
| Intervention performed for restenosis | |
| Yes | 13 (87) |
| No | 2 (13) |
| New stent placed for restenosis | |
| Yes | 5 (33) |
| No | 10 (67) |
| Stent removal required | |
| Yes | 1 (7) |
| No | 14 (93) |
| Care site | |
| Outpatient | 10 (67) |
| Admitted to hospital | 5 (33) |
| Death within 14 d of tumor overgrowth | |
| Yes | 4 (27) |
| No | 11 (73) |
Data are presented as No. (%).
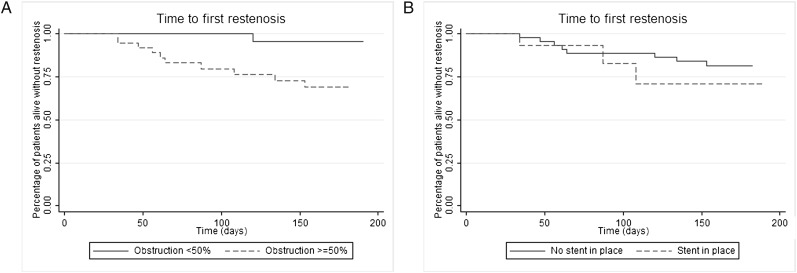
Only preprocedure obstruction ≥ 50% was associated with tumor restenosis on univariate and multivariate analysis (HR = 13.71; 95% CI, 1.75-107.55; P = .013) (Fig 2, Table 6). The incidence rate of restenosis by tumor in patients with obstruction ≥ 50% at baseline was 0.0018 events per person-day. In patients with obstruction < 50%, the incidence rate of restenosis was 0.0002 events per person-day. The incidence rate risk difference, 0.0016 recurrent obstructions per person-day, was significant (95% CI, 0.0005-0.0026; P = .001).
Figure 2.
Kaplan-Meier plots of time to tumor overgrowth. A, Preprocedure obstruction < 50% (solid line) vs preprocedure obstruction ≥ 50% (dashed line). B, No stent in place (solid line) vs stent in place (dashed line).
Table 6.
—Univariate Cox and Extended Cox Models for Time to Tumor Overgrowth
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Sex: male vs female | 0.49 | 0.18-1.37 | .175 |
| Active/ex-smoker vs never smoked | 1.12 | 0.376-3.36 | .835 |
| Preprocedure chemotherapy: yes vs no | 1.89 | 0.53-6.72 | .326 |
| Postprocedure chemotherapy: yes vs no (time varying) | 1.16 | 0.37-3.66 | .806 |
| Preprocedure radiation: yes vs no | 1.70 | 0.61-4.73 | .307 |
| Postprocedure radiation: yes vs no (time varying) | 1.29 | 0.44-3.73 | .642 |
| Lung cancer vs other type of cancer | 0.39 | 0.11-1.40 | .150 |
| Obstruction ≥ 50% preprocedure | 13.71 | 1.75-107.55 | .013 |
| Stent present (time varying) | 1.40 | 0.38-5.19 | .619 |
Overall Survival
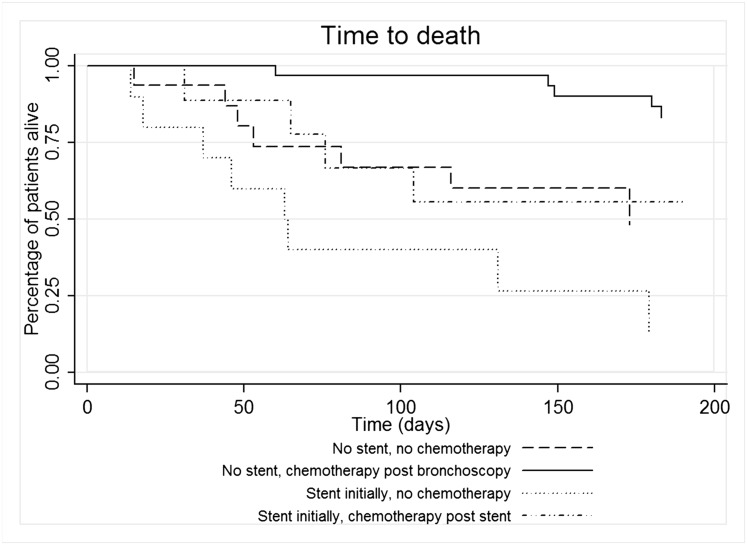
Median follow-up duration was 6 months (range, 0-30 months). Thirty-two deaths occurred among 72 patients. On univariate analysis, poor functional status (Eastern Cooperative Oncology Group performance status score ≥ 2), airway stenting, and lower respiratory tract infection were associated with decreased survival, whereas postbronchoscopy chemotherapy was associated with improved survival (Fig 3, Table 7). In multivariate analysis, only infection (vs no infection) (HR, 11.33; 95% CI, 3.82-33.57; P < .001) and postbronchoscopy chemotherapy (vs no chemotherapy) (HR, 0.20; 95% CI, 0.09-0.45; P < .001) were associated with risk of death.
Figure 3.
Kaplan-Meier plot of time to death. Patients who did not require stenting initially, who received chemotherapy after therapeutic bronchoscopy with ablation, did the best (solid line). Patients who did not require stenting initially, who did not receive chemotherapy (dashed line), and patients who required stenting, who received chemotherapy (dashed-dotted line), had intermediate outcomes. Patients who required stenting, who did not receive chemotherapy (dotted line), had the worst survival (log-rank P < .0001).
Table 7.
—Univariate Cox and Extended Cox Models for Time to Death
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Age (continuous) | 1.01 | 0.99-1.03 | .41 |
| Sex: male vs female | 1.23 | 0.59-2.55 | .59 |
| Days from initial therapy to bronchoscopy | 1.00 | 0.99-1.00 | .29 |
| ECOG ≥ 2 | 2.21 | 1.09-4.47 | .03 |
| Ever smoked | 1.61 | 0.72-3.59 | .25 |
| COPD | 0.56 | 0.17-1.84 | .34 |
| Congestive heart failure history | 0.79 | 0.11-5.81 | .82 |
| Primary lung cancer vs metastatic disease | 1.75 | 0.87-3.53 | .12 |
| Chemotherapy prior to bronchoscopy | 1.70 | 0.73-3.93 | .22 |
| Chemotherapy after bronchoscopy | 0.21 | 0.10-0.45 | < .001 |
| Radiation prior to bronchoscopy | 1.87 | 0.93-3.78 | .08 |
| Radiation after bronchoscopy | 0.70 | 0.34-1.46 | .35 |
| Stent placed initially | 2.87 | 1.40-5.89 | .004 |
| Baseline airway obstruction > 50% | 1.94 | 0.89-4.20 | .09 |
| Infection: yes vs no (time varying) | 11.07 | 3.84-31.96 | < .001 |
See Table 4 legend for expansion of abbreviations.
Discussion
To our knowledge, this is the first study to quantify the differences in infection rates in patients undergoing therapeutic bronchoscopy between those who had stents placed for malignant central airway obstruction and those who did not. Our findings suggest that stent placement is associated with a higher incidence rate of lower respiratory tract infections than is therapeutic bronchoscopy without stent placement. These infections are associated with an increased risk of death and a high case fatality ratio. We also found an association between the severity of airway obstruction prior to intervention and the risk of subsequent restenosis due to tumor. These findings should be balanced against the potential benefits of airway stenting on a case-by-case basis.
Quantification of the incremental risk difference associated with airway stenting is clinically important because it is the risk difference that is relevant when deciding whether a stent should be placed. Previous studies have described the incidence of stent-related infections.3,9 However, no studies have documented the baseline risk of infection in patients with malignant airways obstruction with ablation without stenting. Without knowledge of the baseline risk of infection, we cannot know the incremental risk of infection due to the stent. Without knowledge of the incremental risk, we cannot arrive at a meaningful risk-benefit analysis. The key numbers that this report provides are the incidence rate of infection in patients without stents (0.0011 infections per day at risk) and the incidence rate of infection in patients with stents (0.0057 infections per day at risk). Once we know the baseline risk and the risk with stents, we can calculate the incremental risk (0.0046 infections per day at risk).
This risk may seem low risk, but it is not. The probability that a patient will remain infection free for 30 days with a stent is (1 − 0.0057)30, which is 84%. Therefore, 16% of patients with stents will develop one or more respiratory tract infections by 30 days, which is consistent with other reports from the literature.3,9 Conversely, patients without stents have a 97% chance of remaining infection free for 30 days, so there is a 3% monthly baseline risk of infection in this population. The incremental risk due to stents is 0.0046 more infections per person-day at risk, corresponding to a 13% risk difference per month (number of stents to induce one infection: eight). This is clinically significant because previous studies using the same definition of respiratory tract infections have shown that they are associated with significant morbidity and mortality.9 We found similar results in this study, with 26% of patients with respiratory tract infections dying within 2 weeks of their infection (Table 3).
Our findings built on those of other investigators who have studied infectious complications following therapeutic bronchoscopy. Airway stenting has been shown to be associated with frequent bacterial colonization and infection.3,10 A systematic review of 501 patients with airway stents found that 93 (19%) experienced a stent-associated respiratory tract infection.3 A more recent study of airway stenting in patients with malignant disease documented that the incidence rate of infection varied by stent type, ranging from 0.004 to 0.012 infections per person-day at risk.9 But the incidence rate of infection in patients with malignant airway obstruction who do not undergo airway stenting has not been studied in as much depth; indeed, we found only one such study, which looked at 20 patients undergoing therapeutic rigid bronchoscopy using ablative techniques without stenting. That analysis demonstrated that there was no significant bacterial colonization as assessed by protected-specimen brush cultures.11 To our knowledge, however, no studies before ours have compared the incidence rates of infections in patients undergoing therapeutic bronchoscopy with ablative techniques alone, with ablative techniques plus stenting.
To our knowledge, our study is also the first to use incidence rates and survival analysis methods to quantify the risk difference between patients with stents and those without stents for the outcome of respiratory tract infections. Most other studies of infection in patients undergoing therapeutic bronchoscopy have used incidence proportions.3‐8,12‐14 As we have shown in our previous work, incidence proportions have significant limitations when used to analyze long-term complications; the incidence rate is a more appropriate measurement because it measures events per person-time at risk.9
An additional benefit of using survival analysis methods with time-varying covariates is that we can control for factors that vary over time, such as the degree of airway obstruction and stent removal. In theory, patients with more severe airway obstruction due to tumor would be at increased risk of postobstructive pneumonia. There was a trend suggesting that patients undergoing stenting did have more severe obstruction before the procedure; however, we demonstrated that postprocedure airway patency was not significantly different between the groups, and, if anything, there was a trend suggesting that patients with stents had better airway patency than those without stents with similar degrees of baseline obstruction. Because the at-risk period begins after the procedure, this demonstrates that the observed difference in infection incidence rates between patients with and without stents was not due to differences in airway obstruction, because the patients with stents had good luminal patency after the procedure. The observed association between preprocedure airway obstruction > 50% and infection rates was therefore merely because of the fact that patients with more severe obstruction were more likely to receive stents. Stenting did alleviate the obstruction, but it also increased infection risk. Thus, even after controlling for baseline obstruction and subsequent restenosis, stents were an independent risk factor for infection.
Our results provide additional insight into the risk portion of this decision process: Stenting is associated with increased, nontrivial risk. The clinical implication is that if ablative techniques effectively reopen the airway and there is a good chance that the tumor may respond to chemotherapy and/or radiation, a strategy of initially holding off on stenting may be warranted so that chemotherapy and/or radiation can be given a chance to work. Stenting can be held in reserve in these cases, provided there is careful airway surveillance. If the disease progresses and airway obstruction recurs despite chemotherapy and radiation, stenting is warranted. This approach minimizes the risks of infection while maintaining airway patency. Similarly, stenting is rarely needed if the degree of obstruction is < 50% to begin with, and should be avoided unless there are other indications for it, such as tracheal-esophageal fistula or to isolate an uncontrollable bleeding tumor.
Although we were able to quantify the risk portion of this decision process, we were not as successful in quantifying the benefits of stenting as measured by restenosis rates. We did find that restenosis was associated with more severe obstruction at baseline, but we failed to demonstrate that airway stenting significantly impacted restenosis rates; however, it is likely that these findings represent significant selection bias, as well as residual confounding. Patients judged by their physicians to be at higher risk of restenosis are more likely to have stents placed than are other patients. This is consistent with the observed trend of stent placement being more common in patients with more severe airway obstruction. It is also probable that physicians are more likely to place stents in patients whom they believe have little chance of responding to subsequent chemotherapy or radiation therapy than in other patients. If all good chemotherapy and radiation options have been exhausted, stenting is one of the few viable alternatives for treating malignant airway obstruction. Although we controlled for radiation therapy and chemotherapy before and after airway interventions, it is likely that there was significant residual confounding. Our retrospective design is not suitable for answering this question, and probably the only way to address this issue would be to conduct a randomized controlled trial comparing ablation and stenting with ablation alone.
Similarly, it is possible that physicians would choose to place stents more frequently in patients with less reserve and more severe comorbidities and that these comorbidities, in turn, would lead to the development of more infections. Although we controlled for COPD, congestive heart failure, type of cancer, performance status, chemotherapy, and radiation, residual confounding in this regard is still a possibility. So it may be that some of the observed increase in infection rates is due to differences in comorbidities that resulted from the patient selection process.
Additional limitations of this study include the relatively small sample size and the single-center design. Because of the small sample size, we could not control for different stent types, although our prior work has suggested that different stents have different rates of infection.9 Our findings may also not apply to patients with benign diseases, so the results should be interpreted carefully.
Conclusions
In summary, this is, to our knowledge, the first study to demonstrate that in patients with malignant airway disease, therapeutic bronchoscopy with stent placement is associated with a higher risk of lower respiratory tract infection than is therapeutic bronchoscopy without stent placement. The risk difference, which is the relevant number for clinical decision-making, is 0.0046 more infections per person-day with stenting than without stenting. This corresponds to an incremental risk difference of 13% per month (number of stents to induce one infection: eight). The case fatality rate for patients with infections was 26%, and infection was associated with an increased risk of death. We are hopeful that our findings will facilitate more informed decision-making regarding the risks and benefits of stent placement in patients with malignant airway obstruction. Future studies will need to evaluate both infection rates and tumor restenosis prospectively.
Acknowledgments
Author contributions: Dr Ost is the guarantor of this article and takes responsibility for the integrity of the work as a whole.
Dr Grosu: contributed to the data collection, interpretation, and analysis; and writing and editing of the manuscript.
Dr Eapen: contributed to the writing and editing of the manuscript.
Dr Morice: contributed to the writing and editing of the manuscript.
Dr Jimenez: contributed to the writing and editing of the manuscript.
Dr Casal: contributed to the writing and editing of the manuscript.
Dr Almeida: contributed to the writing and editing of the manuscript.
Dr Sarkiss: contributed to the writing and editing of the manuscript.
Dr Ost: contributed to the study concept and design; data audits and analysis; statistics; writing and editing of the manuscript, and overall study supervision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: In the past 3 years, Dr Eapen has received unrestricted educational grants from the Olympus Corporation and has provided consulting services to the Pentax Corporation. Dr Jimenez is an investigator for the intrapleural catheter daily vs three times a week drainage study (NCT00761618) funded by CareFusion Corporation. Drs Grosu, Morice, Casal, Almeida, Sarkiss, and Ost have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: All work was performed at The University of Texas MD Anderson Cancer Center, Houston, Texas. The authors thank Kathryn Carnes, BS, and Amelia Scholtz, BS, for editorial assistance.
Abbreviations
- HR
hazard ratio
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Stöhr S, Bolliger CT. Stents in the management of malignant airway obstruction. Monaldi Arch Chest Dis. 1999;54(3):264-268. [PubMed] [Google Scholar]
- 2.Alazemi S, Lunn W, Majid A, et al. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest. 2010;138(2):350-356. [DOI] [PubMed] [Google Scholar]
- 3.Agrafiotis M, Siempos II, Falagas ME. Infections related to airway stenting: a systematic review. Respiration. 2009;78(1):69-74. [DOI] [PubMed] [Google Scholar]
- 4.Breitenbücher A, Chhajed PN, Brutsche MH, Mordasini C, Schilter D, Tamm M. Long-term follow-up and survival after Ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration. 2008;75(4):443-449. [DOI] [PubMed] [Google Scholar]
- 5.Wassermann K. How much incidence is enough? Chest. 2001;120(2):686-687. [DOI] [PubMed] [Google Scholar]
- 6.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010;182(5):589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84(6):1870-1877. [DOI] [PubMed] [Google Scholar]
- 8.Chung F-T, Chen H-C, Chou C-L, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg. 2011;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noppen M, Piérard D, Meysman M, Claes I, Vincken W. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med. 1999;160(2):672-677. [DOI] [PubMed] [Google Scholar]
- 11.Noppen M, Piérard D, Meysman M, Herreweghe RV, Vincken W. Absence of bacterial colonization of the airways after therapeutic rigid bronchoscopy without stenting. Eur Respir J. 2000;16(6):1147-1151. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Shin JH, Song HY, Shim TS, Yoon CJ, Ko GY. Benign tracheobronchial strictures: long-term results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol. 2007;188(4):1033-1038. [DOI] [PubMed] [Google Scholar]
- 13.Husain SA, Finch D, Ahmed M, Morgan A, Hetzel MR. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg. 2007;83(4):1251-1256. [DOI] [PubMed] [Google Scholar]
- 14.Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124(5):1993-1999. [DOI] [PubMed] [Google Scholar]