Abstract
Idiopathic pulmonary fibrosis (IPF), a heterogeneous disease with respect to clinical presentation and rates of progression, disproportionately affects older adults. The diagnosis of IPF is descriptive, based on clinical, radiologic, and histopathologic examination, and definitive diagnosis is hampered by poor interobserver agreement and lack of a consensus definition. There are no effective treatments. Cellular, molecular, genetic, and environmental risk factors have been identified for IPF, but the initiating event and the characteristics of preclinical stages are not known. IPF is predominantly a disease of older adults, and the processes underlying normal aging might significantly influence the development of IPF. Yet, the biology of aging and the principles of medical care for this population have been typically ignored in basic, translational, or clinical IPF research. In August 2009, the Association of Specialty Professors, in collaboration with the American College of Chest Physicians, the American Geriatrics Society, the National Institute on Aging, and the National Heart, Lung, and Blood Institute, held a workshop, summarized herein, to review what is known, to identify research gaps at the interface of aging and IPF, and to suggest priority areas for future research. Efforts to answer the questions identified will require the integration of geriatrics, gerontology, and pulmonary research, but these efforts have great potential to improve care for patients with IPF.
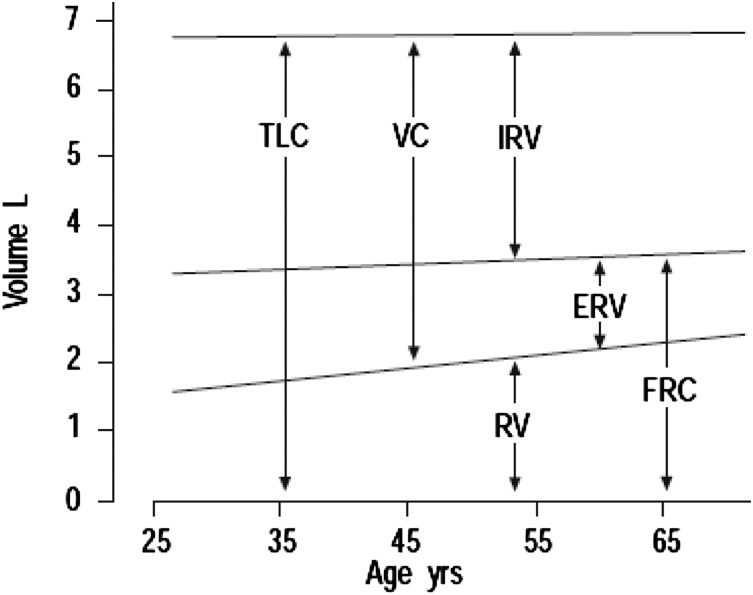
The aging lung loses mass and undergoes several functional and structural changes (Table 1). Lung function declines with age, as shown by changes in gas exchange and airflow limitation (Fig 1). Older adults, defined as those aged ≥ 60 years, become more rapidly limited during exercise, and 50% of adults > 70 years of age become exercise-limited by dyspnea, compared with only 20% of younger adults. In addition, older adults experience a loss of static elastic recoil resulting from non-smoking-related, emphysema-like changes.2 Knowledge of the aging respiratory system remains incomplete, as most data come from studies done in the 1970s or earlier. Yet these structural and functional changes suggest that both inflammation and age-associated emphysema contribute to the development of other lung diseases.
Table 1.
—Age-Associated Changes in Lung Structure and Function
| Hyperinflation with increased dead space |
| Declining FEV1, arising primarily from reduced FVC1 |
| Loss of respiratory muscle strength |
| Stiffening of chest wall |
| Increased residual volume |
| Loss of static elastic recoil2 |
| Loss of central nervous system activity |
| Decreased ventilator response to isocapnic hypoxia. |
| Morphologic changes3 |
| Reduced alveolar surface area |
| Fewer capillaries per alveolus |
| Decreased small-airway diameter |
| Alterations in the composition of the lung matrix |
Figure 1.
Age-related changes in measures of pulmonary function. In general, decreases in elasticity result in increased residual volumes and decreased vital capacity as TLC does not change in the absence of substantial lung disease or comorbidity (eg, severe kyphosis). ERV = expiratory reserve volume; FRC = functional residual capacity; IRV = inspiratory reserve volume; RV = residual volume; TLC = total lung capacity; VC = vital capacity.
Interstitial lung diseases form a heterogeneous group of nonmalignant, noninfectious processes of the lower respiratory tract characterized by dyspnea and cough, crackles on chest examination, restrictive ventilatory impairment, diffuse interstitial opacities on chest images, and alveolointerstitial inflammation with eventual fibrosis on histologic examination. Idiopathic interstitial pneumonias represent about 40% of interstitial lung diseases in the United States, and of these more than half are cases of idiopathic pulmonary fibrosis (IPF). According to a recent joint definition published by the American Thoracic Society and the European Respiratory Society, IPF is a specific form of chronic, progressive, fibrotic interstitial pneumonia of unknown cause, which occurs in adults and is limited to the lung. It is associated with a histopathologic and radiologic pattern of usual interstitial pneumonia (UIP)4 and “honeycombing,” respectively.5
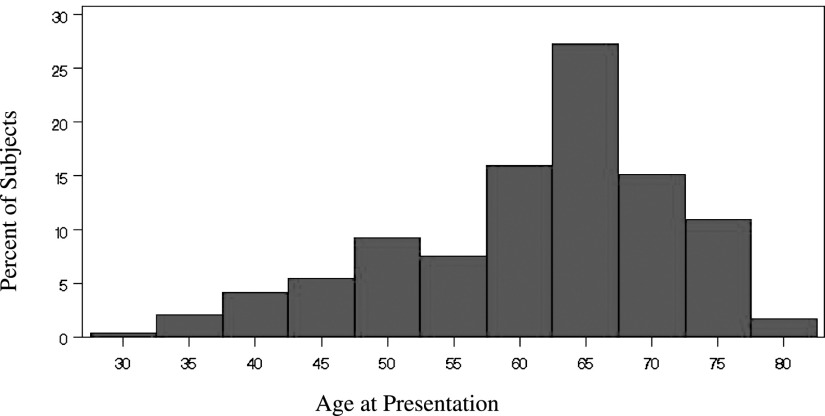
IPF is a relatively rare disease, with an incidence of 10.7 per 100,000 per year for men and 7.4 per 100,000 per year for women. At any given time, there are 35,000 to 50,000 cases of IPF in the United States. However, the occurrence of IPF increases in both prevalence and incidence in the sixth decade of life (Fig 2).7 Symptoms typically occur at age 50 to 70 years, and most patients are > 60 years of age at the time of clinical presentation.8,9 IPF is often fatal—the 5-year survival rates for IPF are worse than those for any other lung disease except cancer6—but individuals with IPF have variable disease courses. Some patients experience rapid declines, which have been associated with poor survival,10 and others experience slow declines but often succumb to acute exacerbations.11,12 It is difficult to predict from onset how long a patient will live or maintain pulmonary function or who will experience acute exacerbations. Moreover, even in retrospect, it is not clear why some patients live longer than others. Large clinical trials have yielded conflicting results with respect to lung function and blood gas measures, and the various patterns of progression, the relationship between age and frequency of acute exacerbations, and triggers of exacerbations are poorly understood.
Figure 2.
Onset of idiopathic pulmonary fibrosis (IPF) usually occurs between the ages of 50 and 70 years, but the mean age in this population of patients with IPF was 61.4 years (n = 238, age range 27-79 years). Sixty-five percent of the subjects were ≥ 60 years of age (< 50 years, n = 36; 50-59 years, n = 48; 60-70 years, n = 108; > 70 years, n = 46). (Data from King et al6).
Diagnosis
IPF diagnosis relies on CT scanning of the chest or surgical lung biopsy. Yet patients often expect dyspnea to be a characteristic of normal aging; they thus limit their activity to accommodate IPF symptoms and do not consult with their physicians. For patients who do consult with them, physicians are often reluctant to perform diagnostic tests, particularly surgical lung biopsies, because of the patients’ advanced age and because no standard treatment has clear benefit. Moreover, the diagnosis of IPF is best established by consensus among radiologists, pathologists, and clinicians.,5,13 However, interobserver agreement, both within and across disciplines, remains poor.14
Diagnosis and prognosis would therefore benefit from better predictors. Genomic signatures might be a predictor. A microarray analysis of lung samples or peripheral blood mononuclear cells from patients with IPF shows clear differences in expression among 2,000 genes,15-17 including genes activated by transforming growth factor β (TGF-β) and genes encoding collagen, matrix metalloproteinases, surfactant proteins, and epithelial proteins. In these studies, assessing the altered expression of as few as 23 to as many as 1,772 genes predicts diagnosis with some degree of accuracy. Microarray data also might differentiate between the accelerated and stable forms of IPF18 and distinguish an IPF from other types of interstitial pneumonia. Thus, genomic signatures could be useful not only in diagnosing IPF but also in prioritizing patients for treatment and monitoring the effects of treatment. Some evidence suggests that expression patterns of markers such as matrix metalloproteinase 7 or toll-like receptor-9 might be useful in prognosis.17,19
Animal Models for IPF Research
Much of what is known about IPF pathogenesis comes from studies using animal models, such as mice treated with bleomycin or fluorescein isothiocyanate,20 mice overexpressing TGF-β,21 and viral infections to model potential exacerbating factors. Those models mimic acute injury and some of the histologic features of IPF (eg, collagen accumulation, architectural tissue distortion, epithelial cell hyperplasia, and isolated fibroblastic foci).22 However, none of these models mimic the entire disease. In particular, because the initiating factor for IPF is unknown, no experimental approach models early, preclinical stages of the disease. In fact, a comparison of long-term studies of TGF-β and bleomycin models over 100 days found that many animals that show acute lung injury and fibrosis immediately following or up to 28 days after bleomycin challenge revert to normal over time (M. Kolb, personal communication).23 Thus spontaneous reversals could hamper interpretation of these animal study results. In addition, a metaanalysis of trials examining > 200 promising drugs suggests that current animal models are not useful for evaluating the clinical management of IPF.24
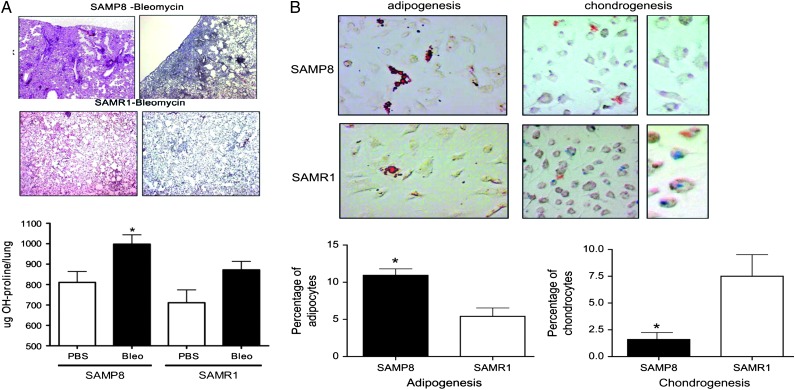
Animal studies are also challenging because of differences between models and humans in terms of disease assessments and age. Human assessments focus on symptoms, exercise testing, imaging, echocardiograms, and bronchoscopy, whereas animal assessments focus on histology, morphometry, collagen tissue content, and matrix gene expression. In addition, most rodent studies are conducted in mice or rats aged 6 to 12 weeks, the equivalent of about 10 to 12 years in humans. Some attempts to mimic older age have been made. For example, senescence-prone mice (SAMP8 in Fig 3) have been used to mimic age and indeed develop more profound fibrosis than do younger mice,25 but it is not clear whether the mechanisms underlying “age-related” differences in mice are the same as those in humans, and other abnormalities in these specific mice might impede interpretation of study results. Novel imaging techniques and development of a clinical-pathologic-radiologic score based on several assessments have been proposed as ways to overcome these concerns.
Figure 3.
(A) Mice with accelerated aging process (SAMP8) develop more pronounced fibrosis in response to bleomycin, as shown by histology and hydroxyproline content, compared with mice that are resistant to aging (SAMR1). Fibrosis in these animals is accompanied by the presence of high numbers of mesenchymal progenitor cells in the blood fibrocytes (not shown). (hematoxylin and eosin and Masson trichrome; magnification × 10 and × 40.) (B) Bone marrow-derived mesenchymal stem cells, which are less differentiated than fibrocytes, do not differentiate into chondrocytes in aging mice as they do in senescence-resistant mice, suggesting that they might already be “destined” to become fibrocytes and thus promote fibrosis in aging individuals. (alcian blue for chondrocytes [blue cells] and oil red O staining for adipocytes [red cells]; magnification × 20 and × 100.) (Reprinted with permission from Xu et al.25)
Cellular and Molecular Factors in IPF
Some understanding of the mechanisms underlying IPF, particularly with respect to alveolar cells, has come from studies of familial pulmonary fibrosis. Mutations in genes encoding proteins involved in the synthesis and metabolism of surfactant have been associated with chronic lung disease, and in adults, mutations in surfactant protein C (SP-C) have been associated with pulmonary fibrosis with a UIP pattern.26 In vitro, transfection of mutant SP-C results in an abundance of insoluble protein aggregates in the lung, similar to the amyloid fibrils seen with Alzheimer disease in the brain, suggesting that SP-C mutations hinder the protein secretory pathway and stress the endoplasmic reticulum (ER). In mouse models, SP-C mutations targeted to alveolar type 2 cells result in cytotoxic protein aggregates that perturb lung development in association with elevated ER stress and cell death. Markers of ER stress and apoptosis are also seen in epithelial cells from patients with sporadic IPF.27
Cytoprotective responses of the ER decline with age. The number of ER chaperones that facilitate protein folding are reduced, and those that remain are especially vulnerable to oxidation because of declines in glutathione expression.28,29 In addition, autophagy, one mechanism by which the cell rids itself of misfolded proteins, declines with age and contributes to cellular senescence. Mutant protein aggregates are thus expected to accumulate in older adults, and it is likely that infection or other processes that overwhelm autophagy exacerbate that accumulation. Thus, the usual paradigm of chemical chaperones might in fact be deleterious for patients with IPF, and lysosomotropic drugs, which block autophagy and are recommended for pediatric lung conditions,30,31 could contribute to further accumulation of misfolded protein aggregates in IPF.
The associations between SP-C mutations, protein aggregates, and lung disease are consistent with a central role for alveolar epithelium in the pathogenesis of IPF, wherein dysregulated epithelial repair following injury might create a profibrotic environment.32 Epithelial-mesenchymal transition (EMT), an extreme form of epithelial cell plasticity in which fully differentiated epithelial cells adopt a mesenchymal phenotype, might also contribute fibroblasts following epithelial stress or injury in adult tissues.33 Although type 2 alveolar epithelial cells typically serve as progenitors for type 1 cells in vitro, type 1-like cells can revert to a type 2 cell phenotype under certain culture conditions.32,34 EMT is marked by loss of polarity and epithelial markers and acquisition of fibroblast/myofibroblast markers, such as a spindle-shaped morphology and a migratory phenotype. Treatment with TGF-β induces EMT in alveolar epithelial cells,33,35 and EMT has been observed in animal models of injury and in lung samples from patients with IPF.36,37 EMT might represent a mechanism for survival in response to stress or injury, which, in the context of aging, might include increased oxidant stress, increased production of TGF-β, excessive inflammatory responses to viral infections, and changes in extracellular matrix. However, it is not clear how age itself affects EMT of alveolar epithelial cells nor to what extent EMT contributes to the pathogenesis of pulmonary fibrosis.
Bone marrow-derived cells have also been shown to regulate the pathogenesis of pulmonary fibrosis. Mesenchymal stem cells (MSCs) appear to be reparative, as suggested by their ability to localize to the injured lung, engraft as alveolar epithelial cells, suppress inflammation,38 reduce collagen deposition,39 and induce growth factors. Fibrocytes, however, appear to be pathogenic. Transgenic mice that express green fluorescent protein only in bone marrow progenitor cells show abundant green fluorescent protein-expressing fibroblasts in the lung following bleomycin challenge.40 Adoptive transfer of purified fibrocytes worsens lung injury in mouse models.41 Herpesvirus exacerbation of lung fibrosis has been associated with enhanced fibrocyte recruitment,42 and human fibrocytes migrate to injured lungs in mouse models.43 Moreover,44 patients with IPF exhibit more lung fibrocytes than do healthy volunteers,24,45 and fibrocytes obtained from patients with IPF are more proliferative.
Both MSCs and fibrocytes are recruited by the same chemokine signals, suggesting that the repair of lung injury requires a balancing act between reparative MSCs and pathogenic fibrocytes. Evidence suggests that aging alters the balance; MSC recruitment appears to be favored in younger individuals, whereas fibrocyte recruitment is favored in older individuals.46 In senescence-prone mice, increased recruitment of fibrocytes and more fibrosis is observed following injury, and MSCs isolated from these mice are less able to migrate.25 Thus chemokine- or chemokine receptor-targeted therapies for IPF might increase the risk for infection or impair wound repair, and treatments designed to increase the number of MSCs might work only during the inflammatory phase immediately following injury. In addition, treatments designed to limit fibrocytes might prove problematic, because chemokine receptor expression and cellular subsets are poorly defined, and not all patients with IPF show elevations in circulating fibrocytes.
Genome linkage scans in families affected by the familial form of pulmonary fibrosis, and further analysis of these families and of individuals with IPF, have revealed mutations in telomerase, an enzyme that protects telomeres from damage and whose activity declines with age.47 These results are consistent with the first reports of mutations in TERT, the protein component of telomerase; these mutations were described in a family affected by dyskeratosis congenita, a rare disorder characterized partly by pulmonary fibrosis.48 All mutations identified in the more recent analyses are loss-of-function mutations leading to reduced telomerase activity and decreased telomere length.49 Thus IPF is the third “telomeropathy” identified (Table 2). However, even in families affected by telomerase mutations, pulmonary fibrosis still appears to be an age-associated disease, suggesting a requirement for a second hit to trigger disease onset. Aside from age, gender and smoking history appear to modify the effects of telomerase mutations.
Table 2.
—Telomeropathies
| Characteristic | DKC | Bone Marrow Failure | IPF |
| Mode of inheritance | X-linked recessive | Autosomal dominant | Autosomal dominant |
| Autosomal dominant | Autosomal recessive | ||
| Autosomal recessive | |||
| Age of onset | 10-30 y | All ages | > 50 y |
| Skin phenotype | Yes | No | No |
| Bone marrow failure | Yes | Yes | Anemia |
| Somatic manifestations | Yes | Rare | Osteoporosis |
| Cirrhosis | |||
| Cancer | Yes | Yes | Unknown |
| Mutations | DKC1 > TERC > TERT | TERC > TERT | TERT > TERC |
| Shortened telomeres | Yes | Yes | Yes |
Adapted from Garcia et al.50 DKC = dyskeratosis congenita; IPF = idiopathic pulmonary fibrosis; TERC = telomerase RNA component; TERT = telomerase reverse transcriptase.
Environmental Factors and IPF
Smoking is a major risk factor for the development of IPF. Most patients are current or former smokers at the time of IPF diagnosis,8,9 and in families affected by the familial form of pulmonary fibrosis, ever smoking is the largest risk factor associated with eventual development of the disease.51 Smoking status affects FVC, gas exchange, and blood gases, and although its role in the natural history of IPF is not clear, smoking likely exerts synergistic effects with other factors. Exposure to cigarette smoke augments the effects of bleomycin injury in animal models,52 and smoking delays the clearance of toxins such as aluminosilicate. Studies in rodents also suggest that exposure to cigarette smoke alters gene expression in both the airways and parenchyma and that altered expression patterns could revert to normal when the exposure ends.53,54
Viral infection might also play a role in the cause of IPF, as suggested by findings from animal studies. Herpesviruses have been positively associated with equine pulmonary fibrosis,55 and studies in animal models indicate that infection with a murine form of Epstein-Barr virus (EBV) can trigger and exacerbate progressive fibrosis.42,56-59 In addition, several studies have found EBV proteins and DNA in lung samples from patients with IPF,60,61 although others have observed EBV DNA in control lungs and in fibrotic lungs associated with systemic sclerosis.62 In one patient with mild symptoms and a higher EBV load, lung function was stabilized and viral load decreased upon initiation of antiviral therapy.63 Viral infection might trigger IPF by augmenting the effects of ER stress or by promoting fibrocyte recruitment and fibrotic growth. Viral reactivation also might represent a chronic injury to the lung.
IPF and Comorbidities
Functional status is probably the most important predictor of health outcomes in older patients, and it becomes more predictive than specific diagnoses or laboratory measures as people age.64 Among patients with IPF waiting for lung transplants, performance on the 6-min walk test is more predictive of survival than lung function.65 Further, functional disability, depression, and cognitive function all predict mortality risk in older people.64,66 Disease-associated functional decline arises both from the insidious decline associated with disease progression and accelerated declines associated with hospitalization.67,68 Functional measures could thus enhance traditional measures in predicting outcomes and response to therapy among patients with IPF. A minimal dataset of a few such measures (Table 3)69 could provide a solid understanding of a patient’s function without excessive time burden. However, more study is needed to assess geriatric measures of function in patients with IPF.
Table 3.
—A Suggested Minimum Dataset for Functional Status Assessment in Older Adult Cohorts Including Those With IPF69
| Measure | Estimated Time Requirement, min |
| ADL | 2 |
| Instrumental ADL (eg, handling money, medication, housework) | 2 |
| Mobility (eg, 6-min walk) | 6 |
| Geriatric syndromes (falls and incontinence) | 1 |
| Mini-Mental State Examination (cognitive function) | 5 |
| Depression (Geriatric Depression Scale) | 3 |
| Social functioning (social support or social isolation, depending on purpose of the study) | 2 |
The proposed dataset is intended for research purposes. A minimum clinical dataset would likely include fewer measures. ADL = activities of daily living. See Table 2 legend for expansion of other abbreviation.
Comorbidities may play a role in the heterogeneity seen among patients with IPF. As suggested by Medicare data,70 older individuals are likely to have at least three chronic conditions, and evidence suggests that the presence of these comorbidities influences both the eligibility of patients with IPF for lung transplants and their survival while on the waitlist. In addition, comorbidities, and the medications prescribed for them, can often contribute to symptoms and thus hamper diagnosis and treatment.71 Vascular or coronary artery disease, obstructive sleep apnea, diabetes,71-74 corticosteroid-associated osteoporosis, and sarcopenia are commonly observed in patients with IPF.75-81
The incidence of and mortality risk associated with pulmonary hypertension (PH), a common feature of advanced IPF, increases in individuals > 60 years of age.82-84 The pathogenesis of PH in these patients is not clear. Retrospective studies of patients with IPF indicate that a resting mean pulmonary artery pressure > 25 mm Hg, as measured by right-heart catheterization, remains the definitive diagnostic method for PH rather than ECG or high-resolution CT scan.83 On the basis of this definition, PH is associated with poorer survival in patients with IPF.85 In addition, patients with combined pulmonary fibrosis and emphysema syndrome have a poor prognosis, which might be associated with higher pulmonary vascular resistance and PH.86-88
Gastroesophageal reflux disease (GERD) is also a common comorbidity in patients with IPF,73 and the likelihood of having both IPF and GERD increases in patients > 60 years of age.89,90 Patients with IPF and GERD are at higher risk for hospitalizations from any cause and from respiratory illness, perhaps resulting, at least in part, from the contribution of uncontrolled reflux and microaspiration to pulmonary fibrosis. Some case reports suggest that controlling reflux might stabilize IPF.90,91 These observations suggest a hypothesis in which recurrent epithelial injury caused by microaspiration of gastric juice and contents causes alveolar damage and pulmonary fibrosis. However, the prevalence of microaspiration in patients with IPF is not known, and it is not clear whether microaspiration represents an intrinsic risk factor or causes acute exacerbations of IPF. Moreover, whether GERD is more common in older adults is not known.
Treatment
Treatment benefit is often measured in terms of average change in a cohort of patients. This approach assumes a fairly homogeneous population. With IPF, however, there is likely a heterogeneous distribution of treatment benefit, with some patients deriving benefit from any given therapy and others deriving no benefit or even harm.92 The 2000 American Thoracic Society/European Respiratory Society consensus statement on the management of IPF pointed out that there was little evidence of effectiveness for any treatment,13 and there are no high-quality studies of antiinflammatory treatments.93 The use of the antioxidant N-acetylcysteine, in combination with prednisone and azathioprine, has been associated with a slower decline in FVC over 12 months94 compared with prednisone and azathioprine alone,95 but the clinical significance of these results is unclear. Studies of novel therapies, such as pirfenidone96,97 bosentan,98 and sildenafil, have shown no clear treatment benefit.
Common treatment approaches for IPF include pulmonary rehabilitation, long-term oxygen therapy, and treatment of comorbidities. Pulmonary rehabilitation appears to benefit both symptoms and function.99 The treatment of IPF-associated PH remains controversial. Lung transplant, usually the end stage in IPF treatment, is still a fairly new approach. Until 2005, the number of deaths while waiting for a transplant was higher for the lung than for any other organ, and the risk for waitlist mortality was highest among patients with IPF. With the implementation of the Lung Allocation System, which scores patients based on their risk for waitlist mortality and assigns higher allocation scores for IPF,100 transplantation volumes have increased, waiting times have decreased, and IPF is now the most common indication for transplant. Although short- and long-term survival have improved, patients with IPF continue to exhibit the worst post-transplant outcomes.101 It should be noted, however, that the mortality rate among these patients has decreased since 1998, and the Lung Allocation System allows transplantation in critically ill patients, among whom survival is the worst. The optimal timing for transplant for patients with IPF is not known.
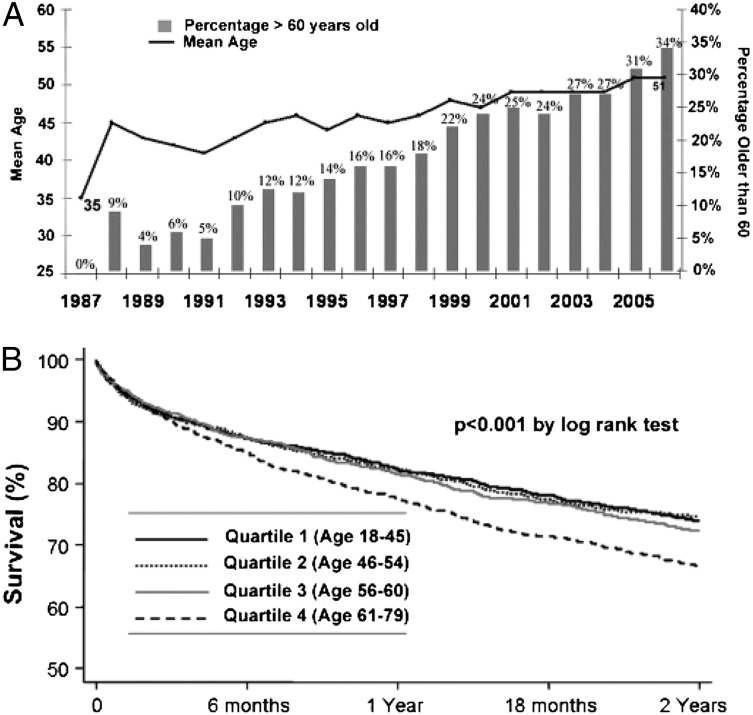
The International Society for Heart and Lung Transplantation guidelines list age > 65 years of age as a relative contraindication for lung transplantation. However, the average age of lung transplant recipients is 51 years. The percentage of those > 60 years of age has grown since 1987, and patients > 65 years of age represent the largest growing segment of transplant recipients (Fig 4).102,103 The survival rate among patients aged 61 to 74 years is about 78% at 1 year and 69% at 2 years.104 Mortality is slightly increased among patients > 65 years of age, with a survival rate of 71% (Fig 4). Thus, although it is likely that advanced age and IPF confer the highest mortality risk, it is not clear that advanced age should be a contraindication for transplantation. Moreover, quality of life, functional status, neurocognition and psychologic status, and the effects of comorbidities on transplant outcomes are still poorly understood. GERD appears to be a risk factor for organ rejection and bronchiolitis obliterans,105,106 the most common cause of death posttransplant, but more study of post-transplant complications in patients with IPF is needed. In addition, it is not clear whether immunosuppression methods should differ for patients of advanced age.
Figure 4.
Patients aged > 60 years form the largest growing segment of transplant recipients, with an increase of 648% since 1996 and 175% since 2004 (A). Although survival is lower in this group, the absolute difference at 2 years is < 10% when compared with any of the younger cohorts (B).
Recommended Research Priorities
Specific research questions and potential research cohorts appear in Table 4. Increasing knowledge about the natural history of IPF is chief among research priorities, as this will begin to allow identification, at the time of diagnosis, of individuals at highest risk for progression. In addition, the present diagnostic criteria for IPF are descriptive, might misclassify phenotypically similar but etiologically distinct conditions, and might miss early disease. A precise biologically based screening tool, based on increased understanding of underlying mechanisms and the identification of relevant biomarkers, is needed to improve the sensitivity and specificity of diagnosis. Symptom management and interactions between IPF and comorbidities also should be explored further. Therapeutic trials should include mechanistic substudies, which will require sustainable trial networks and phase I/II studies, and cell-based therapies and multicomponent approaches should be explored.
Table 4.
—Recommended Research Questions
| Area of Study | Research Questions | Needs |
| Epidemiology | 1. Are there patient subsets that could potentially be diagnosed as “at risk” for IPF? | 1. Appropriately matched control groups (eg, age-matched, commonly encountered comorbidities in IPF, such as pulmonary hypertension or GERD.) |
| 2. How can the first complaints or symptoms of IPF be differentiated from normal aging with greatest accuracy? | 2. Standardized outcome measures among various fibrotic diseases, which will allow investigators of different studies to collect the same types of information. | |
| 3. What are the effects of comorbidities and functional status on outcomes and management of IPF? | ||
| Pathophysiology | 1. Does IPF represent multiple disorders with a common outcome? | 1. Appropriately aged animal models. |
| 2. Are nonspecific interstitial pneumonia, which appears in younger individuals, and UIP, which typically appears in older individuals, components of the same pathway? | 2. Reagents to identify different cell types in more easily obtained specimens, such as peripheral blood, sputum, and BAL. | |
| 3. What are the effects of immune response and immune senescence on IPF? | 3. Fresh tissue from lungs removed during transplant, as well as from control lungs. | |
| 4. Is IPF a disease of senescence or of accumulated insult? | 4. Longitudinal studies of the familial form of IPF. | |
| 5. How does age affect IPF-related cellular processes, such as EMT of alveolar cells? | ||
| 6. What is the relative contribution of alveolar epithelial cell EMT to pathogenesis of human fibrosis? | ||
| 7. Is EMT largely a manifestation of epithelial injury, and is it protective against apoptosis or senescence? | ||
| 8. Which intrinsic and extrinsic factors (matrix, oxidant, immune) associated with aging might predispose alveolar epithelial cells to undergo EMT? | ||
| 9. Can prevention or reversal of EMT affect progression of pulmonary fibrosis? | ||
| 10. Can EMT be prevented or reversed, and does this affect progression of pulmonary fibrosis? | ||
| 11. What is the intrinsic susceptibility of the IPF lung to stress, and how arecytoprotective measures affected when stress has already occurred? | ||
| 12. What role does autophagy, which is markedly influenced by age, play in IPF pathogenesis? | ||
| Diagnosis | 1. What is the applicability of genomic and proteomic profiles in screening populations for IPF or determining prognosis or response to therapy? | 1. Imaging and blood markers to better identify fibrotic UIPs and to distinguish disease processes, abnormal healing responses, and nonresponse to treatment. |
| 2. Can biomarkers of aging provide additional diagnostic or prognostic value? | 2. Better definition of radiographic findings (eg, “honeycombing”); will require improved interobserver agreement. | |
| Treatment | 1. What are the effects of age and comorbidities on treatment response and side effects? | 1. Inclusion of mechanistic studies in therapeutic trials. |
| 2. Are there multicomponent or cell-based therapies that would be effective against IPF? | 2. Sustainable trial networks. | |
| 3. Phase I/II studies. |
Available cohorts: IPFnet, LTRC, COPD cohorts, industry cohorts, Cardiovascular Health Study, Women’s Health Study, Health and Retirement Study, Baltimore Longitudinal Study of Aging, Lung Cancer Early Detection Study, immunologic cohorts, Multi-ethnic Study of Atherosclerosis. Future cohorts should require consensus diagnoses made by communication among clinicians, radiologists, pathologists, and geriatricians. EMT = epithelial-mesenchymal transition; GERD = gastroesophageal reflux disease; LTRC = Lung Tissue Research Consortium; UIP = usual interstitial pneumonia. See Table 2 for expansion of other abbreviation.
Although IPF is assumed to be a disease of aging, it is not clear what aspects of age are associated with the disease. Previously identified senescence markers appear to have little or no validity when applied to studies of IPF. In addition, the effects of age on the distribution of cell types in the lung, as well as on the cellular processes related to fibroproliferation, are not known. Future studies will require control groups of appropriate age and physiologic status to separate the effects of IPF from those of normal aging. Thus traditional age restrictions on clinical trial participation should be lifted. In addition, appropriately aged animal models will be needed in basic science and preclinical studies, and the selection of the specific model will depend on the research question. Reagents to facilitate the study of different cell types also are needed; for example, fibrocytes are particularly difficult to study.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Castriotta is a consultant for Blue Cross and Blue Shield of Texas and has received past research support from Cephalon, Inc. Dr Eldadah is a federal employee with the National Institutes of Health. Dr King serves as a consultant to the following pharmaceutical or medical device companies: Actelion, AstraZeneca, Boehringer Ingelheim, Centocor, Chronoger, CV Therapeutics, Domantis Limited, FibroGen, Genzyme, Human Genome Sciences, Huya Bioscience, InterMune, Millennium Pharmaceuticals, Merck, GlaxoSmithKline, Gilead, Novartis, Hoffman-La Roche, Inc., and Serono. He also serves on the advisory committees for Actelion and InterMune and on the data safety monitoring boards for Centocor and GlaxoSmithKline. Dr Nayfield is a federal employee with the National Institutes of Health. Dr Reynolds is a federal employee with the National Institutes of Health. Dr Schmader has received grant support from Merck and Wyeth and serves as a consultant for Merck and GlaxoSmithKline. Dr High has received grant support from Merck, Pfizer, Optimer, Chimerix, and Afexa Life Sciences, and serves as a consultant for Optimer and GlaxoSmithKline. Drs Foster, Halter, Hazzard, Kiley, McFarland Horne, and Toews have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Charles Clayton, Erika Tarver, Catherine Scott, and Nancy Woolard for their assistance with organizing the workshop.
Additional information: The e-Appendix 1 can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/138/3/693/suppl/DC1.
Abbreviations
- EBV
Epstein-Barr virus
- EMT
epithelial-mesenchymal transition
- ER
endoplasmic reticulum
- GERD
gastroesophageal reflux disease
- IPF
idiopathic pulmonary fibrosis
- MSC
mesenchymal stem cell
- PH
pulmonary hypertension
- SP-C
surfactant protein C
- TERT
telomerase reverse transcriptase
- TGF-β
transforming growth factor β
- UIP
usual interstitial pneumonia
*A complete list of workshop participants is located in the e-Appendix 1.
Funding/Support: This workshop was supported by a grant from the John A. Hartford Foundation to the Association of Specialty Professors [Grant 2006-0239].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Griffith KA, Sherrill DL, Siegel EM, Manolio TA, Bonekat HW, Enright PL. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163(1):61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 2.Brown RH, Pearse DB, Pyrgos G, Liu MC, Togias A, Permutt S. The structural basis of airways hyperresponsiveness in asthma. J Appl Physiol. 2006;101(1):30–39. doi: 10.1152/japplphysiol.01190.2005. [DOI] [PubMed] [Google Scholar]
- 3.Harding R, Pinkerton K, Plopper C. The Lung: Development, Aging and the Environment. London, England: Elsevier, Ltd; 2004. [Google Scholar]
- 4.Liebow A. Definition and classification of interstitial pneumonias in human pathology. Prog Respir Res. 1975;8:1–33. [Google Scholar]
- 5.American Thoracic Society European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 7.Chest 2002 Scientific Highlights. Abstracts of original investigations and case reports. 2-7 November 2002, San Diego, California, USA. Chest. 2002;122(4) Suppl:1S–269S. [PubMed] [Google Scholar]
- 8.Myers JL, Katzenstein AL. Beyond a consensus classification for idiopathic interstitial pneumonias: progress and controversies. Histopathology. 2009;54(1):90–103. doi: 10.1111/j.1365-2559.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selman M, Carrillo G, Estrada A, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2(5):1–11. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collard HR, Moore BB, Flaherty KR, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez FJ, Safrin S, Weycker D, et al. IPF Study Group The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 pt 1):963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KR, Andrei AC, King TE, Jr, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175(10):1054–1060. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo A, Gibson K, Cisneros J, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2(9):891–903. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selman M, Pardo A, Barrera L, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173(2):188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon K, Bailey NW, Yang J, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PLoS One. 2009;4(4):1–14. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneghin A, Choi ES, Evanoff HL, et al. TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem Cell Biol. 2008;130(5):979–992. doi: 10.1007/s00418-008-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33(1):9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 21.Lee CG, Cho SJ, Kang MJ, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200(3):377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vannella KM, Moore BB. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):1–11. doi: 10.1186/1755-1536-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ask K, Labiris R, Farkas L, et al. Comparison between conventional and “clinical” assessment of experimental lung fibrosis. J Transl Med. 2008;6:16. doi: 10.1186/1479-5876-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40(3):362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Gonzalez ET, Iyer SS, et al. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64(7):731–739. doi: 10.1093/gerona/glp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson WE, Crossno PF, Polosukhin VV, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 27.Korfei M, Ruppert C, Mahavadi P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(8):838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8(3):150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009;20(1):23–37. doi: 10.1515/revneuro.2009.20.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Paiva MA, Amaral SM. Chronic interstitial lung disease in children. J Pediatr (Rio J) 2007;83(3):233–240. doi: 10.2223/JPED.1635. [DOI] [PubMed] [Google Scholar]
- 31.Chan EY, Dell SD. Pediatric interstitial lung disease masquerading as difficult asthma: management dilemmas for rare lung disease in children. Can Respir J. 2005;12(6):317–320. doi: 10.1155/2005/413758. [DOI] [PubMed] [Google Scholar]
- 32.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3(4):364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 33.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol. 1995;12(5):497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- 35.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 36.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180(7):657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(2):213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 41.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillan TR, Moore BB, Weinberg JB, et al. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med. 2008;177(7):771–780. doi: 10.1164/rccm.200708-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 46.Mora AL, Rojas M. Aging and lung injury repair: a role for bone marrow derived mesenchymal stem cells. J Cell Biochem. 2008;105(3):641–647. doi: 10.1002/jcb.21890. [DOI] [PubMed] [Google Scholar]
- 47.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102(44):15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 2007;35(22):7406–7416. doi: 10.1093/nar/gkm644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele MP, Speer MC, Loyd JE, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cisneros-Lira J, Gaxiola M, Ramos C, Selman M, Pardo A. Cigarette smoke exposure potentiates bleomycin-induced lung fibrosis in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2003;285(4):L949–L956. doi: 10.1152/ajplung.00074.2003. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson CS, Docx C, Webster R, et al. Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation. Am J Physiol Lung Cell Mol Physiol. 2007;293(5):L1183–L1193. doi: 10.1152/ajplung.00105.2007. [DOI] [PubMed] [Google Scholar]
- 54.Churg A, Zhou S, Preobrazhenska O, Tai H, Wang R, Wright JL. Expression of profibrotic mediators in small airways versus parenchyma after cigarette smoke exposure. Am J Respir Cell Mol Biol. 2009;40(3):268–276. doi: 10.1165/rcmb.2007-0367OC. [DOI] [PubMed] [Google Scholar]
- 55.Williams KJ, Maes R, Del Piero F, et al. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol. 2007;44(6):849–862. doi: 10.1354/vp.44-6-849. [DOI] [PubMed] [Google Scholar]
- 56.Mora AL, Woods CR, Garcia A, et al. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L711–L721. doi: 10.1152/ajplung.00007.2005. [DOI] [PubMed] [Google Scholar]
- 57.Mora AL, Torres-González E, Rojas M, et al. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(4):466–473. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mora AL, Torres-González E, Rojas M, et al. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am J Respir Crit Care Med. 2007;175(11):1139–1150. doi: 10.1164/rccm.200610-1426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pozharskaya V, Torres-González E, Rojas M, et al. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One. 2009;4(10):1–10. doi: 10.1371/journal.pone.0007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mora AL, Roman J. Virus-related interstitial lung disease. In: King TE Jr, Schwarz MI, editors. Interstitial Lung Disease. 5th ed. Hamilton, Ontario, Canada: BC Decker; 2010. pp. 251–265. [Google Scholar]
- 61.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166(4):510–513. doi: 10.1164/rccm.2103058. [DOI] [PubMed] [Google Scholar]
- 62.Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000;55(11):958–961. doi: 10.1136/thorax.55.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41(6):2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 65.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(6):659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehta KM, Yaffe K, Langa KM, Sands L, Whooley MA, Covinsky KE. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. J Gerontol A Biol Sci Med Sci. 2003;58(5):M461–M467. doi: 10.1093/gerona/58.5.m461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 69.Gallo J, Bogner H, Fulmer T, et al. Handbook of Geriatric Assessment. 4th ed. Sudbury, MA: Jones and Bartlett; 2006. [Google Scholar]
- 70.Anderson G, Horvath J, Knickman JR, Colby DC, Schear S, Jung M. Chronic Conditions: Making The Case For Ongoing Care. Baltimore, MD: Johns Hopkins University, Robert Wood Johnson Foundation’s Partnership for Solutions; 2002. [Google Scholar]
- 71.Boyd CM, Weiss CO, Halter J, Han KC, Ershler WB, Fried LP. Framework for evaluating disease severity measures in older adults with comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):286–295. doi: 10.1093/gerona/62.3.286. [DOI] [PubMed] [Google Scholar]
- 72.Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest. 2003;123(6):2007–2011. doi: 10.1378/chest.123.6.2007. [DOI] [PubMed] [Google Scholar]
- 73.Gribbin J, Hubbard R, Smith C. Role of diabetes mellitus and gastro-oesophageal reflux in the aetiology of idiopathic pulmonary fibrosis. Respir Med. 2009;103(6):927–931. doi: 10.1016/j.rmed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Sancho Figueroa MC, Carrillo G, Perez-Padilla R, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respir Med. 2009;104(2):305–309. doi: 10.1016/j.rmed.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zisman DA, Kawut SM, Lederer DJ, et al. Serum albumin concentration and waiting list mortality in idiopathic interstitial pneumonia. Chest. 2009;135(4):929–935. doi: 10.1378/chest.08-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med. 2008;178(12):1257–1261. doi: 10.1164/rccm.200805-725OC. [DOI] [PubMed] [Google Scholar]
- 78.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 79.Lederer DJ, Caplan-Shaw CE, O’Shea MK, et al. Racial and ethnic disparities in survival in lung transplant candidates with idiopathic pulmonary fibrosis. Am J Transplant. 2006;6(2):398–403. doi: 10.1111/j.1600-6143.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 80.Kizer JR, Zisman DA, Blumenthal NP, et al. Association between pulmonary fibrosis and coronary artery disease. Arch Intern Med. 2004;164(5):551–556. doi: 10.1001/archinte.164.5.551. [DOI] [PubMed] [Google Scholar]
- 81.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 82.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration. 2008;76(3):288–294. doi: 10.1159/000114246. [DOI] [PubMed] [Google Scholar]
- 83.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132(3):773–779. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 85.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 86.Cottin V, Le Pavec J, Prévot G, et al. GERM”O”P Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–111. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 87.Cottin V, Nunes H, Brillet PY, et al. Groupe d’Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM O P) Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 88.Mejía M, Carrillo G, Rojas-Serrano J, et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 89.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 90.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129(3):794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 91.Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131(2):438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82(4):661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richeldi L, Davies HR, Ferrara G, Franco F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2003;(3):CD002880. doi: 10.1002/14651858.CD002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demedts M, Behr J, Buhl R, et al. IFIGENIA Study Group High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 95.Felton VM, Borok Z, Willis BC. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L805–L812. doi: 10.1152/ajplung.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 97.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 98.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 99.Ferreira A, Garvey C, Connors GL, et al. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest. 2009;135(2):442–447. doi: 10.1378/chest.08-1458. [DOI] [PubMed] [Google Scholar]
- 100.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 101.McCurry KR, Shearon TH, Edwards LB, et al. Lung transplantation in the United States, 1998-2007. Am J Transplant. 2009;9(4 Pt 2):942–958. doi: 10.1111/j.1600-6143.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 102.Levine SM. Transplant/Immunology Network of the American College of Chest Physicians A survey of clinical practice of lung transplantation in North America. Chest. 2004;125(4):1224–1238. doi: 10.1378/chest.125.4.1224. [DOI] [PubMed] [Google Scholar]
- 103.Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of United Network for Organ Sharing data. J Am Coll Surg. 2009;208(3):400–409. doi: 10.1016/j.jamcollsurg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 104.Meyer DM, Edwards LB, Torres F, Jessen ME, Novick RJ. Impact of recipient age and procedure type on survival after lung transplantation for pulmonary fibrosis. Ann Thorac Surg. 2005;79(3):950–957. doi: 10.1016/j.athoracsur.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 105.Cantu E, III, Appel JZ, III, Hartwig MG, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78(4):1142–1151. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 106.Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125(3):533–542. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]