Abstract
Alvinocaridid shrimps are endemic species inhabiting hydrothermal vents and/or cold seeps. Although indirect evidences (genetic and lipid markers) suggest that their larval stages disperse widely and support large scale connectivity, larval life and mechanisms underlying dispersal are unknown in alvinocaridids. Here we provide for the first time detailed descriptions of the first larval stage (zoea I) of four alvinocaridid species: Rimicaris exoculata and Mirocaris fortunata from the Mid-Atlantic Ridge, Alvinocaris muricola from the Congo Basin and Nautilocaris saintlaurentae from the Western Pacific. The larvae were obtained from onboard hatching of brooding females (either at atmospheric pressure or at habitat pressure in hyperbaric chambers) and from the water column near adult habitats, sampled with plankton pumps or sediment traps. Major characteristics of the alvinocaridid larvae include undeveloped mandible and almost complete absence of setation in the inner margin of the mouth parts and maxillipeds. Although the larvae are very similar between the four species studied, some morphological features could be used for species identification. In addition, undeveloped mouthparts and the large amount of lipid reserves strongly support the occurrence of primary lecithotrophy in the early stage of alvinocaridids. Although lecithotrophy in decapod crustaceans is usually associated with abbreviated larval development, as a mechanism of larval retention, morphological and physiological evidences suggest the occurrence of an extended and lecithotrophic larval stage in the Alvinocarididae. These traits permit the colonization of widely dispersed and fragmented environments of hydrothermal vents and cold seeps. Distribution of larval traits along the phylogenetic reconstruction of the Alvinocarididae and related families suggest that lecithotrophy/planktotrophy and extended/abbreviated development have evolved independently along related families in all potential combinations. However, the Alvinocarididae is the only taxa with a combination of lecithotrophy and extended larval development.
Introduction
Shrimps of the family Alvinocarididae inhabit deep-waters in the Atlantic, Pacific and Indian Oceans, usually at depths greater than 1000 m [1]. They occur at hydrothermal vents and/or cold-seeps, and could represent the dominant species in these ecosystems, even in some cases forming large aggregations of thousands of individuals per m2 [2,3]. Some species such as Rimicaris exoculata harbour symbiotic bacteria in their gill chambers and within their guts, which supply nutrients to the shrimp in a complex mutualistic association [4], whereas other species depend on grazing on chemoautotrophic bacteria and detritus in their adult stage [2].
However, populations of these shrimps exhibit high genetic connectivity along the Mid-Atlantic Ridge [5], between different vent and cold seeps systems in the Atlantic [6] and in vents systems of the Western Pacific and Indian Oceans [7,8]. This suggests a notable ability for dispersion and migration in widely spaced habitats. Juveniles and adult stages of alvinocaridids inhabit close to the vents and cold seeps due to their dependence on bacterial-detrital grazing or to their need to supply their symbiotic bacteria with reduced compounds, limiting adult migration [4,6]. Colonization of new habitats and connectivity along their geographic range must be promoted by larval forms. However, information about larval stages is scarce, limited to reports of post-larvae in plankton samples [9,10], estimations of larval development mode based on egg size [11], and on board observations of larvae hatched from brooding females [10, 12–17]. In general, published data on larval forms of alvinocaridid shrimps include only brief descriptions or some illustrations, but without clues about important larval traits, differences between species or comparison with other caridean shrimps.
As other carideans, brooding females of alvinocaridids maintain their eggs below the abdomen during the embryonic development until the hatching, which is followed by a planktonic larval stage [15,17,18]. Larval history appears to occur in the water column, where, after a series of molts, post-larval stages recruit in the benthic system of hydrothermal vents and cold seeps. However no information is available about the duration, dispersion or development of larvae, and no field samples of larval stages have been reported at the present (except briefly by Miyake [19]). The absence of larval descriptions in this group is a gap to be resolved, because it could help to identify larvae in the plankton and elucidate mechanisms of dispersion and larval history. Also larval morphology can bring important cues about the early life history of the species [20] and provide useful morphological features to help interpreting both phylogenies [21,22] and ecology [23,24]. In this paper we describe the first larval stage of four genera of Alvinocarididae obtained from hatching of gravid females and some larvae identified from field plankton samples.
Material and Methods
Collection and preparation of larval stages
Brooding females of Rimicaris exoculata, Mirocaris fortunata, Nautilocaris saintlaurentae and Alvinocaris muricola were collected from hydrothermal vents on the Mid-Atlantic Ridge (R. exoculata and M. fortunata), in the Western Pacific (N. saintlaurentae), and at cold seeps of the Congo Basin (A. muricola) (Table 1). Shrimps were collected with a suction sampler connected to the submersible (Nautile or ROV VICTOR 6000), and in most of cases, were brought on board the research vessel (N/O L’Atalante or Pourquoi Pas?) without pressure compensation. Adult females with eggs in advanced stage of development were examined, and in some of them, eggs had hatched (maybe due to recovery stress). After hatching, active larvae, rests of eggs and no-motile larvae were fixed in 4% buffered formalin, 10% glutaraldehyde, or 96% ethanol. Adult females were preserved and identified by external morphology [25,26] and by DNA mitochondrial COI sequence (for N. saintlaurentae).
Table 1. Cruise and sample information for the larvae herein studied.
| Species | Sample | Location | Site | Coordinates | Cruise | Depth (m) | Date | Sampling gear |
|---|---|---|---|---|---|---|---|---|
| R. exoculata | Lab. hatched | MAR | Logatchev | 14°45'N; 44°57'W | Serpentine | 3037 | March 2007 | Suction sampler |
| R. exoculata | Lab. hatched | MAR | TAG | 26°08' N; 44°49' W | BICOSE | 3635 | January 2014 | Suction sampler |
| R. exoculata | Lab. hatched, with habitat pressure | MAR | TAG | 26°08' N; 44°49' W | BICOSE | 3635 | February 2014 | Suction sampler, pressure compensation |
| R. exoculata | Plankton samples | MAR | TAG | 26°08' N; 44°49' W | BICOSE | 3637 | February 2014 | Plankton pump |
| A. muricola | Lab. hatched | Gulf of Guinea | pockmark Regab | 05°48'S; 09°42' W | Biozaïre 2 | 3150 | November 2001 | Suction sampler |
| A. muricola | Plankton samples | Gulf of Guinea | pockmark Regab | 5°48' N; 9°42' W | Congolobe | 3150 | January 2012 | Sediment trap |
| M. fortunata | Lab. hatched | MAR | Lucky Strike | 37°17' N; 32°17' W | Momarsat | 1739 | September 2013 | Suction sampler |
| N. saintlaurentae | Lab. hatched | Wallis and Futuna | Fatu Kapa [30] | 14°N; 177°'W | Futuna 3 | 1554 | June 2012 | Suction sampler |
For the laboratory reared larvae, sampling information refers to the brooding female.
Brooding females of R. exoculata collected during the BICOSE cruise in January 2014 at the TAG site of the MAR were brought on board with pressure compensation in the PERISCOP chamber [27] and were maintained in the BALIST chamber [28] at 300 bars and 5°C during 24 hours. Hatching occurred in the pressure chamber and active larvae were separated after a short period of decompression. Larvae were maintained in the PICCEL chamber [29] at 300 bars and 8°C during 96 hours. Larvae hatched at habitat pressure were compared with the larvae hatched at atmospheric pressure in order to assess the potential effects of hatching at low pressure on larval morphology.
Additionally, larvae collected in deep-water plankton samples from different locations were studied. Larvae of alvinocaridid shrimps (6 specimens) from the Congo Basin were collected using sediment traps deployed at 3150 m depth at the cold seep site REGAB in the Congo Basin. On the MAR, a larval pump SALSA (Serial Autonomous Larval Sampler- Ifremer) deployed at the TAG site at 3637 m captured 6 specimens of alvinocaridid larvae, 3 of which are included in the present study (Table 1). The morphology of these larvae was compared with the larvae obtained from hatching on board for species identification.
For larvae obtained from on board hatching, from 8 to 15 larvae of each species were fully dissected, and mounted on slides for observations and drawings with a Leica-Leitz microscope with camera lucida. Measurement of total length (TL) and carapace length (CL) were performed for each specimen and variation in the number of setae and spines were noted, including variation between the appendices of the left and right side in the same specimen. For the larvae obtained from field samples, some specimens were dissected for observation and drawings. For general considerations during dissection, mounting, description and illustration we followed Clark et al. [31].
Some specimens from both laboratory hatching and field samples were prepared for Scanning Electron Microscopy (SEM) analyses. Specimens previously fixed in 2.5% glutaraldehyde (seawater) or in 4% formalin were washed (3 times, 5 min each) in distilled water and post-fixed in 0.8% osmium tetroxide (OsO4) for 1 h. Later the samples were washed 3 times in water and fixed again in OsO4 for 1h, rinsed (6 times, 5 min each) in distillated water and dehydrated in graded ethanol series (10, 25, 35, 50, 65, 70, 80, 90, 96, 100%, 5 min each, 3 times for the last 5 grades). Samples were submitted to a critical point (Leica EM CPD300), sputter in gold, and observed in SEM.
DNA extraction, amplification, sequencing and phylogenetic reconstruction
Extractions were performed from whole larvae, or 1–2 pleopods for the adult specimens collected at MAR and Western Pacific hydrothermal vents, using the CTAB method (cetyl trimethyl ammonium bromide [32]). Amplifications of the COI gene were performed in a 50 μL solution of 1X reaction buffer, 2 mM MgCl2, 0.25 mM dNTPs, 0.6 mM of each primer (LCOI1490 and HCOI2198 [33]), 1.25 U Taq polymerase and 1–4 μL extracted DNA (depending on the DNA concentration). We performed 35 cycles of amplification with an annealing temperature of 52°C. Similarly, amplifications of the 18S rRNA gene were performed in a 50 μL solution of 1X reaction buffer, 2 mM MgCl2, 0.4 mM dNTPs, 0.5 mM of each primer (18S1 Forward and 1498 Reverse [34]), 1.25 U Taq polymerase and 1 μL extracted DNA. We performed 30 cycles of amplification with an annealing temperature of 51°C. All PCR amplifications were conducted on a GeneAmp PCR system 9700 (Applied Biosystems). PCR products were purified and sequenced at Macrogen, Inc. (Netherlands) using the amplification primers for COI and for the 18S gene the following primers: 18S-3F, 18S-bi, 18S-1F, 18S-5r, 18S-1373F, 18S-505r [34–36].
Other sequences included in the phylogenetic reconstructions were obtained from different studies, mostly related with phylogeny and population genetics on alvinocaridids [5–7,37–41], and available in genbank (S1 Table and S2 Table). For the phylogenetic reconstructions, the sequences were aligned using MUSCLE [42]. Phylogenetic trees were constructed using Bayesian Inference (BI) in BEAST version 1.8 [43]. Configurations of the evolutionary model for each data set were selected according to the best-fit obtained from Model Generator [44]. The selected model of nucleotide substitution for the COI was HKY + I + G, considering a speciation Yule process. Whereas for the 18S, the best-fit model was HKY + G, with a speciation Yule process. 206 generations trees with sampling each 1000 generations were performed, the first 25% of the trees were discarded, the rest of the trees were summarized using Treeannotator. Robustness of the inferred trees was evaluated using posterior probabilities. For the COI phylogenetic reconstruction, few sequences (8 of the 201 COI sequences analyzed) obtained from Genbank were discarded due inconsistences (outgroup sorting, and non-monophyletic distribution of the same species) observed in early analyses.
Relationship of the DWCC phylogenetic reconstruction and larval traits
In order to interpret larval morphology traits in an ecological and evolutionary context, literature data on larval traits in related families were also examined. 18S rRNA nuclear gene was used to reconstruct a phylogeny of alvinocaridid shrimps and related families. According to Braken et al. [39], Li et al. [40] and Aznar-Cormano et al. [41] Alvinocarididae are closely related to 7 other families of mainly deep-water Caridea (Oplophoridae, Nematocarcinidae, Agostocarididae, Campylonotidae, Pasiphaeidae, and Psalidopodidae, with some differences between authors), herein mentioned as deep-water caridean clade (DWCC, although these are not the only deep-water families and include a few shallow-water taxa). Sequences of these major taxa were obtained from GenBank, including those used in the phylogenetic analyses of the former studies [39–41] and others as well (S2 Table). Moreover we obtained new sequences of the 18S gene for R. chacei, M. fortunata and N. saintlaurentae. Rather than bringing a new phylogenetic proposal for the group, our intent was to set an evolutionary framework to analyze the occurrence of larval traits in the DWCC.
In order to compare the phylogenetic relationships along the DWCC and the occurrence of larval traits, we sorted the larval information related to the species considered in the phylogenetic reconstruction. We considered general traits of the larval morphology as indicators of some aspects of the early larval biology. Undeveloped mouth parts (absence of incisive and molar processes in the mandible, and lack or poor setation and spination in the maxillule and maxilla) was considered as indicator of primary lecithotrophy, because the larvae is not able to take external source of food and so relies on its lipid reserves [45]. On the contrary, larvae with developed mouth parts were categorized as planktotrophic, because the larvae have the ability to capture and ingest food when it becomes available. However some planktotrophic larvae could also hatch with large amount of lipid reserves (usually accomplished by large eggs > 1 mm), which could enhance their nutritional flexibility [20]. Unfortunately the descriptions of lipid reserves in the group are scarce and range between just “oil drops” to isotopic analysis, and a more accurate classification distinguishing facultative lecithotrophic from planktotrophic larvae was limited in some cases.
Moreover the presence of pereiopods and/or pleopods in hatching larvae was considered as a trait related with abbreviated development [20,45]. In general, decapod crustacean larvae hatch without pereiopods or pleopods. The development of these structures occurs usually as the larvae molt into more advanced stages. The occurrence of these structures in the first stage suggests advanced development and reduction of the larval instars. For some species considered in our phylogenetic analysis, no information is available regarding the larval development. In these cases, traits were inferred from other species of the same genus with existing knowledge about larval traits (but without genetic data that prevented their inclusion in our dataset) (S2 Table). Although larvae of the same genus usually show interspecific differences in spines and setae, there is no evidence of variation within genera for the major larval traits herein considered (i.e. mouth parts development, occurrence of pereiopods and pleopods) in the DWCC.
Ethics statement
No specific permissions were required to collect the samples used here during the Serpentine and BICOSE cruises (international waters). During the cruises Biozaïre 2 and Congolobe, samples were collected with permission from the Gabon Government. Samples were collected during the cruise MOMARSAT with permission of the Azores Regional Government, Portugal, and during the Futuna cruise with permission of the French Government. The study did not involve endangered or protected species.
Results
Description of first larval stage in alvinocaridids shrimps
Rimicaris exoculata Williams and Rona, 1986
Mid-Atlantic Ridge (Figs 1, 2, 3A–3D, 3G and 3H). Dimensions: LC = 0.61±0.06 mm; LT = 2.41±0.12 mm.
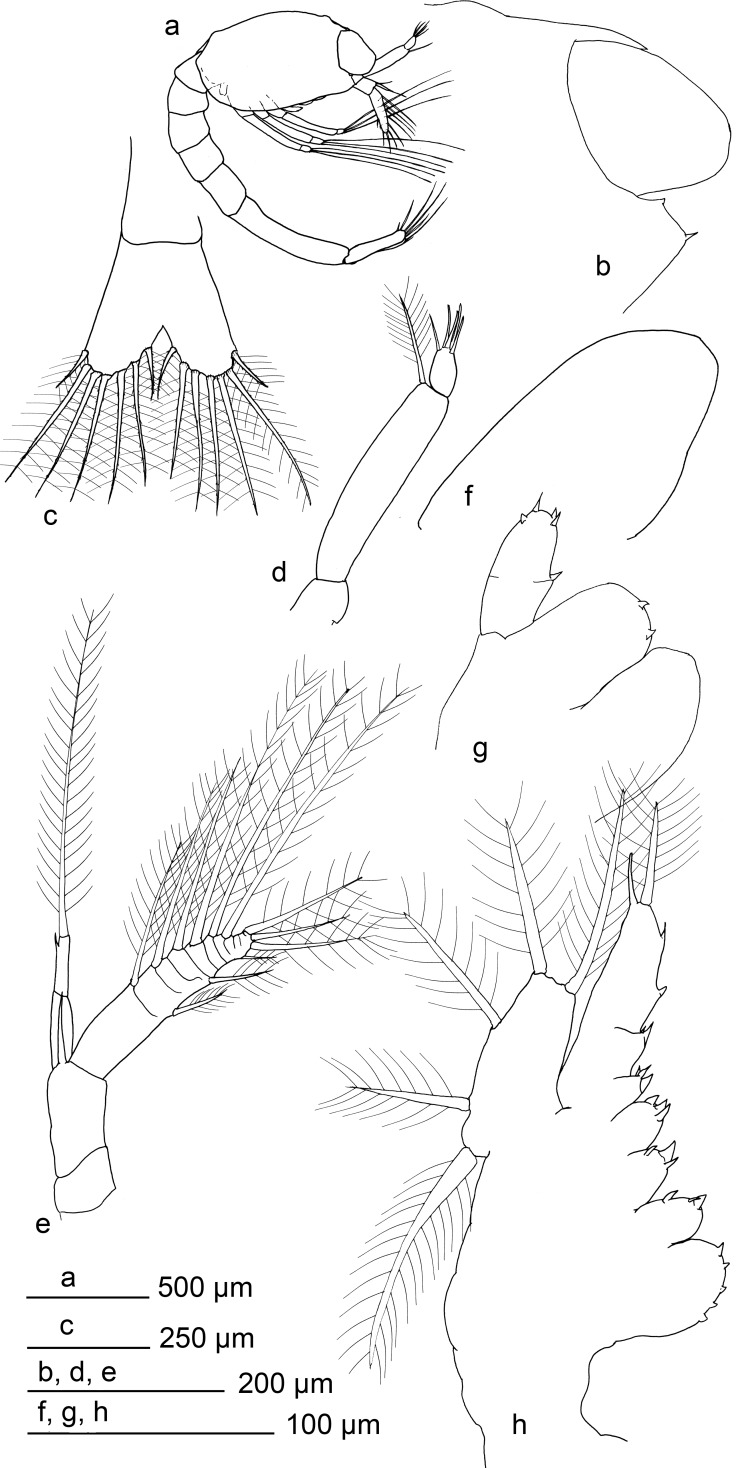
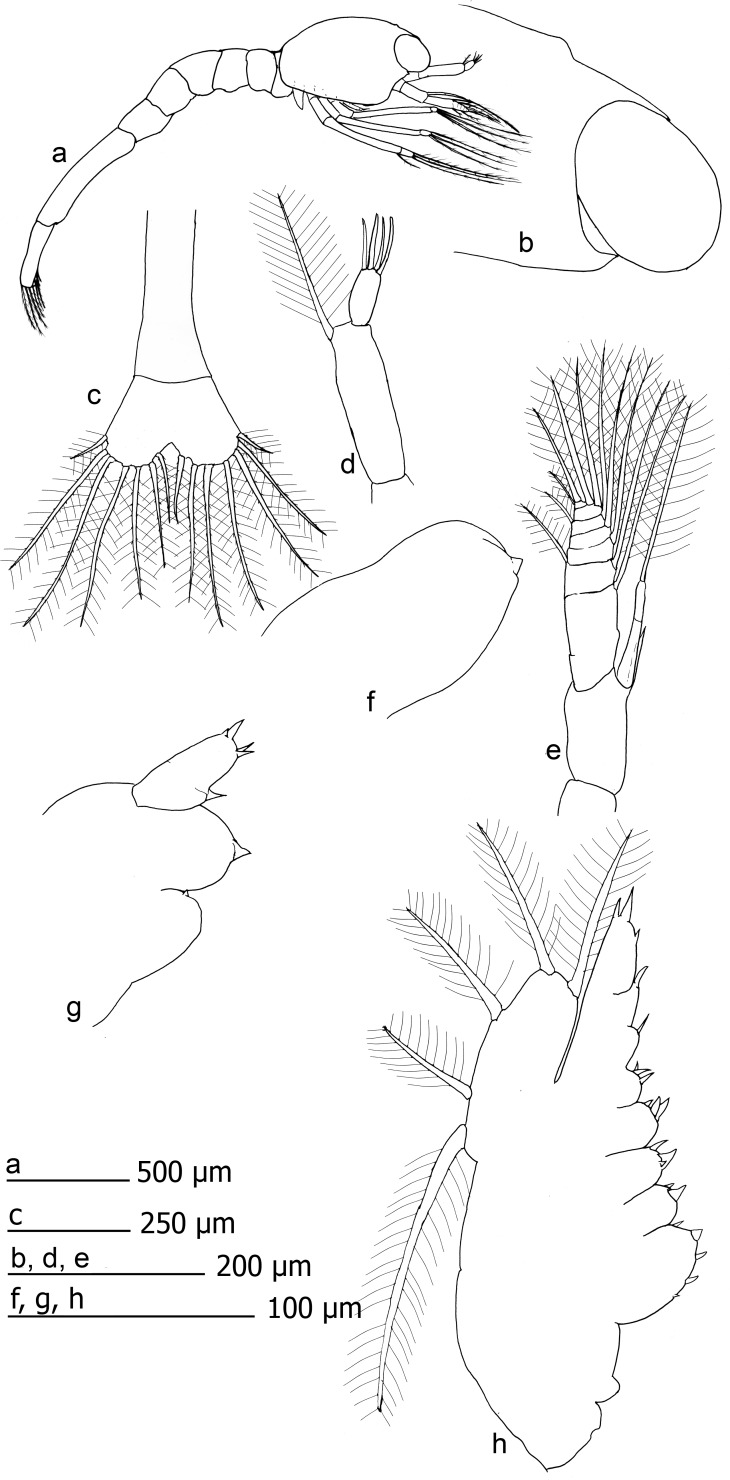
Fig 1. Rimicaris exoculata zoea I.
a) habitus, b) distal section of carapace, c) telson, d) antennule, e) antennae, f) mandible, g) maxillule, h) maxilla.
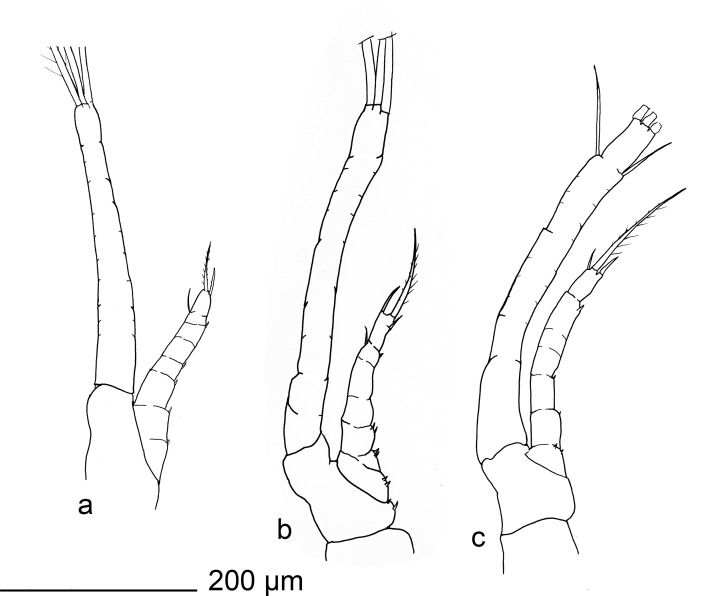
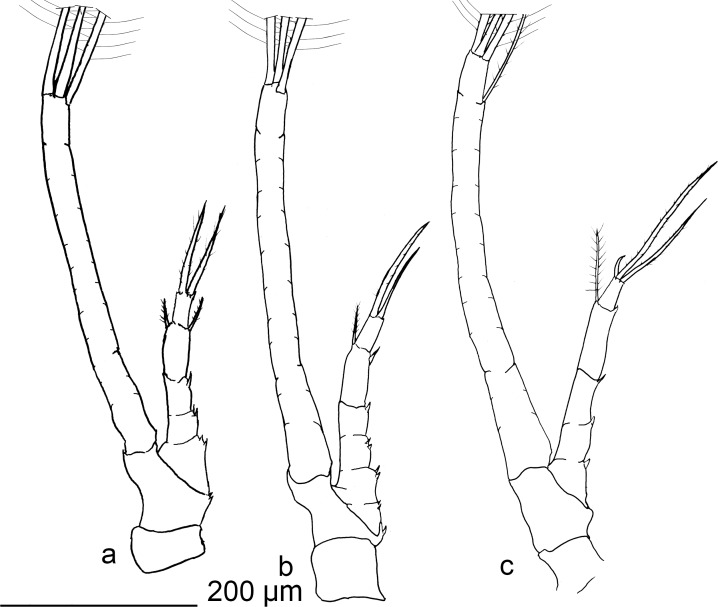
Fig 2. Rimicaris exoculata zoea I.
maxilipeds, a) first, b) second, c) third.
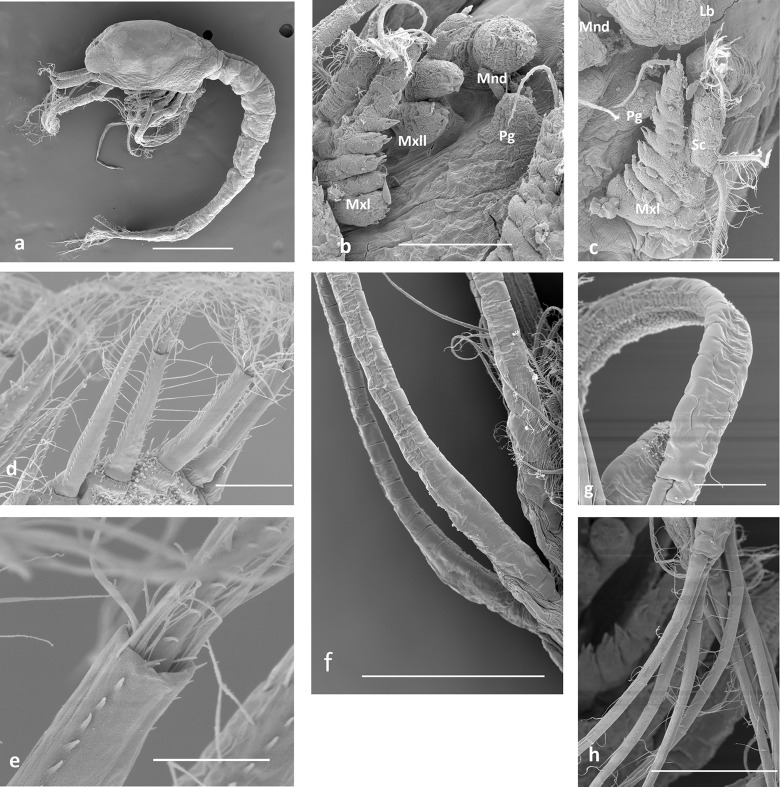
Fig 3. SEM details in some alvinocaridid zoea I larvae.
A.- Rimicaris exoculata, lateral view; b, c.- R. exoculata, mouth parts; d, e.- R. exoculata, plumodenticulate seta of telson; f.- Mirocaris fortunata, exopods of 1–3 maxillipeds; g, h.- R. exoculata, exopod of 2nd maxilliped and distal setae. Mnd, mandibule; Mxll, maxillule; Mxl, maxilla; Pg, paragnathe; Lb, labrum.
Carapace with tiny rostrum, sharp but hidden between the eyes, reaching around a half of the eyes length. Eyes sessile. A small anterior-dorsal hump present. Pterisgostomial spine present, followed by small sub-ocular spine. Posterior margin bilobulated in dorsal view. Antennulae uniramous, peduncle unsegmented with 1 distal setulose setae. Distal segment (outer flagellum) with 0–1 setae simple and 3–5 aesthetascs. Basal segment of the antennae (peduncle) unsegmented, armed with a large spine inserted near to the endopod, and approximately 0.6 times the length of the endopod. Endopod bi-articulated, second article with subdistal spine, absent in some specimens, and large setulose setae. Exopod distally segmented and armed with large setae (setulosae), 7 in the inner margin, 3 distally inserted and 2 smaller in the outer margin.
Labrum and paragnathes present, thumb-like. Mandible thumb-like, unsegmented. Incisive and molar processes absents, 0–3 small distal spines. Palp absent. Maxillule with 0–4 spines in coxal endite. Basal endite with 2–3 spines. Endopod composed by two segments, with mesial spine in the inner margin and 2–4 distal spines. Maxilla with endite coxal and basal bilobulated, coxal endite with 3–8 spines in the first lobule and 1–3 spine in the second. Basal endite with 2–4 spines in the first lobule and 1–3 spines in the second. Endopod unsegmented, with two proximal small lobes armed by 1–3 spines and 1–2 spines respectively. Followed by 2 subdistal spines, one distal spine in the outer margin and distal setulose setae 2–5 times the size of the distal spine. Margin of the scaphognathite with 5 marginal setulose setae.
Maxillipeds 1–3 similar in shape. Endopod with irregular segmentation, between 5–6 articles. Distal article with 1–3 plumodenticulate setae, occasionally with additional spine. Inner margin of endopod with 2–6 disperse, small spinules, sometimes with small subdistal setae. Exopod with superficial and incomplete segmentation, with a shallow furrow-like surface in the inner face and flat outer surface. Distally the exopod is armed with 3 large setulose setae. Pereiopod only as rudimentary bud, without segmentation, unarmed.
Abdomen with 6 somites, first segment overlap with the carapace. Last segment larger than others, thinner and dorsally compressed. Setae and spines absent. Ventral humps present in the 1–5 somites, pleopods and uropods absents. Telson bilobulated, distal margin with 7 plumodenticulate setae in each lobe.
Remarks: The larvae hatched in the BALIST chamber and maintained at 300 bars during 96 h did not show differences with the specimens hatched at atmospheric pressure. Although some variation occurs between specimens in the number of spines on the mouth parts and on the maxillipeds, their range completely overlap between the larvae hatched at atmospheric pressure or at habitat pressure. Hatching at atmospheric pressure does not appear to have an effect on the morphology of the larvae of R. exoculata and we assume that larvae of other species obtained after hatching at atmospheric pressure reflect then the morphology of those at their habitats.
Mirocaris fortunata (Martin and Christiansen, 1995)
Mid-Atlantic Ridge (Figs 3F and 4). Dimentions: LC = 0.56±0.027 mm; LT = 2.32±0.08 mm.
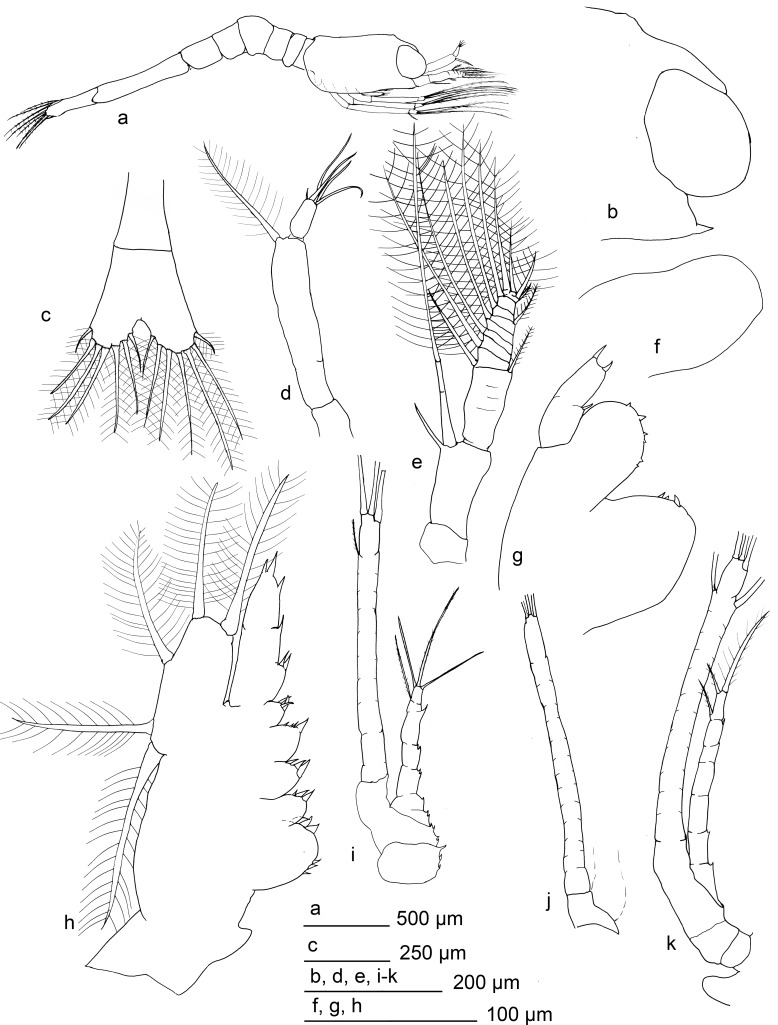
Fig 4. Mirocaris fortunata zoea I.
a) habitus, b) distal section of carapace, c) telson, d) antennule, e) antennae, f) mandible, g) maxillule, h) maxilla, i-k) first to third maxillipeds respectively.
Carapace with tiny rostrum, sharp but hidden between the eyes, reaching around a half of the eyes length. Eyes sessile. A small anterior-dorsal hump present. Pterisgostomial spine present. Posterior lateral margin bilobulated in dorsal view. Antennulae uniramous, peduncle unsegmented, setulose setae inserted distally. Distal joint (outer flagellum) with 3–5 aesthetascs and, eventually, 1 setae. Antennae with basal segment (peduncle) unsegmented, armed with a distal spine 0.6 times the size of the endopod. Endopod bi-articulated, second article with tiny subdistal spine, sometimes absent, and large setulose setae. Exopod distally segmented and armed with large setae (setulosae), 7 in the inner margin, 2–3 distally inserted and 2 smaller in the outer margin.
Mandible thumb-like, unsegmented. Incisive and molar processes absents, unarmed or with 1–2 small spines. Palp absent. Labrum and paragnathes present, thumb-like, unarmed. Maxillule with coxal endite with 2–4 distal spines. Basal endite with 3–6 spines, some minute. Endopod composed by two segments, with mesial spine in the inner margin and 2 distal spines. Maxilla with two lobules in coxal and basal endites. Coxal endite with 5–6 spines in the first lobule and 2–3 spines in the second. Basal endite with 3 and 4 spines in each lobule. Endopod unsegmented, with two proximal small lobes armed with 3 and 2 spines respectively, followed by 2 subdistal spines and two distal spines. Margin of the scaphognathite with 5 marginal setulose setae.
Maxillipeds 1–3 similar in shape. Endopod with segmentation irregular, uncomplete. Subdistal setae present followed by 2–3 distal setae, irregular in size, setulose. Inner margin of endopod with 5–6 disperse, small spinules. The first maxilliped shows up to 13 spines between the margin of the endopod and coxal-basal margin. Exopod with superficial incomplete segmentation, with 3 large setulose setae distally inserted. Inner and outer faces of exopod show a flat surface, inner also with small furrow. Some specimens also with 1–2 thin sub-distal setae. Pereiopods only as rudimentary bud, without segmentation, unarmed.
Abdomen with 6 somites, first segment overlap with the carapace. Last somite larger and laterally thinner. Setae and spines absent. Ventral humps present in the 1–5 somites, pleopods and uropods absent. Telson bilobulated, distal margin with 7 plumodenticulate setae in each lobe.
Nautilocaris saintlaurentae Komai & Segonzac, 2004
Western Pacific (Figs 5 and 6). Dimensions: LC = 0.58±0.004 mm; LT = 2.20±0.01 mm.
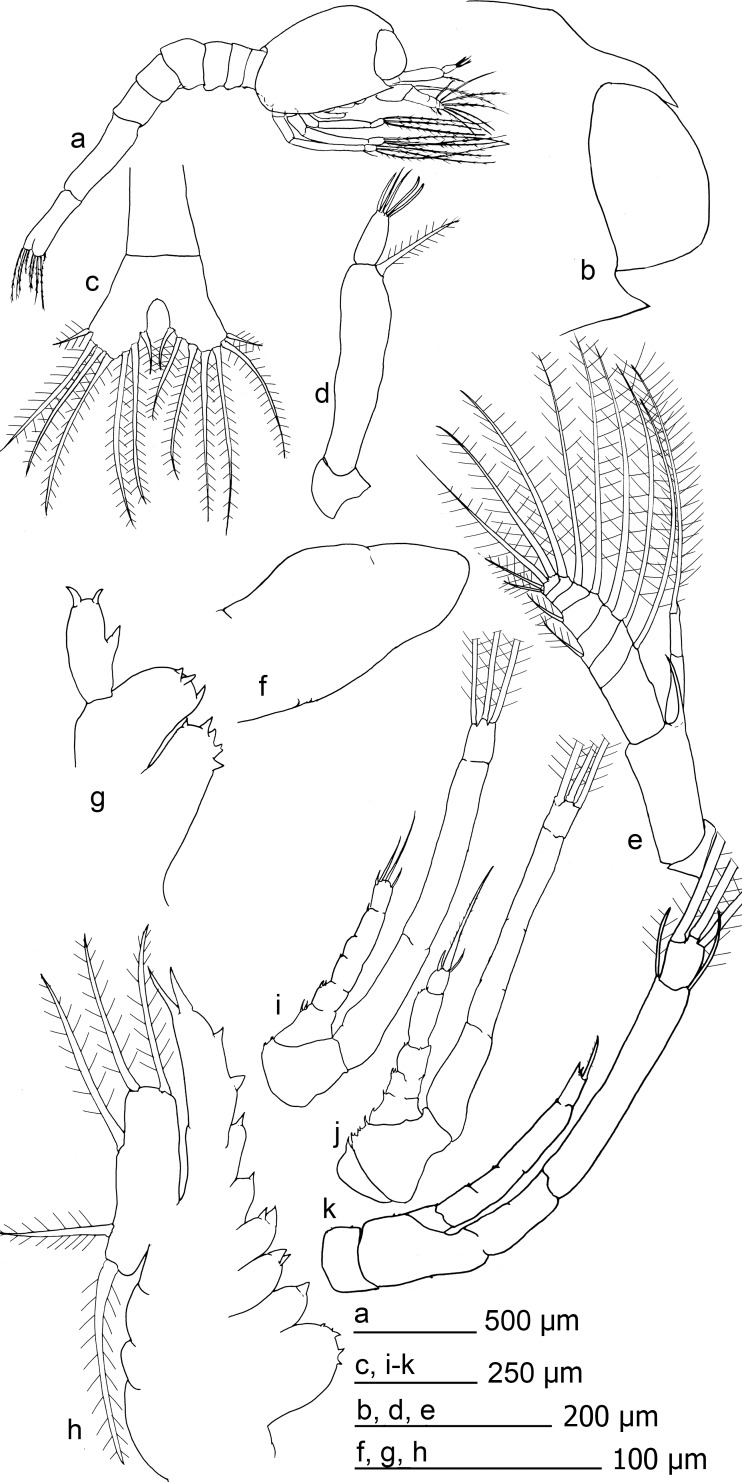
Fig 5. Nautilocaris saintlaurentae zoea I.
a) habitus, b) distal section of carapace, c) telson, d) antennule, e) antenna, f) mandible, g) maxillule, h) maxilla.
Fig 6. Nautilocaris saintlaurentae zoea I.
maxillipeds, a-c) first to third respectively.
Carapace armed with tiny rostrum, sharp but hidden between the eyes, reaching around a half of the eyes length. Eyes sessile. A small anterior-dorsal hump present. Pterisgostomial spine present. Posterior margin laterally bilobulated. Antennulae uniramous, peduncle unsegmented, setulose setae inserted close to distal joint. Distal segment (outer flagellum) with 3–5 aesthetascs. Basal segment of the antennae (peduncle) unsegmented, with large spine inserted near to the endopod (0.6–1.1 times the size of the endopod). Endopod bi-articulated, second article usually with small subdistal spine and large setulose setae. Exodop distally segmented and armed with large setae (setulosae), 6–7 in the inner margin, 2–3 distally inserted, and 2 smaller in the outer margin.
Mandible thumb-like, unsegmented. Incisive and molar processes absents, unarmed or with 1–2 small spines. Palp absent. Labrum and paragnathes present, thumb-like, unarmed. Maxillule with coxal endite with 0–5 small spines. Basal endite with 1–4 spines, some minute. Endopod composed by two segments, with 1–2 mesial spines in the inner margin and 2–4 distal spines. Maxilla with endite coxal and basal bilobulated, coxal endite with 4–8 spines in the first lobule and 2–3 spines in the second. Basal endite with 2–3 spines in the first lobule and 2–4 in the second. Endopod unsegmented, with two proximal small lobes armed with 1–3 and 1–2 spines respectively, followed by 2 subdistal spines. Distal margin with two spines, a single spine in some specimens. Margin of the scaphognathite with 5 setulose setae.
Maxillipeds 1–3 similar in shape. Endopod with segmentation irregular, 3–6 joints. Distal joint with 2–3 setae, and 1–2 small subdistal setae, all setulose. Inner margin of endopod with 0–8 disperse, small spinules. Exopod show superficial segmentation at lateral sides and flat inner and outer faces. Small furrow also on the inner face of expodod. Three large setulose setae inserted distally. Some specimens also with 1–2 sub-distal setae. Pereiopods only as rudimentary bud, unarmed.
Abdomen with 6 somites, first segment overlap with the carapace. Last somite larger and laterally thinner. Setae and spines absent. Ventral humps present in the 1–5 somites, pleopods and uropods absent. Telson bilobulated, distal margin with 7 plumodenticulate setae in each lobe.
Alvinocaris muricola Williams, 1988
Eastern Atlantic, Congo Basin (Fig 7). Dimensions: LC = 0.57±0.02 mm; LT = 2.13±0.07 mm.
Fig 7. Alvinocaris muricola zoea I.
a) habitus, b) distal section of carapace, c) telson, d) antennule, e) antenna, f) mandible, g) maxillule, h) maxilla, i-k) first to third maxillipeds respectively.
Carapace with tiny rostrum, sharp but hidden between the eyes, reaching around a half of the eyes length. Eyes sessile. A small anterior-dorsal hump present. Pterisgostomial spine present. Posterior margin laterally bilobulated. Antennulae uniramous, peduncle unsegmented, large setulose setae inserted close to distal joint. Distal segment (outer flagellum) with 5 aesthetascs. Antennae with basal segment (peduncle) unsegmented, armed by a large spine inserted near to the endopod. Endopod bi-articulated, second article with distal spine and large setulose setae. Exopod distally segmented and armed with large setae (setulosae), 6 in the inner margin, 2 large and 2 small setae distally inserted, 2 small setae inserted in the outer margin.
Mandible thumb-like, unsegmented. Incisive and molar processes absents, unarmed. Palp absent. Labrum and paragnathes present, thumb-like, unarmed. Maxillule with coxal endite with 5 strong spines, one bifid. Basal endite with 3 spines. Endopod composed by single segment, with mesial spine in the inner margin and 2 curved spines distally. Maxilla with coxal and basal endites bilobulated, coxal endite with 5 small and 1 large spines in each lobule, and basal endite with 2 spines in each lobule. Endopod unsegmented, with two proximal lobes armed with 1 spine each followed by 2 subdistal spines, one distal small and thick setae (setulose) and distal spine. Margin of the scaphognathite with 5 setulose setae.
Maxillipeds 1–3 similar in shape. Endopod with segmentation irregular, composed by 4–8 joints. Distal joint with 1 large and 2–3 small setae. Inner margin of endopod with 6–8 disperse, small spinules and 4–5 spinules in the basis. Exopod superficially segmented in lateral sides, distally armed 3 large setulose setae and occasionally with 1–2 small sub-distal setae. Inner and outer faces of the endopod flat, with small furrow in the inner surface. Pereiopods only as rudimentary bud, without segmentation, unarmed.
Abdomen with 6 somites, first segment overlaps with the carapace. Last somite larger and laterally thinner. Setae and spines absent. Ventral humps present in the 1–5 somites, pleopods and uropods absent. Telson bilobulated, distal margin with 7 plumodenticulate setae in each lobe.
Morphological identification of alvinocaridid larvae collected by plankton samplers
The larvae collected with the plankton pump on the MAR show the same morphological features as the larvae hatched from R. exoculata females onboard. These characters include the setae at the tip of the endopod of the maxilla and the small subocular spine, which are distinct from other species herein studied. No other species except R. exoculata exhibit a subocular spine in the carapace, although this small spine was, rarely, absent in some R. exoculata specimens. Moreover the setae at the tip of the endopod of the maxilla is present in R. exoculata and A. muricola, but in the latter species, it is variable in shape from a spine to a small and thick setae with few setulae. Other species, M. fortunata and N. saintlaurentae only show two spines at the tip of the endopod.
The shrimp larvae collected with the sediment traps in the Congo Basin shows similar characters as those of specimens of A. muricola that hatched onboard. Although all alvinocaridid species herein studied are very similar to each other and the knowledge of alvinocaridid larval morphological variation is still low, a thick short setae at the tip of the endopod of the maxilla was observed only in A. muricola and in the specimens collected with sediment traps. Moreover A. muricola is the only alvinocaridid species in the Congo cold seeps. The other species with a distal setae in the maxilla endopod, R. exoculata, shows a long setae and a large spine besides, and also usually a small subocular spine, which is absent in A. muricola.
Molecular identification of alvinocaridid larvae
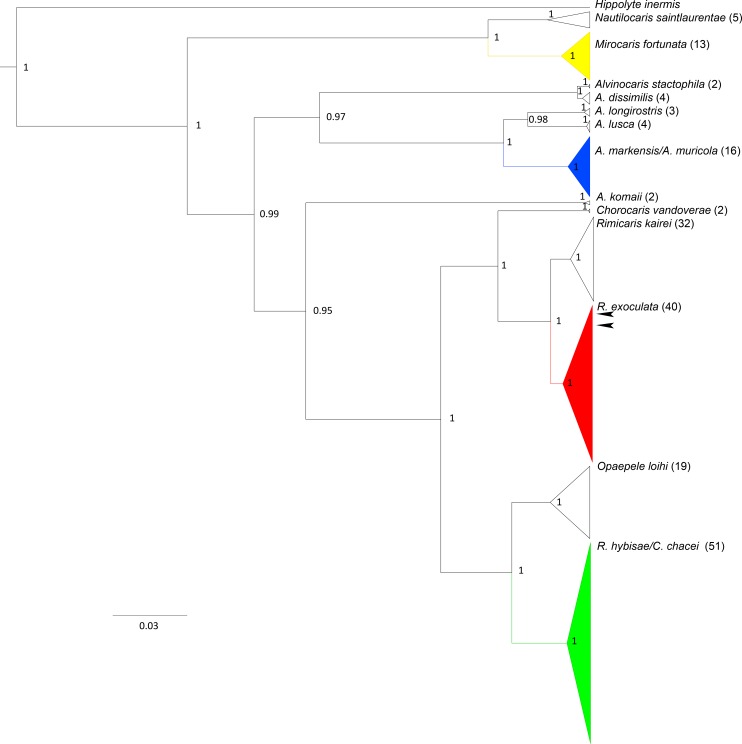
The phylogenetic tree obtained from Bayesian inferences with the COI gene shows three very divergent clades (Fig 8). The first clade consists of the genera Mirocaris and Nautilocaris, and includes the N. saintlaurentae hatching female considered in the present study. It is a sister group of the rest of Alvinocarididae. The second clade includes all species of Alvinocaris (6 spp.) except A. komaii. This group includes the sequence of Alvinocaridid larvae studied by Koyama et al. [10] which is affiliated with sequences of Alvinocaris longirostris. The third clade includes the genera Rimicaris, Chorocaris and Opaepele, in addition to one species of Alvinocaris (A. komaii). This 3-clade configuration is consistent with previous phylogenetic reconstruction based in COI gene [37], including the position of A. komaii [46]. The two species complexes formed by Alvinocaris muricola/markensis and by Rimicaris chacei/hybisae, both proposed by Teixeira et al. [6] and confirmed by Vereshchaka et al. [1] also appear in our phylogenetic analysis. This tree topology was expected since we used the same data as those presented by former authors, and it is further supported in our analysis which also includes a new sequences of R. hybisae obtained by Plouviez et al. [38]. All the species and species-complexes in our analysis are well supported by the posterior probabilities of the Bayesian Inference. The larvae collected with the larval pump at the TAG site on the MAR and identified from their morphology as R. exoculata, fall in our tree with the other R. exoculata samples. The large divergence with other alvinocaridid species from the MAR brings no doubt about the genetic confirmation of the morphological identification of these larvae.
Fig 8. Phylogenetic relationships of Alvinocarididae shrimps based on the Bayesian Inference of COI gene using HYK + I + G evolutionary model.
Species or monophyletic species-complex are cartooned and MAR species are in colors. Arrows shown the position of the larvae collected in the plankton samples of the MAR. Number of sequences in parentheses.
Larval traits along the DWCC
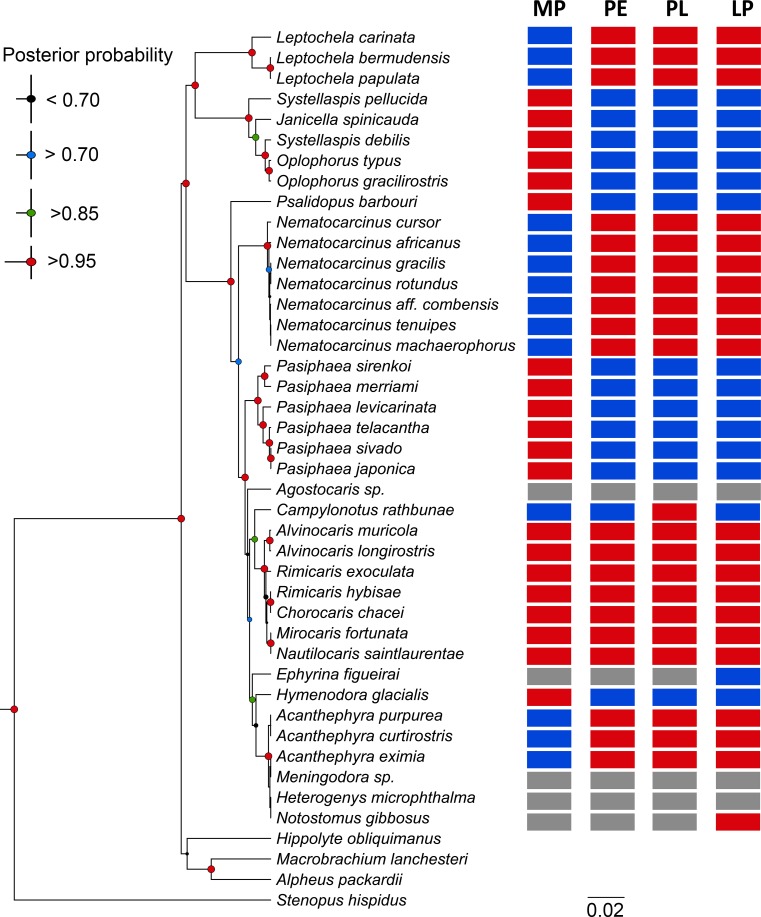
Strong phylogenetic reconstructions of the DWCC based on several nuclear and mitochondrial loci are already published, but they do not necessarily include species with published information on larval traits. Here we selected species from different DWCC families with such knowledge as well as genetic information (most of the time 18S rRNA sequence) available. Our phylogenetic reconstruction of the DWCC based on the 18S gene (Fig 9) shows some differences with previous reconstructions [39–41], particularly in the position of Acanthephyridae and some families represented by single species (Campylonotidae, Psalidopodidae and Agostocarididae). These differences are attributed to the taxa coverage and locus selection. However the general topology of the tree is in agreement with some of the important phylogenetic features in the group previously suggested. Particularly, our tree also reflects the separation of Acanthephyridae sensu stricto from Ophoploridae [39,41,47], the polyphyletic status of Pasiphaeidae [39], and retains the monophyletic status of Nematocarcinidae [39,40] (although our analysis includes a single genus) and Alvinocarididae [1, 37,39–41,46–48]. Moreover in all genera with more than one species (except Acanthephyra and Systellaspis) the genetic relationship was closer within genus. Since we recover here the main trends previously proposed in broader analyses, we consider our tree suitable to make inferences about larval morphology and ecology.
Fig 9. Phylogenetic relationships between Alvinocarididae and related families and distribution of larval traits along the tree.
Phylogenetic reconstruction is based on the Bayesian Inference of 18S gene using HYK + G evolutionary model. Larval traits of first zoeal stage: MP, mouth parts developed (blue), non-developed (red); PE, pereiopods present (blue), absent (red); PL, pleopods present (blue), absent (red); LP, larval development abbreviated (blue), extended (red). Gray squares, non-information available.
Based on general traits of the first larval stage (mouth parts development and pereiopod or pleopod development), DWCC considered here could be separated in four main groups: 1: lack of pereiopod and pleopods, and developed mouth parts, 2: presence of pereiopod or pleopods and developed mouth parts, 3: presence of pereiopod or pleopods and undeveloped mouth parts, 4: lack of pereiopod and pleopods, and undeveloped mouth parts. The first group includes species from several families (Pasiphaeidae: Leptochela, Nematocarcinidae: Nematocarcinus, Acanthephyridae: Acanthephyra). Larval traits associated with planktotrophic and extended development are considered plesiomorphic due to their general distribution in decapod crustaceans in both shallow and deep-water habitats and their occurrence in the basal taxa of Caridea (Disciadidae and Rhynchocinetidae) according Bracken et al. [39] and Aznar-Cormano et al. [41]. The second group includes Campylonotidae. Facultative primary lecithotrophy is also present in this group [49]. The third group, with abbreviated larval development (hatching in advanced stage) and undeveloped mouth parts, typically exhibits lecithotrophy, which is found in several families (Ophophoridae, Pasiphaeidae: Pasiphaea, Acanthephyridae: Hymenodora and Psalidopodidae). The forth combination of traits is found only in Alvinocarididae.
Discussion
Comparison of larvae between alvinocaridid species
Early stages of Alvinocarididae exhibit particular features such as a notorious lack of development in mouth parts and few setation in the inner margin of the maxillipeds. This observation is not an artifact due to abnormally precocious hatching caused by recovery stress. The absence of a cover layer over the appendices and telson confirms that larvae analyzed here are at first zoeal stage instead of a prezoea stage [50,51] that could occur just after hatching. Moreover, the similarity between the larvae of R. exoculata obtained on board from hatching at atmospheric pressure or in pressurized chambers and in the plankton pump also demonstrates that the low degree of development of mouth parts is neither the product of an ontogenetic anomaly caused by the pre-hatching decompression nor abnormal early hatching of undeveloped larvae.
The absence of setation on the mouth parts (except for the scaphonagtite, and the tip of the endopod of the maxilla) and on the endopod margin of the maxillipeds, with the almost complete absence of spines on the carapace and abdomen bring a configuration very similar between the larvae herein studied. Spinulation of mouth parts shows intraspecific variation that overlaps interspecific variation. However R. exoculata can be separated from the other species by the presence of a spine and setulose setae on the distal section of the endopod of the maxilla and a small sub-ocular spine on the carapace, additional to the pterigostomian spine. The other species studied exhibit two spines at the tip of the endopod of the maxilla, or one spine and one thick setae (with few or no setulae), but only the pterigostomian spine on the carapace. In addition, the distal spine projected on the external margin of the endopod of the maxilla is usually larger in R. exoculata than in other species. A. muricola can be distinguished by the occurrence of a thick setae at the tip of the endopod of the maxilla, although this character showed some variation from small simple spine-like setae to large setae occasionally with few setulae (resembling R. exoculata). The intraspecific variation in spinulation completely overlaps between M. fortunata and N. saintlaurentae, limiting the distinction of larvae of the 2 species based on morphological characters (Table 2).
Table 2. Variation in larval structures in Alvinocaridid larvae.
| R. exoculata | M. fortunata | N. saintlaurentae | A. muricola | |||||
|---|---|---|---|---|---|---|---|---|
| MF | Range | MF | Range | MF | Range | MF | Range | |
| Carapace | ||||||||
| Subocular spine | p | ra-p | a | a | a | |||
| Antennule | ||||||||
| Aesthetascs | 4 | 3–5 | 4 | 3–5 | 4 | 3–5 | 4 | 4–5 |
| Other spines or setae | 0 | 0–1 | 1 | 0–1 | ||||
| Antenna | ||||||||
| Basal spine ratio/endopod | 0.6 | 0.4-.06 | 0.6 | 0.6–0.7 | 0.8 | 0.6–1.1 | 0.6 | 0.4–0.6 |
| Endopod, Nbr of joints | 2 | 1–2 | 2 | 1–2 | 2 | 2 | ||
| Spine in the last joint | l, sd | a-l, sd-d | s, d | a-s | s, sd | s-l | d, l | s-l |
| Mandible | ||||||||
| Nbr of spines | 1 | 0-3s | 0 | 0–2 | 0 | 0–2 | 0 | 0–1 |
| Maxillule | ||||||||
| Nbr spines coxal endite | 2 | 0–4 | 3 | 2–4 | 4 | 0–5 | 5 | 3–7 |
| Nbr spines basal endite | 2 | 2–3 | 3 | 3–6 | 2 | 1–4 | 3 | 1–3 |
| Subdistal spine endopod | 1 | 1–2 | 1 | 1 | 1–2 | 1 | 1–3 | |
| Nbr of distal spines | 2 | 2–4 | 2 | 3 | 2–4 | 2 | ||
| Maxilla | ||||||||
| Spines lobe 1 coxal | 5 | 3–8 | 6 | 5–6 | 5 | 4–8 | 5 | 4–8 |
| Spines lobe 2 coxal | 3 | 1–3 | 3 | 2–3 | 3 | 2–3 | 2 | 2–3 |
| Spines lobe 1 basal | 3 | 2–4 | 3 | 2–3 | 3 | 2–3 | 3 | 2–3 |
| Spines lobe 2 basal | 3 | 2–4 | 3 | 2–4 | 3 | 2–4 | 3 | 2–4 |
| Spines lobe 1 endopod | 3 | 1–3 | 3 | 3 | 1–3 | 3 | 1–3 | |
| Spines lobe 2 endopod | 2 | 1–2 | 1 | 1–2 | 2 | 1–2 | 1 | 1–2 |
| Inner distal projection | st | sp | sp | st | 1–2 | |||
| Size of inner distal projection | l | m-l | S | s | s | 1–2, s-l | ||
| Outer distal spine | m | m-l | S | s | a-s | s | a-l | |
| Maxilliped 1 | ||||||||
| Endopod segmentation | 5 | 5–6 | 4 | 4–6 | 6 | i5-6 | 5 | |
| Distal setae | 2pd | 1-2pd+ 0–1, s+ 1-3sp | 2pd | 2-3pd +1sp | 3pd | 2-3pd +1-2sp | 1pd+1sp | 1-2sp |
| Spinules inner margin | 6 | 2–8 | 2 | 2–8 | 5 | 3–11 | 4 | 2–5 |
| Spinules in the basis | 2 | 0–8 | 4 | 0–4 | 3 | 0–5 | 3 | 0–4 |
| Exopod segmentation | 3 | 3 | 3 | 2–3 | 3 | |||
| Distal setae | 3 | 3 | 1–3 | 3 | 2–3 | 3 | 2–3 | |
| Subdistal Setae | 0 | 0–1 | 1 | 0–1 | 0 | 0–1 | 0–1 | 0–2 |
| Maxilliped 2 | ||||||||
| Endopod segmentation | 6 | 5–6 | i4 | 3–6 | i6 | 3–6 | 6 | 5–6 |
| Distal setae | 2 | 1–3+0-1s+ 0-1sp | 2 | 2–3+1sd, s | 2 | 2–3+1sd, s | 1 | 1–2+0-2s+1-2sp |
| Spinules inner margin | 5 | 0–5 | 4 | 4–10 | 3 | 3–8 | 3 | 3–4 |
| Spinules in the basis | 0 | 0–3 | 0 | 0–3 | 1 | 0–4 | 3 | 2–3 |
| Exopod segmentation | 3 | 3 | 2–4 | 3 | 2–3 | 3 | ||
| Distal setae | 3 | 3 | 3–4 | 3 | 2–3 | 0 | ||
| Subdistal Setae | 0 | 0 | 0–3 | 1 | 0–1 | |||
| Maxilliped 3 | ||||||||
| Endopod segmentation | 5 | 5–6 | i4 | 4–6 | i6 | 3–6 | 6 | 5–6 |
| Distal setae | 2 | 1–2+ 0-1s+ 0-1sp | 2 | 2–3+1sd | 2 | 2–3+1sd | 2 sp | 0–2+0-1sp |
| Spinules inner margin | 3 | 0–5 | 5 | 4–11 | 0 | 0–4 | 2 | 2–4 |
| Spinules in the basis | 0 | 0–2 | 0 | 0–2 | 0 | 3 | 2–3 | |
| Exopod segmentation | 3 | 3 | 3 | 3 | ||||
| Distal setae | 3 | 3 | 3 | 2–4 | 3 | |||
| Subdistal Setae | 0 | 0–2 | 0 | 0–2 | 1 | 1–2 | 0 | |
Columns show the mean or most frequent number or character (MF) and range. For each larval feature (only features with variation is considered). Keynote: a, absent; p, present; ra, rarely absent; s, small; m, medium; l, large; st, setae; sp, spine; d, distal; sd, subdistal; i, irregular. X-X denote a range and X+X denote additional structure (e.g. Distal setae: 0–2+0-1s = from 0 to 2 setae and from 0 to 1 small setae).
The larvae collected in plankton samples from Regab in the Gulf of Guinea are identified as first larval stage of A. muricola. These larvae, as well as those collected from a gravid female from the same site, showed a higher degree of morphological variations than zoea from the three other species herein studied. These variations were not related to growth and molting to the next larval stages since we did not observe changes in size or new structures (i.e. more setation or changes in size and shape in the appendices) as it would be expected after molting, as compared with the larvae obtained from onboard hatching. Adults and juveniles of A. muricola exhibit a wide range of morphological variations in some characters such as rostrum and carapace width [25]. A. muricola is the only alvinocaridid species known to inhabit the Congo basin cold seeps so far. However, this species is also found in the Barbados Prism, the Gulf of Mexico and the Blake Diapir [52]. Moreover, phylogenetic studies based on both mitochondrial (COI) and nuclear (18S rRNA) genes suggest that A. muricola and A. markensis from hydrothermal vents on the MAR are a single species [6]. A. muricola thus seems to be a morphologically plastic species, with plasticity also occurring in larvae as observed here, widely distributed and able to colonize different habitats, although morphological variability has not been explicitly related to specific locations or habitats so far.
Larvae collected with the plankton pump at the TAG vent field on the MAR were identified as first zoea stage of R. exoculata based on their external morphology. Both specimens examined show a tiny subocular spine and large setae at the tip of the endopod of the maxilla, which is consistent with the description in R. exoculata. This identification is also confirmed by COI barcode. The occurrence of ovigerous females bearing eggs close to hatching (pers. observation) explain the presence of early larval stages in the water column near the vent, which probably originates from TAG and have not yet dispersed away.
The close similarities between the larvae of Rimicaris, Mirocaris, Nautilocaris and Alvinocaris and their differences with other carideans (see next section), support the monophyletic status of Alvinocarididae previously suggested based in molecular phylogeny [37,39–41] and morphology, although genetic evidence suggests that the generic relationships within the family require a revision [1, 6,37]. Unfortunately, our taxa coverage and the overlapping of larval characters between the species herein studied preclude any approximation of within family phylogenetic relationship based on larval morphology.
Morphology and larval biology of alvinocaridids
The absence of both masticatory processes in the mandible and setation in mouthparts that could participate in the process of capture and manipulation of food suggests that the alvinocaridid first larval stage is a non-feeding larva. This contrasts with previous hypothesis of planktotrophic larval nutrition in Alvinocarididae [9,11,18,53] based on indirect (egg size) evidence [11]. Although egg size in alvinocaridids is relatively smaller than in other species with lecithotrophic larvae [54], the accumulation of triacylgycerols, wax esters and monounsaturated fatty acids in eggs of alvinocaridid species [55] supports the hypothesis of primary lecithotrophy in this family. The occurrence of lipid storage in early stages is common in decapod larvae for standing eventual starvation or even lecithotrophy [20], but the suppression of feeding structures is rare. Lipid reserves in a lecithotrophic larvae or lacking of developed feeding structures has been documented previously in land crabs [56,57], symbiotic crabs [58], symmetric hermit crabs [59–61], galatheids [62,63], alpheid shrimps [64,65], freshwater shrimps (Atydae, [66]) and in some deep water Oplophoridae, Acanthephyridae and Pasiphaeidae [66, 67]. Larvae of these species also exhibit developed pleopods or pereiopods, which are common in late larvae, suggesting abbreviated development. These features are associated with restricted larval dispersal due to the short development period and larval or postlarval retention [56,58,64,68]. In alvinocaridids, although the early larval stage is lecithotrophic, the absence of pleopod and pereiopod does not support abbreviated development and genetic connectivity also suggest high dispersal [5,6,51]. Moreover, Koyama et al. [13] report that the early stage (zoea I) larvae of Alvinocaris sp. (which belong to A. longirostris according to our COI analysis) could survive for at least 74 days under laboratory conditions (atmospheric pressure, 4.5°C).
Related taxa (Pasiphaeidae) do not develop mouthparts during the larval stage and could survive without food source until the post-larvae [69], however these larvae also have abbreviated development that could be completed in 12 days (at 13°C). In alvinocaridids, the large amount of lipid reserve could be not enough to support the survivorship and growth in a complete extended larval development. According to Pond et al. [55] adults and eggs of M. fortunata have a similar lipid composition, dominated by monounsaturated and saturated fatty acids, lipids being transferred from the parent during vitellogenesis. However, between adults of both R. exoculata and M. fortunata and their respective juveniles, a shift in lipid composition occurs, with lipids in the juveniles dominated by polyunsaturated fatty acids and with different isotopic signature [55,70]. This suggests the accumulation of new lipid reserves before the recruitment to the vents, which could supply, at least partially the period between the recruitment, maturity and the acquisition of symbiotic bacteria [16]. Since the generation of new lipid reserves usually requires feeding, according to Pond et al. from a photosynthetic carbon origin [55,70], it is expected that the early lecithotrophic period would be followed by a feeding period during the larval development.
Although lipid composition of R. exoculata, R. chacei, A. markensis and M. fortunata at juvenile stage suggest feeding from photosynthetic source [55,70], similar to bathypelagic shrimps living close to hydrothermal vents in the MAR [71], it is still unclear which habitat the larval stages use. Post-larval stages of Alvinocarididae have been collected at a long distance from their potential origin (> 100 km) [9,70,72] in bathypelagic habitat between 1990–3060 m. Although Alvinocarididae early stages seem to tolerate large pressure variation, with larvae that can, in some cases, hatch and survive at atmospheric pressure ([12,13], present study), temperature tolerance may constrain the upper limit of the bathypelagic habitat [12]. An alternative hypothesis to explain the presence of lipids with photosynthetic isotopic signature in alvinocaridid shrimp is the feeding on particles descending to the aphotic zone, which are found near to the hydrothermal plumes [73] or in the open sea. At the present there is no direct evidence to support the occurrence of alvinocaridid larvae in the photic zone.
Differences in larval morphology of alvinocaridid larvae with others caridean shrimps
General characters in the Alvinocarididae first larvae include tiny rostrum hidden between the eyes, pterisgostomial spine present, setation absent in mouth parts (except for scaphognathite and occasionally the tip of the endopod of the maxilla), mandible thump-like unarmed or with only 1–2 small spines, incisive and molar processes absent, maxilliped 1–3 similar in form and size, three large setae in the distal join of the exopod and 1–3 distal setae in the tip of the endopod, inner margin of endopod only with spinules. Since the present study is the first detailed description of early stage of alvinocaridid shrimps larvae, no other information is available yet for comparisons within the family.
In order to compare the larval morphology of Alvinocarididae with other carideans, it is important to consider their phylogenetic relationships. Although this family had been included in the Superfamily Bresilioidea [74], molecular evidence did not support monophyly at this level [39,41] or relationship between Alvinocarididae and Bresiliidae [41]. Phylogenetic relationships proposed by Tokuda et al. [75] suggest monophyly of Alvinocarididae with Palaemonidae, however general phylogenetic reconstruction of carideans using both mitochondrial and nuclear genes support the phylogenetic relationship of Alvinocarididae with Oplophoridae, Acanthephyridae, Nematocarcinidae, Pasiphaeidae [39–41], and also Agostocarididae, Psalidopodidae [39] and Campylonotidae [40]. Although there are evidences of polyphyly within Pasiphaeidae, all members tested of this family belong to the same general clade [39–41].
Related with a close morphological comparison of the larvae, Thatje et al. [76] highlighted the larval similarities between Campylonotidae and Oplophoridae sensu lato (Oplophoridae+Acantephyridae) based on the absence of external setae in maxillule, occurrence of four well developed endites on the maxilla and presence of exopods in all pereiopods. These structures of the maxillule and maxilla are described in the first larval stage of Oplophoridae, Acanthephyridae, Nematocarcinidae, Psalidopodidae and Campylonotidae [49,76–79], except for the occurrence of external setae in some Nematocarcinidae. Although setal interpretation is not possible for Psalidopodidae, due to description of late embryonic stage [79], both pairs of coxal and basal endites of the maxilla are present in this larva. Additionally, in three genera of Oplophoridae (Systellaspis, Janicella and Oplophorus), two Acanthephyridae (Hymenodora and Acanthephyra) and two Pasiphaeidae (Pasiphaea and Parapasiphae) a first larval stage with undeveloped mouth parts has been described [67,69,77]. Although the occurrence of pleopods at this stage for all previous taxa suggest also abbreviated larval development [66,77], which also had been suggested for Psalidopodidae [79,80]. The combination of lack of development of mandible and maxillule, the almost complete absence of setation at the inner margin of maxillule, maxilla and maxillipeds and the absence of pleopods all advocate for a new larval configuration in marine caridean shrimps.
Scattered distribution of the genera with lecithotrophic larvae or abbreviated development in the present phylogenetic reconstruction suggests that reduction of mouth parts development and abbreviated development evolved independently along major taxa. No pattern is observed comparing the distribution of the larval traits in the phylogenetic reconstructions proposed previously [39–41,47], although differences in the position of the taxa occurs between studies due to gene and taxa coverture. However, combinations of larval traits are consistent within families or monophyletic units (for Pasiphaeidae), except for Hymenodora glacialis and Ephyrina figuerai, which show different traits compared to other Acanthephyridae. Polyphyletic taxa, such as Oplophoridae (sensu lato) (Ophophoridae + Acanthephyridae) [39,41,47] and Pasiphaeidae [39], exhibit distinct larval trait combinations, which further support the genetic evidence that split them into monophyletic groups. Concerning Acanthephyridae, the species with abbreviated development are in a sister position of other Acanthephyridae with extended development, although the information about the larval traits in this group is incomplete. Larval traits seem to evolve through different events to the acquisition of the larval form, however these events are not observed at genera or species level of diversification, at least for most of the DWCC. The sequence of acquisition of different larval traits cannot be determined because the relationships between the monophyletic units are still not fully resolved, and there are discrepancies between the previous studies.
Although low variation in the larval traits is observed within the families (or monophyletic units) in the DWCC, other carideans show differences at this level. For instance, three distant and diverse monophyletic families as Alpheidae, Pandalidae and Atyidae [39–41] have species with planktotrophic and extended development but also species with lecithotrophic and abbreviated development [65,81,82]. This variation is associated with the distribution of the species in different habitats and the acquisition of different mechanisms for dispersal or for retention of the offspring. In alvinocaridids the restriction of the species to hydrothermal vents and cold seeps habitats and the common characteristics of these systems (deep waters, fragmented and widely distributed and dominance of bacterial chemosynthesis) could be related to the lack of diversification of larval traits.
Conclusion
Alvinocaridid first larval stage is very distinctive from other decapod crustaceans because of the combination of undeveloped mouth parts and lack of pereiopods and pleopods, which suggest lecithotrophy but extended larval development. The larvae are very similar between the four genera and species studied, but minor morphological structures could be used for species identification. The larval traits observed in Alvinocarididae contrast with other larval models in decapod crustaceans, where lecithotrophy is associated to abbreviated development and some degree of larval retention. This model is consistent with a wide dispersal in the oligotrophic bathypelagic environment to colonize fragmented habitats such as hydrothermal vents and cold seeps. In the DWCC the scattered distribution of traits associated with lecithotrophic/planktotrophic and abbreviated/extended development suggests that they evolved independently in different combinations.
Supporting Information
(PDF)
The list includes information on larval traits of species not considered in the phylogenetic reconstruction, but used to make inferences on closely related species included in our phylogenetic reconstruction but without available data on larval traits (see methods).
(PDF)
Acknowledgments
We thank Valérie Cueff-Gauchard (LMEE-Ifremer) for her assistance in sequencing of N. saintlaurentae, and Nicolas Gayet (LEP-Ifremer) for his assistance in SEM images. We thank Bruce Shillito for shrimp recovery and incubation in PERISCOP and BALIST. We also thank the chief scientists, captains and crew of the N/O Atalante and Pourquoi pas? for the oceanographic cruises BIOZAÏRE 2–2001, SERPENTINE-2007, CONGOLOBE-2012, FUTUNA 3–2012, MOMARSAT-2013 and BICOSE-2014, during which our samples were collected, as well as the crew of the ROV Victor 6000 and Nautile. We thank Cindy L. Van Dover for providing tissues of Rimicaris hybisae.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under the MIDAS project, grant agreement n° 603418, and from the French Agence Nationale de la Recherche (ANR) under the CARNOT Programme, grant CARN 018-01 EDROME, and under the CONGOLOBE project, grant n° 11 BS 56 030 02. Pressure equipments used in this study were funded by a European Community program EXOCET/D (FP6-GOCE-CT-2003-505342), by an ANR grant, BALIST ANR-08-BLAN-0252, and by the University Pierre et Marie Curie, under the BQR UPMC 2008. Financial support was also provided by the Eramet and Technip companies (Futuna cruise). IHA received PhD funding from the Fundación Gran Mariscal de Ayacucho (www.fundayacucho.gob.ve), PhD grant E-223-85-2012-2, and from Campus France (http://www.campusfrance.org), grant 796045K. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vereshchaka AL, Kulagin DN, Lunina AA (2015) Phylogeny an new classification of hydrothermal vents and cold seep shrimps of the family Alvinocarididae (Decapoda). PLoS ONE 10(7): e0129975 10.1371/journal.pone.0129975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desbruyères D, Almeida A, Biscoito M, Comtet T, Khripounoff A, Le Bris N, et al. (2000) A review of the distribution of hydrothermal vent communities along the northern Mid-Atlantic Ridge: dispersal vs. environmental controls. Hydrobiologia 440: 201–216. [Google Scholar]
- 3. Segonzac M, de Saint Laurent M, Casanova B (1993) L'énigme du comportement trophique des crevettes Alvinocarididae des sites hydrothermaux de la dorsale médio-atlantique. Cah Biol Mar 34: 535–571. [Google Scholar]
- 4. Ponsard J, Cambon-Bonavita M-A, Zbinden M, Lepoint G, Joassin A, Cobari L, et al. (2013) Inorganic carbon fixation by chemosynthetic ectosymbionts and nutritional transfers to the hydrothermal vent host-shrimp Rimicaris exoculata . ISME J 7: 96–109. 10.1038/ismej.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teixeira S, Serrão EA, Arnaud-Haond S (2012) Panmixia in a fragmented and unstable environment: the hydrothermal shrimp Rimicaris exoculata disperses extensively along the Mid-Atlantic Ridge. PLoS ONE 7: e38521 10.1371/journal.pone.0038521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teixeira S, Olu K, Decker C, Cunha RL, Fuchs S, Hourdez S, et al. (2013) High connectivity across the fragmented chemosynthetic ecosystems of the deep Atlantic Equatorial Belt: efficient dispersal mechanisms or questionable endemism? Mol Ecol 22: 4663–4680. 10.1111/mec.12419 [DOI] [PubMed] [Google Scholar]
- 7. Beedessee G, Watanabe H, Ogura T, Nemoto S, Yahagi T, Nakagawa S, et al. (2013) High Connectivity of Animal Populations in Deep-Sea Hydrothermal Vent Fields in the Central Indian Ridge Relevant to Its Geological Setting. PLoS ONE 8: e81570 10.1371/journal.pone.0081570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thaler AD, Plouviez S, Saleu W, Alei F, Jacobson A, Boyle EA, et al. (2014) Comparative Population Structure of Two Deep-Sea Hydrothermal-Vent-Associated Decapods (Chorocaris sp. 2 and Munidopsis lauensis) from Southwestern Pacific Back-Arc Basins. PLoS ONE 9: e101345 10.1371/journal.pone.0101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herring PJ (1998) North Atlantic midwater distribution of the juvenile stages of hydrothermal vent shrimps (Decapoda: Bresiliidae). Cah Biol Mar 39: 387–390. [Google Scholar]
- 10. Gebruk AV, Galkin SV, Vereshchaka AL, Moskalev LI, Southward AJ. 1997. Ecology and biogeography of the hydrothermal vent fauna of the Mid-Atlantic Ridge. Adv Mar Biol 32: 93–144. [Google Scholar]
- 11. Van Dover CL, Factor JR, Williams A, Berg CJ (1985) Reproductive patterns of decapod crustaceans from hydrothermal vents. Bull Biol Soc Wash 5: 223–227. [Google Scholar]
- 12. Tyler PA, Dixon DR (2000) Temperature/pressure tolerance of the first larval stage of Mirocaris fortunata from Lucky Strike hydrothermal vent field. J Mar Biol Asso UK 80: 739–740. [Google Scholar]
- 13. Koyama S, Nagahama T, Ootsu N, Takayama T, Horii M, Konishi S, et al. (2005) Survival of deep-sea shrimp (Alvinocaris sp.) during decompression and larval hatching at atmospheric pressure. Mar Biotech 7: 272–278. [DOI] [PubMed] [Google Scholar]
- 14. Miyake H, Kitada M, Tsuchida S, Okuyama Y, Nakamura K-i (2007) Ecological aspects of hydrothermal vent animals in captivity at atmospheric pressure. Mar Ecol 28: 86–92. [Google Scholar]
- 15. Ramirez-Llodra E, Segonzac M (2006) Reproductive biology of Alvinocaris muricola (Decapoda: Caridea: Alvinocarididae) from cold seeps in the Congo Basin. J Mar Biol Asso UK 86: 1347–1356. [Google Scholar]
- 16. Guri M, Durand L, Cueff-Gauchard V, Zbinden M, Crassous P, Shillito B, et al. (2012) Acquisition of epibiotic bacteria along the life cycle of the hydrothermal shrimp Rimicaris exoculata . ISME J 6: 597–609. 10.1038/ismej.2011.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nye V, Copley JT, Tyler PA (2013) Spatial variation in the population structure and reproductive biology of Rimicaris hybisae (Caridea: Alvinocarididae) at hydrothermal vents on the Mid-Cayman Spreading Centre. PLoS ONE 8: e60319 10.1371/journal.pone.0060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramirez-Llodra E, Tyler PA, Copley JTP (2000) Reproductive biology of three caridean shrimp, Rimicaris exoculata, Chorocaris chacei and Mirocaris fortunata (Caridea: Decapoda), from hydrothermal vents. J Mar Biol Asso UK 80: 473–484. [Google Scholar]
- 19. Miyake H, Kitada M, Itoh T, Nemoto S, Okuyama Y, Watanabe H, et al. (2010) Larvae of deep sea chemosynthetic ecosystem animals in captivity. Cah Biol Mar 51: 441–450. [Google Scholar]
- 20. Anger K (2001) The biology of decapod crustacean larvae; Koenemann S, editor: CRC Press. [Google Scholar]
- 21. Campos E, Hernandez-Avila I (2010) Phylogeny of Calyptraeotheres Campos, 1990 (Crustacea, Decapoda, Brachyura, Pinntoheridae) with the description of C. pepeluisi new species from the tropical Mexican Pacific. Zootaxa 2691: 41–52. [Google Scholar]
- 22. Terosi M, Cuesta JA, Wehrtman IS, Mantelatto FL (2010) Revision of the larval morphology (Zoea I) of the family Hippolytidae (Decapoda Caridea), with a description of the first stages of the shrimp Hippolyte obliquimanus Dana, 1852. Zootaxa 2624: 49–66. [Google Scholar]
- 23. Mullineaux LS, Adams DK, Mills SW, Beaulieu SE (2010) Larvae from afar colonize deep-sea hydrothermal vents after a catastrophic eruption. Proc Natl Acad Sci USA 107: 7829–7834. 10.1073/pnas.0913187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metaxas A (2004) Spatial and temporal patterns in larval supply at hydrothermal vents in the northeast Pacific Ocean. Limnol Oeanogr 49: 1946–1956. [Google Scholar]
- 25. Komai T, Segonzac M (2005) A revision of the genus Alvinocaris Williams and Chace (Crustacea: Decapoda: Caridea: Alvinocarididae), with descriptions of a new genus and a new species of Alvinocaris . J Nat His 39: 1111–1175. [Google Scholar]
- 26. Komai T, Segonzac M (2008) Taxonomic review of the hydrothermal vent shrimp genera Rimicaris Williams & Rona and Chorocaris Martin & Hessler (Crustacea: Decapoda: Caridea: Alvinocarididae). J Shellfish Res 27: 21–41. [Google Scholar]
- 27. Shillito B, Hamel G, Duchi C, Cottin D, Sarrazin J, Sarradin P-M, et al. (2008) Live capture of megafauna from 2300m depth, using a newly designed pressurized recovery device. Deep Sea Res Part 1 Oceanogr Res Pap 55: 881–889. [Google Scholar]
- 28. Ravaux J, Hamel G, Zbinden M, Tasiemski AA, Boutet I, Léger N, et al. (2013) Thermal limit for metazoan life in question: in vivo heat tolerance of the pompeii worm. PLoS ONE 8: e64074 10.1371/journal.pone.0064074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pradillon F, Shillito B, Chervin J-C, Hamel G, Gaill F (2004) Pressure vessels for in vivo studies of deep-sea fauna. High Press Res 24: 237–246. [Google Scholar]
- 30.Fouquet Y, Alix AS, Birot D, Chéron S, Charlou JL, Donval JP, et al (2015) Discovery of extensive hydrothermal fields in the Wallis and Futuna back-arc environment (SW Pacific). Marine Mineral Ressources in a Sustainable World. 13th SGA Biennial Meeting 2015, Proceedings, vol 3: 1223–1226.
- 31. Clark PF, Calazans D, Pohle GW (1998) Accuracy and standardization of brachyuran larval descriptions. Invertebr Reprod Dev 33: 127–144. [Google Scholar]
- 32. Doyle JJ (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- 33. Folmer O, Black M, Hoeh W, Lutz RA, Vrijenhoek RC (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biotech 3: 294–299. [PubMed] [Google Scholar]
- 34. Giribet G, Carranza S, Baguñà J, Riutort M, Ribera C (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Mol Biol Evol 13: 76–84. [DOI] [PubMed] [Google Scholar]
- 35. Whiting MF (2002) Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool Scr 31: 93–104. [Google Scholar]
- 36. Pradillon F, Schmidt A, Peplies Jr, Dubilier N (2007) Species identification of marine invertebrate early stages by whole-larvae in situ hybridisation of 18S ribosomal RNA. Mar Ecol Progr Ser 333: 103–116. [Google Scholar]
- 37. Shank TM, Black MB, Halanich KM, Lutz RA, Vrijenhoek RC (1999) Miocene radiation of the deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): Evidence from mithochondrial cytochrome oxidase subunit I. Mol Phylogen Evol 13: 244–254. [DOI] [PubMed] [Google Scholar]
- 38. Plouviez S, Jacobson A, Wu M, Van Dover CL (2015) Characterization of vent fauna at the Mid-Cayman Spreading Center. Deep Sea Res Part 1 Oceanogr Res Pap 97: 124–133. [Google Scholar]
- 39. Bracken HD, De Grave S, Felder D (2009) Phylogeny of the infraorder Caridea based on mitochondrial and nuclear genes (Crustacea: Decapoda) In: Martin JW, Crandal KA, Felder D, editors. Decapod Crustacean Phylogenetics: CRC Press; pp. 281–305. [Google Scholar]
- 40. Li CP, De Grave S, Chan T-Y, Lei HC, Chu KH (2011) Molecular systematics of caridean shrimps based on five nuclear genes: Implications for superfamily classification. Zool Anz 250: 270–279. [Google Scholar]
- 41. Aznar-Cormano L, Brisset J, Chan T-Y, Corbari L, Puillandre N, Utge J, et al. (2015) Hierarchical taxonomic sampling is a necessary but not sufficient condition for resolving inter-families relationships in Caridean decapods. Genetica 145: 195–205. [DOI] [PubMed] [Google Scholar]
- 42. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29: 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keane T, Creevey C, Pentony M, Naughton T, McLnerney J (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rabalais NN, Gore RH (1985) Abbreviated development in decapods In: Wenner AM, Schram FR, editors. Larval Growth. Rotterdam: Balkema; pp. 67–126. [Google Scholar]
- 46. Zelnio KA, Hourdez S (2009) A new species of Alvinocaris (Crustacea: Decapoda: Caridea: Alvinocarididae) from hydrothermal vents at the Lau Basin, Southwest Pacific, and a key to the species of Alvinocarididae. Proc Biol Soc Wash 122: 52–71. [Google Scholar]
- 47. Chan T, Lei HC, Li CP, Chu KH (2010) Phylogenetic analysis using rDNA reveals polyphyly of Oplophoridae (Decapoda: Caridea). Invertebr Syst 24: 172–181. [Google Scholar]
- 48. Komai T, Segonzac M (2003) Review of the hydrothermal vent shrimp genus Mirocaris, redescription of M. fortunata and reassessment of the taxonomic status of the family Alvinocarididae (Crustacea: Decapoda: Caridea). Cah Biol Mar 44: 199–215. [Google Scholar]
- 49. Thatje S, Lovrich GA, Anger K (2004) Egg production, hatching rates, and abbreviated larval development of Campylonotus vagans Bate, 1888 (Crustacea: Deacapoda: Caridea), in subantartic waters. J Exp Mar Bio Ecol 301: 15–27. [Google Scholar]
- 50. Pohle GW, Telford M (1981) The larval development of Dissodactylus crinitichelis Moreira, 1901 (Brachyura: Pinnotheridae) in laboratory culture. Bull Mar Sci 31: 753–773. [Google Scholar]
- 51. Hong SY (1988) The prezoeal stage in various decapod crustaceans. J Nat His 22: 1041–1075. [Google Scholar]
- 52. Olu K, Cordes EE, Fisher CR, Brooks JM, Sibuet M, Desbruyères D, et al. (2010) Biogeography and potential exchanges among the Atlantic equatorial belt cold-seep faunas. PLoS ONE 5: e11967 10.1371/journal.pone.0011967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Copley JTP, Young CM (2006) Seasonality and zonation in the reproductive biology and population structure of the shrimp Alvinocaris stactophila (Caridea: Alvinocarididae) at a Louisiana Slope cold seep. Mar Ecol Prog Ser 315: 199–209. [Google Scholar]
- 54. Bauer R (2004) Remarkable shrimps: adaptations and natural history of Carideans: University Oklahoma Press. [Google Scholar]
- 55. Pond DW, Segonzac M, Bell MV, Dixon DR, Fallick AE, Sargent JR (1997) Lipid and lipid carbon stable isotope composition of the hydrothermal vent shrimp Mirocaris fortunata: evidence for nutritional dependence on photosynthetically fixed carbon. Mar Ecol Prog Ser 157: 221–231. [Google Scholar]
- 56. Anger K, Schuh M (1992) Bioenergetics of abbreviated larval development in the bromeliad crab, Metopaulias depressus (Decapoda: Grapsidae). Comp Biochem Physiol 103A: 507–518. [Google Scholar]
- 57. Gonzalez-Gordillo I, Anger K, Schubart C (2010) Morphology of the larval and first juvenile stage of two Jamaican endemic crab species with abbreviated development, Sesarma windsor and Metopaulias depressus (Decapoda: Brachyura: Sesarmidae). J Crust Biol 30: 101–121. [Google Scholar]
- 58. Bolaños JA, Cuesta JA, Hernandez G, Hernandez J, Felder DL (2004) Abbreviated larval development of Tunicotheres moseri (Rathbun, 1918) (Decapoda: Pinnotheridae), a rare case of parental care among brachyuran crabs. Sci Mar 68: 373–384. [Google Scholar]
- 59. Konishi K, Imafuku M (2000) Hatching of the symmetrical hermit crab Pomatocheles jeffreyssi Miers, 1879: the first information on polylchelids larva (Anomura: Pylochelidae). Crustacean Res 29: 65–69. [Google Scholar]
- 60. Saito T, Konoishi K (2002) Description of the first stage zoea of the symmetrical hermit crabs Pylocheles mortensenii (Boas, 1926) (Anomura, Paguridea, Pylochelidae). Crustaceana 75: 621–628. [Google Scholar]
- 61. McLaughlin PA, Lemaitre R (2008) Larvae of two species of Trozocheles (Decapoda: Anomura: Paguroidea: Pylochelidae: Trizochelinae), description of the adult of one, and preliminary implications of development on pylochelid phylogeny. Zootaxa 1911: 52–68. [Google Scholar]
- 62. Clark PF, Ng PKL (2008) The lecithotrophic zoea of Chirostylus ortmanni Miyake & Baba, 1968 (Crustacea: Anomura: Galatheoidea: Chirostylidae) decribed from laboratory hatched material. Raffles Bul Zool 56: 85–94. [Google Scholar]
- 63. Thatje S, Smith KE, Marsh L, Tyler P (2015) Evidence for protracted and lecithotrophic larval development in the yeti crab Kiwa tyleri from hydrothermal vents of the East Scotia Ridge, Southern Ocean. Sex Early Dev Aquat Org 1: 109–116. [Google Scholar]
- 64. Dobkin S (1967) The first post-embryonic stage of Synalpheus brooksi Coutière. Bull Mar Sci 15: 450–462. [Google Scholar]
- 65. Knowlton RE (1973) Larval development of the snapping shrimp Alpheus heterochaelis Say, reared in the laboratory. J Nat His 7: 273–306. [Google Scholar]
- 66. Gurney R, Lebour MV (1941) On the Larvae of certain Crustacea Macrura, mainly from Bermuda. Zool J Linn Soc 41: 89–181. [Google Scholar]
- 67. Williamson DI (1960) Larval stages of Pasiphaea sivado and some other Pasiphaeidae (Decapoda). Crustaceana 1: 331–341. [Google Scholar]
- 68. Duffy JE (1996) Eusociality in a coral-reef shrimp. Nature 381: 512–514. [Google Scholar]
- 69. Nanjo N, Konishi K (2009) Complete larval development of the Japanese glass shrimp Pasiphaea japonica Omori, 1976 (Decapoda: Pasiphaeidae) under laboratory conditions. Crustacean Res 38: 77–89. [Google Scholar]
- 70. Dixon DR, Dixon LRJ, Pond DW (1998) Recent advances in our understanding of the life history of bresiliid vent shrimps on the MAR. Cah Biol Mar 39: 383–386. [Google Scholar]
- 71. Pond DW, Sargent JR, Fallick AE, Allen C, Bell MV, Dixon DR (2000) δ13C values of lipids from phototrophic zone microplankton and bathypelagic shrimps at the Azores sector of the Mid-Atlantic Ridge. Deep Sea Res Part 1 Oceanogr Res Pap 47: 121–136. [Google Scholar]
- 72. Herring PJ, Dixon DR (1998) Extensive deep-sea dispersal of postlarval shrimp from a hydrothermal vent. Deep Sea Res Part 1 Oceanogr Res Pap 45: 2105–2118. [Google Scholar]
- 73. Cowen JP, Bertram MA, Wakeham SG, Thomson RE, William Lavelle J, Baker ET (2001) Ascending and descending particle flux from hydrothermal plumes at Endeavour Segment, Juan de Fuca Ridge. Deep Sea Res Part 1 Oceanogr Res Pap 48: 1093–1120. [Google Scholar]
- 74. De Grave S, Pentcheff ND, Ahyong S, Chan T, Crandal KA, Dworschak PC, et al. (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool Suppl 21: 1–109. [Google Scholar]
- 75. Tokuda G, Yamada A, Nakano K, Arita N, Yamasaki H (2006) Occurrence and recent long-distance dispersal of deep-sea hydrothermal vent shrimps. Biol Lett 2: 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thatje S, Bacardit R, Romero MC, Tapella F, Lovrich GA (2001) Description and key to the zoeal stages of the Campylonotidae (Decapoda: Caridea) from the Magellan region. J Crustacean Biol 21: 492–505. [Google Scholar]
- 77. Gurney R (1942) Larvae of decapod Crustacea. Royal Soc Lond 129: 1–306. [Google Scholar]
- 78. Hendrickx M, Garcia-Guerrero M (2007) Description of the first zoea of Acanthephyra brevicarinata Hanamura, 1984 (Caridea: Oplophoridae) from the deep water of the Gulf of Mexico. Contr Stud E Pacific Crustaceans 4: 31–36. [Google Scholar]
- 79. Martin JW (1985) A late embryo of the deep water shrimp Psalidopus barbourdi Chace, 1939 (Decapoda: Caridea). Crustaceana 59: 299–302. [Google Scholar]
- 80. Chace FA, Holthuis LB (1978) Psalidopus: the scissorfoot shrimps (Crustacea: Decapoda: Caridea). Smithson Contr Zool 277: 1–22. [Google Scholar]
- 81. Thatje S, Bacardit R (2000) Larval development of Austropandalus grayi (Cunninghem, 1871)(Decapoda, Caridea, Pandalidae) from the southwestern Atlantic Ocean. Crustaceana 73: 609–628. [Google Scholar]
- 82. Benzie JAH, Silva PKd (1983) The abbreviated larval development of Caridina singhalensis Ortmann, 1894 (Decapoda, Atyidae). J Crustacean Biol 3: 117–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The list includes information on larval traits of species not considered in the phylogenetic reconstruction, but used to make inferences on closely related species included in our phylogenetic reconstruction but without available data on larval traits (see methods).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.