Abstract
Background:
Survivors of acute lung injury (ALI) and other critical illnesses often experience substantial posttraumatic stress disorder (PTSD) symptoms. However, most questionnaires have not been validated against a PTSD diagnostic reference standard in this patient population. Hence, in the current study of survivors of ALI, we evaluated the Impact of Events Scale-Revised (IES-R), a questionnaire measure of PTSD symptoms, against the Clinician-Administered PTSD Scale (CAPS), the current state-of-the-art PTSD diagnostic reference standard, which also provides a quantitative assessment of PTSD symptoms.
Methods:
We evaluated the IES-R questionnaire vs the CAPS diagnostic interview in 60 of 77 consecutively recruited survivors of ALI from two prospective cohort studies of patients 1 to 5 years after ALI.
Results:
The IES-R total score (range: 0.0-3.2) and the CAPS total severity score (range: 0-70) were strongly related (Pearson r = 0.80, Spearman ρ = 0.69). Using CAPS data, eight of the 60 patients (13%) had PTSD at the time of assessment, and an additional eight patients had partial PTSD (total prevalence, 27%). In a receiver operating characteristics curve analysis with CAPS PTSD or partial PTSD as criterion variables, the area under the curve ranged from 95% (95% CI, 88%-100%) to 97% (95% CI, 92%-100%). At an IES-R threshold of 1.6, with the same criterion variables, sensitivities ranged from 80% to 100%, specificities 85% to 91%, positive predictive values 50% to 75%, negative predictive values 93% to 100%, positive likelihood ratios 6.5 to 9.0, negative likelihood ratios 0.0 to 0.2, and efficiencies 87% to 90%.
Conclusions:
The IES-R appears to be an excellent brief PTSD symptom measure and screening tool in ALI survivors.
Critically ill patients face tremendous physical and psychologic stresses in the ICU, including respiratory insufficiency, painful procedures, delirium with associated psychotic experiences, and reduced autonomy and ability to communicate. For these reasons, posttraumatic stress disorder (PTSD) is a major clinical concern for ICU survivors,1‐5 and a brief, valid PTSD measure would be extremely useful to clinicians and researchers evaluating these patients.
The Impact of Event Scale (IES),6 a self-report questionnaire, is the most widely used measure of PTSD symptoms in critical care outcomes research.2,5 A criticism of the IES is that it only measures reexperiencing (intrusion) symptoms (eg, intrusive memories, nightmares, or flashbacks) and avoidance/numbing symptoms (eg, avoidance of places or topics, or feeling numb about the event), and not hyperarousal symptoms of PTSD (eg, difficulty sleeping or irritability). The authors of the revised version of the IES, the IES-R, added six hyperarousal items and an additional reexperiencing item.7
An important feature of the IES-R is that the measure is “grounded” to a particular trauma (eg, combat, rape, or, as in the current study, critical illness/ICU treatment). Given this feature, as well as its coverage of a variety of PTSD symptoms, we hypothesized that the IES-R might be suitable as a PTSD symptom measure and screening tool in survivors of critical illness. To test this hypothesis, we evaluated the IES-R against the current gold standard quantitative assessment and diagnostic tool in PTSD research, the Clinician-Administered PTSD Scale (CAPS),8 in survivors of acute lung injury (ALI). The CAPS takes roughly 45 min to administer and requires experienced clinicians or appropriately trained paraprofessionals8; thus, the CAPS is impractical for use in routine clinical practice or complex research protocols requiring longitudinal evaluations. In this report, we follow the Standards for Reporting of Diagnostic Accuracy guidelines.9,10
Materials and Methods
Patients
Between July and November 2010, patients were consecutively recruited from two ongoing, prospective cohort studies evaluating the long-term outcomes of survivors of ALI.11 In the ARDS Network Long-Term Outcomes Study (ALTOS; NCT00719446),12 which recruits patients from ARDSNet clinical centers across the United States, 47 patients were eligible after their 1-year follow-up assessments. In the Improving Care of ALI Patients (ICAP) study,13 which follows patients from 13 ICUs at four hospitals in Baltimore, Maryland, 30 patients were eligible after their 2-, 3-, 4-, or 5-year follow-up assessments. Exclusion criteria for both studies included the inability to understand or speak English, no fixed address, and preexisting cognitive impairment, all of which would prevent completion of study assessments. In both cohort studies, trained research staff administered the IES-R at scheduled follow-up visits (eight different staff members, four in each study).
Within 1 week of IES-R administration and blind to the patients’ results, we interviewed patients using the CAPS. CAPS interviewers included a board-certified attending psychiatrist (O. J. B., seven interviews), a fourth-year psychiatry resident (J. W., 51 interviews), and a medical student (A. Y., seven interviews). Prior to the study, the attending psychiatrist trained the other two interviewers in diagnostic interviewing, PTSD and its associated symptoms, and the administration of the CAPS. At the beginning of the study, for quality assurance purposes, the attending psychiatrist and the medical student co-interviewed three patients, and the psychiatry resident and the medical student co-interviewed two patients. Seventy-five percent of the IES-R and CAPS assessments were conducted via telephone, and 25% were conducted face to face. Telephone interviews were useful because many of the participants lived a long distance from Baltimore; previous research has shown that telephone and face-to-face methods are comparable when evaluating PTSD symptoms.14 The Johns Hopkins University School of Medicine Institutional Review Boards X and 5 approved this research (protocols NA_00013113, NA_00025950, and NA_00041630), and patients provided informed consent.
Measures
With the IES-R, respondents are asked to report how distressed or bothered they have been by particular difficulties in the past 7 days: “not at all” (item score 0), “a little bit” (score, 1), “moderately” (score, 2), “quite a bit” (score, 3), or “extremely” (score, 4). The IES-R authors demonstrated the internal consistency of the instrument (α = 0.8-0.9) and short-term test-retest reliability (r = 0.8).15 The IES-R has performed fairly well as a screening instrument for PTSD, with optimal thresholds (item mean scores) between 1.0 and 2.2 in different populations.16‐20 The IES-R has demonstrated concurrent and discriminant validity, as well as a lack of social desirability effects.20,21
The CAPS can be used to assess the presence or absence of a PTSD diagnosis; however, it also provides a dimensional assessment of PTSD symptom severity. Clinical interviewers rate each of the 17 PTSD criteria (five reexperiencing, seven avoidance/numbing, and five hyperarousal criteria) on five-point (0-4) scales reflecting frequency and intensity of symptoms, and severity scores are calculated by adding frequency and intensity scores for each item. A continuous measure of PTSD symptoms is particularly attractive in that psychiatric nosologists increasingly conceptualize illnesses like PTSD in dimensional terms,22 that is, patients show a spectrum of symptom severity, with no clear threshold delineating clinically meaningful symptoms. Indeed, most,23‐31 but not all,32,33 studies of “partial” or “subthreshold” PTSD indicate levels of severity and dysfunction intermediate between full PTSD and no PTSD.34,35 Thus, we also included three established, partial PTSD definitions in the current study: (1) symptoms met the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV B criterion (reexperiencing) and either the C criterion (avoidance/numbing) or D criterion (hyperarousal)17; (2) symptoms met the B criterion as well as one or more C and D symptom criteria26; and (3) symptoms met at least two of the three B, C, and D criteria.36 The CAPS has shown high internal consistency (α = 0.9), interrater reliability (perfect diagnostic agreement, r = 0.9 for symptom severity), test-retest reliability (κ = 0.9-1.0; r = 0.9 for symptom severity), and convergent and discriminant validity.37
When interviewing patients with the CAPS, we assessed symptoms for two time periods: the last week prior to the interview and time since the index ALI hospitalization. As with the IES-R, in the CAPS diagnostic interviews, we focused on PTSD symptoms related to each patient’s critical illness and ICU care. We used the “frequency ≥ 1, intensity ≥ 2 rule”38 to determine the presence or absence of individual CAPS PTSD symptoms. For example, a patient was rated as having difficulty falling or staying asleep if that symptom was present (occurred at least once or twice, eg, in the last week) and was at least moderate in intensity (30-90 min sleep loss).
Statistics
We used the Cronbach α statistic to describe the internal consistency of IES-R and CAPS items. In testing for differences between patients who did or did not complete CAPS interviews, we used t tests (and Mann-Whitney U tests, if appropriate) to test for differences in continuous variables and the χ2 test to evaluate for sex differences. We used Pearson and Spearman correlation coefficients to examine bivariate relationships between continuous variables. Though the Pearson r statistic is typically reported in psychometric studies regardless of variable distributions, we included Spearman ρ because the distributions of IES-R total scores and CAPS total severity scores are skewed to the right. We examined the screening characteristics of IES-R total scores vs last-week CAPS DSM-IV PTSD diagnoses using a receiver operating characteristics (ROC) curve, including the area under the ROC curve (AUC), and sensitivities, specificities, positive and negative predictive values, positive and negative likelihood ratios, and efficiencies (defined as the numbers of true positives and true negatives divided by the numbers of true positives, true negatives, false positives, and false negatives)39 at each IES-R total-score cut point. In addition, we examined IES-R screening characteristics vs last-week CAPS partial PTSD, as defined previously in this report. Finally, we conducted exploratory subgroup analyses to determine whether the AUC varied substantially by age, sex, or time after ALI. We did not exclude any outliers in analyses. We performed all analyses using SPSS, version 19 (IBM).
Results
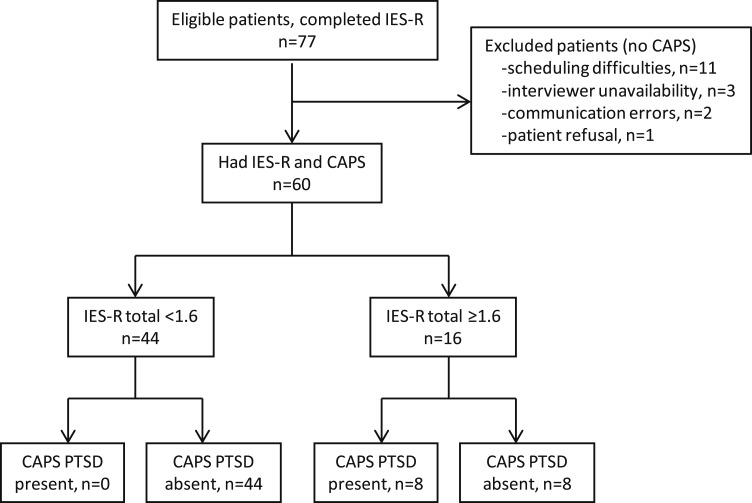
During the study period, 77 patients completed the IES-R, and 60 of these patients completed CAPS interviews. Of the 60 patients who completed CAPS interviews, 35 were from ALTOS (all assessed 1 year after ALI; mean [SD] age: 49 years [12], range: 21-71; 33% male patients), and 25 were from the ICAP study (the numbers of patients assessed 2, 3, 4, and 5 years after ALI were 1, 5, 13, and 6, respectively; mean [SD] age: 54 years [13], range: 26-75; 44% male patients). Reasons patients did not complete CAPS interviews included patient scheduling difficulties within 1 week of the IES-R (n = 11), interviewer unavailability (n = 3), communication errors between the parent study and PTSD study teams (n = 2), and patient declining to be interviewed (n = 1).
The internal consistency of the 22 IES-R item scores was high (α = 0.96). The mean IES-R total scores for the 60 and 17 patients who did and did not complete CAPS interviews were 0.95 (median [SD]: 0.68 [0.89]; range: 0-3.2) and 0.98 (median [SD]: 0.55 [1.0]; range: 0-3.6), respectively (P ≥ .90). Patients did not differ significantly between groups in age or sex. The mean age of those who completed the interviews was 51 years (SD 13, range: 21-75), that of those who did not complete the interviews was 46 years (SD 14, range: 23-75; P = .22). Thirty-eight percent of patients who completed the interviews were men; 53% of those who did not were men (P = .28).
The internal consistency of the 17 CAPS item severity scores was high (α = 0.84). The mean CAPS total severity score was 20 (median [SD], 16 [19]; range: 0-70). In our quality assurance exercise (n = 5), co-interviewers agreed perfectly regarding PTSD diagnosis, and severity scores were highly correlated across interviewers (Pearson r = 0.94, Spearman ρ = 1.0). The most frequent CAPS PTSD symptoms were difficulty sleeping, irritability, and poor concentration (e-Fig 1 (412.4KB, pdf) ). Seventeen of the 60 interviewed patients (28%) had CAPS-diagnosed PTSD since their initial ALI hospitalization, and eight (13%) had CAPS-diagnosed PTSD in the last week. The prevalence of last-week, CAPS-diagnosed, partial PTSD (three different definitions) ranged from 20%17 to 25%36; 16 (27%) of the patients met criteria for any last-week CAPS, partial PTSD definition.
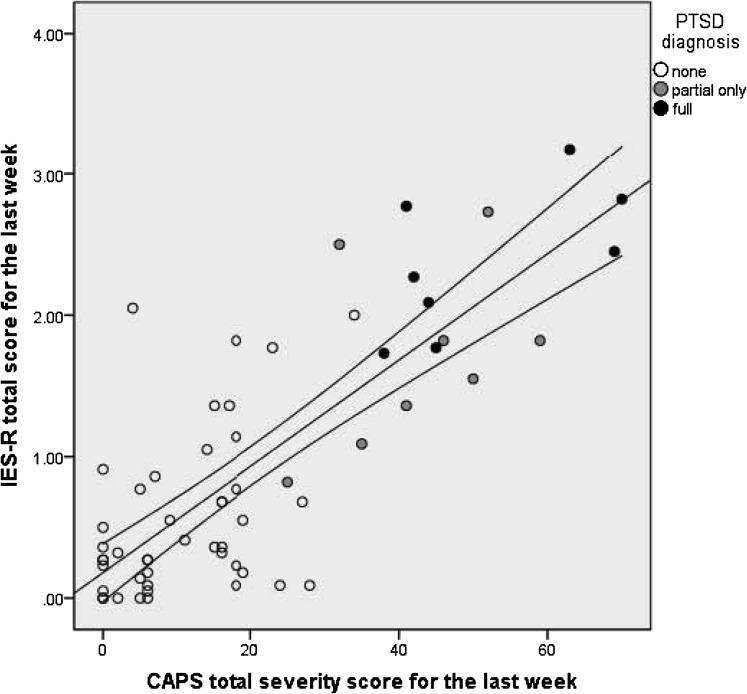
IES-R total scores, which reflect last-week symptom severity, were highly correlated with CAPS last-week total severity scores (Pearson r = 0.80, Spearman ρ = 0.69). Figure 1 shows the bivariate distribution of these scores, with indicators for scores of patients with last-week, CAPS-diagnosed, full or partial PTSD. The screening properties of the IES-R were excellent (Fig 2). The AUC for CAPS-diagnosed DSM-IV PTSD was 95%, and those for partial PTSD ranged from 95% to 97% (Table 1).
Figure 1.
Convergence of two measures of PTSD symptoms in the last week before interview: the IES-R total score vs the CAPS total severity score. Lines indicate the slope and 95% CI of a fitted linear regression line; Pearson r = 0.80 (P < .0005) and Spearman ρ = 0.69 (P < .0005). CAPS = Clinician-Administered PTSD Scale; IES-R = Impact of Events Scale-Revised; PTSD = postraumatic stress disorder.
Figure 2.
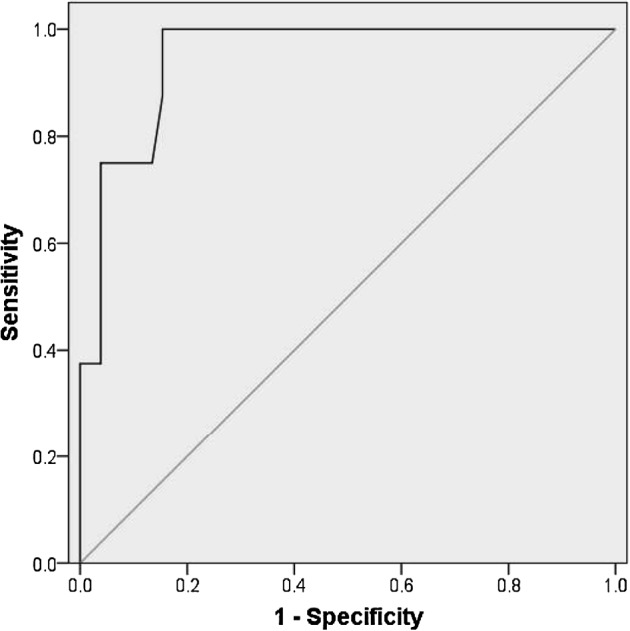
Receiver operating characteristics (ROC) curve for the IES-R total score (test variable) vs CAPS (criterion variable, eight of 60 patients). The area under the ROC curve is 95% (95% CI, 88%-100%). See Figure 1 legend for expansion of abbreviations.
Table 1.
—Sensitivities, Specificities, Predictive Values, Likelihood Ratios, and Efficiencies for IES-R Thresholds Corresponding to Full or Partial PTSDa
| IES-Rb | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | Efficiency |
| DSM-IV PTSD (n = 8), AUC 95%, 95% CI, 88%-100% | |||||||
| 1.0 | 100 | 71 | 35 | 100 | 3.5 | 0.0 | 75 |
| 1.1 | 100 | 75 | 38 | 100 | 4.0 | 0.0 | 78 |
| 1.2 | 100 | 77 | 40 | 100 | 4.3 | 0.0 | 80 |
| 1.4 | 100 | 83 | 47 | 100 | 5.8 | 0.0 | 85 |
| 1.6 | 100 | 85 | 50 | 100 | 6.5 | 0.0 | 87 |
| 1.8 | 75 | 86 | 46 | 96 | 5.6 | 0.3 | 85 |
| 2.0 | 75 | 92 | 60 | 96 | 9.8 | 0.3 | 90 |
| Partial PTSDc (1), n = 12, AUC 97%, 95% CI, 92%-100% | |||||||
| 1.0 | 100 | 77 | 52 | 100 | 4.4 | 0.0 | 82 |
| 1.1 | 100 | 81 | 57 | 100 | 5.3 | 0.0 | 85 |
| 1.2 | 100 | 83 | 60 | 100 | 6.0 | 0.0 | 87 |
| 1.4 | 92 | 88 | 65 | 98 | 7.3 | 0.1 | 83 |
| 1.6 | 92 | 90 | 69 | 98 | 8.8 | 0.1 | 90 |
| 1.8 | 75 | 92 | 69 | 94 | 9.0 | 0.3 | 88 |
| 2.0 | 66 | 96 | 80 | 92 | 16 | 0.4 | 90 |
| Partial PTSD (2), n = 13, AUC 95%, 95% CI, 89%-100% | |||||||
| 1.0 | 92 | 76 | 52 | 97 | 3.9 | 0.1 | 80 |
| 1.1 | 92 | 81 | 57 | 97 | 4.8 | 0.1 | 83 |
| 1.2 | 92 | 83 | 60 | 98 | 5.4 | 0.1 | 85 |
| 1.4 | 85 | 87 | 65 | 95 | 6.6 | 0.2 | 87 |
| 1.6 | 85 | 89 | 69 | 95 | 8.0 | 0.2 | 88 |
| 1.8 | 69 | 91 | 69 | 91 | 8.1 | 0.3 | 87 |
| 2.0 | 66 | 96 | 80 | 92 | 16 | 0.4 | 90 |
| Partial PTSD (3), n = 15, AUC 96%, 95% CI, 91%-100% | |||||||
| 1.0 | 100 | 82 | 65 | 100 | 5.6 | 0.0 | 87 |
| 1.1 | 93 | 84 | 67 | 97 | 6.0 | 0.1 | 87 |
| 1.2 | 93 | 87 | 70 | 98 | 7.0 | 0.1 | 88 |
| 1.4 | 87 | 91 | 76 | 95 | 9.8 | 0.2 | 90 |
| 1.6 | 80 | 91 | 75 | 93 | 9.0 | 0.2 | 88 |
| 1.8 | 67 | 93 | 77 | 89 | 10 | 0.4 | 87 |
| 2.0 | 53 | 96 | 80 | 86 | 12 | 0.5 | 85 |
Data given as % unless otherwise indicated. AUC = area under the receiver operating characteristics curve; DSM = Diagnostic and Statistical Manual of Mental Disorders; IES-R = Impact of Event Scale-Revised; LR− = likelihood ratio of a negative test result; LR+ = likelihood ratio of a positive test result; NPV = negative predictive value; PPV = positive predictive value; PTSD = posttraumatic stress disorder.
Assessed using the Clinician-Administered PTSD Scale.
IES-R total score (mean of all items).
Partial PTSD definitions: (1) symptoms met the DSM-IV B criterion (reexperiencing), as well as either the C (avoidance/numbing) or D (hyperarousal) criterion16; (2) symptoms met the B criterion, as well as one or more C and D symptom criteria25; (3) symptoms met at least two of three B, C, and D criteria.35
Table 1 also shows sensitivities, specificities, positive and negative predictive values, positive and negative likelihood ratios, and efficiencies for a range of IES-R total score thresholds (1.0-2.0), using CAPS-diagnosed, last-week, full PTSD or partial PTSD as criterion variables. Using an IES-R total-score threshold of 1.6, sensitivities ranged from 80% to 100%, specificities 85% to 91%, positive predictive values 50% to 75%, negative predictive values 93% to 100%, positive likelihood ratios 6.5 to 9.0, negative likelihood ratios 0.0 to 0.2, and efficiencies 87% to 90%.
Using the information in Table 1, we concluded that an IES-R total score threshold ≥ 1.6 was optimal for screening purposes in this population of ALI survivors. Figure 3 illustrates, using a flow diagram, the diagnostic accuracy of this threshold in predicting CAPS-diagnosed full PTSD.
Figure 3.
Flow diagram of the diagnostic accuracy of an IES-R total score threshold ≥ 1.6 using CAPS PTSD as the criterion variable. See Figure 1 legend for expansion of abbreviations.
In the exploratory subgroup analyses, we used any last-week, partial PTSD as the criterion variable to maximize power. The AUC did not differ substantially among subgroups. For the 29 patients aged < 51 years at interview, the AUC was 90% (95% CI, 78%-100%), and for the 31 patients aged ≥ 51 years at interview, the AUC was 99% (95% CI, 96%-100%). For the 23 male patients, the AUC was 100%; for the 37 female patients, the AUC was 95% (95% CI, 89%-100%). For the 35 ALTOS patients interviewed 1 year after ALI, the AUC was 94% (95% CI, 86%-100%); for the 25 ICAP study patients interviewed 2 to 5 years after ALI (grouped together to maximize power), the AUC was 97% (95% CI, 90%-100%).
Discussion
To our knowledge, this is the first study to assess the IES-R as a measure of PTSD symptoms in ALI (or other critical illness) survivors. The IES-R appears to be an excellent brief measure of PTSD symptoms in this population; the correlation between the IES-R total score and the CAPS total severity score was high and in the expected range for measures of the same mental health construct (0.7-0.8). In addition, the IES-R appears to be an appropriate screening tool for full or partial PTSD. At an IES-R threshold of 1.6, using full or partial PTSD as criterion variables, sensitivities ranged from 80% to 100%, specificities 85% to 91%, positive predictive values 50% to 75%, negative predictive values 93% to 100%, positive likelihood ratios 6.5 to 9.0, negative likelihood ratios 0.0 to 0.2, and efficiencies 87% to 90%.
To our knowledge, the only self-report questionnaire of PTSD symptoms previously evaluated vs clinician diagnoses in ICU survivors is the Posttraumatic Stress Syndrome 10-Questions Inventory (PTSS-10).40 The PTSS-10 mainly measures hyperarousal symptoms of PTSD (five items), with one item to assess each of the two other major DSM-IV PTSD domains: reexperiencing (“nightmares”) and avoidance (“the need to withdraw from others”).41 The PTSS-10 also includes two mood items (“depression, I feel dejected/down-trodden” and “frequent mood swings”) and one muscle-tension item, none of which are DSM-IV PTSD symptoms. Though the IES-R includes more items, potential advantages include the following: (1) it is “anchored” to a particular trauma (eg, critical illness and ICU care), and (2) it covers a wide range of DSM-IV PTSD symptoms. Recently, Twigg et al41 expanded the PTSS-10 to create the PTSS-14, which includes a flashback item, an additional avoidance item, and two numbness items. The investigators evaluated the PTSS-14 against the Posttraumatic Stress Diagnostic Scale,42 a validated diagnostic questionnaire. The AUC ranged from 82% to 95%.41
This study has potential limitations. First, the positive predictive values from this study of ALI survivors may not generalize well to all critical illness survivors. That is, critical illnesses like ALI and septic shock may be associated with relatively more post-ICU PTSD phenomena,2,4,5 and other PTSD risk factors could also affect prevalences, and thus positive predictive values. Second, we did not evaluate survivors until a year or more after ALI. However, we are encouraged because the AUC did not differ substantially for the group assessed 1 year post-ALI (ie, ALTOS patients) vs the group assessed between 2 and 5 years post-ALI (ie, ICAP study patients). Third, we had limited power in our exploratory subgroup analyses, and limited information on PTSD risk factors such as pre-ALI traumatic events/psychiatric history or post-ALI social support. As noted in a recent review article,43 more studies are needed to examine PTSD screening-tool measurement properties in population subgroups. Fourth, we did not assess the measurement properties of the IES-R in survivors’ family members, who often experience substantial distress as well.44
Given the promising measurement characteristics of the IES-R, we propose that this instrument be used to examine questions regarding the etiology, prevention, and treatment of PTSD symptoms in critical-illness/ICU survivors. For example, to what extent is in-ICU amount/duration of benzodiazepine/opiate administration a marker of pre-ICU risk for post-ICU PTSD symptoms, vs a cause?45 If a need for high amounts of sedation reflects something about the patients themselves (eg, vulnerability to extreme distress in the context of critical illness/intensive care), such patients may benefit from more careful monitoring during recovery. However, if particular sedation strategies are causally related to post-ICU PTSD symptoms, then alternative sedation strategies may be preventive. Also, does corticosteroid administration prevent post-ICU PTSD symptoms generally, as seen previously in particular patient groups,46 and does in-ICU catecholamine administration cause PTSD symptoms?47 The answers could affect the risk-benefit analysis clinicians make when deciding whether to administer corticosteroids. Finally, what other preventive interventions or treatments may be useful? ICU diaries,48 in-ICU social support,49 and in-ICU psychologic interventions50 appear beneficial, but might post-ICU cognitive-behavioral therapy and/or antidepressants aid recovery? These are important questions for future research.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Bienvenu is the guarantor of the manuscript.
Dr Bienvenu: contributed to adapting the introduction to the diagnostic interview to focus on the period of critical illness and intensive care, conducting the diagnostic interviews, and drafting the manuscript and editing it for important intellectual content; served as the principal author; and approved the final draft.
Dr Williams: contributed to adapting the introduction to the diagnostic interview to focus on the period of critical illness and intensive care, conducting the diagnostic interviews, editing the manuscript for important intellectual content, and approved the final draft.
Mr Yang: contributed to adapting the introduction to the diagnostic interview to focus on the period of critical illness and intensive care, conducting the diagnostic interviews, editing the manuscript for important intellectual content, and approved the final draft.
Dr Hopkins: led one of the parent cohort studies, contributed to the design and coordination of the study, editied the manuscript for important intellectual content, and approved the final draft of the manuscript.
Dr Needham: led the parent cohort studies, contributed to the design and coordination of the study, edited the manuscript for important intellectual content, and approved the final draft of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding body supported the collection and maintenance of data in the parent studies. It had no role in the study design; data collection, analysis, and interpretation; writing the manuscript; or the decision to submit the manuscript for publication.
Other contributions: We thank all of the patients who participated in the study and the dedicated research staff who assisted with the study, including Joseph Besho, ScM; Alexander Brown, BS; Jahnavi Chatterjee, MHS; Alexandra Chong, MA; Victor Dinglas, MPH; Elizabeth Fuller, BA; Melissa McCullough, BS; Pedro Mendez-Tellez, MD; Laura Methvin, BA; Kristin Sepulveda, BA; Faisal Siddiqi, MD; and Elizabeth Vayda, MS.
Additional information: The e-Figure can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ALI
acute lung injury
- ALTOS
ARDS Network Long-Term Outcomes Study
- AUC
area under the receiver operating characteristics curve
- CAPS
Clinician-Administered PTSD Scale
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ICAP
Improving Care of ALI Patients
- IES
Impact of Event Scale
- IES-R
Impact of Event Scale-Revised
- PTSD
posttraumatic stress disorder
- PTSS-10
Posttraumatic Stress Syndrome 10-Questions Inventory
- ROC
receiver operating characteristics
Footnotes
Funding/Support: This research was supported by the National Institutes of Health [Grants HL73994, R01 HL88045, and R01 HL091760].
For editorial comment see page 1
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.DiMartini A, Dew MA, Kormos R, McCurry K, Fontes P. Posttraumatic stress disorder caused by hallucinations and delusions experienced in delirium. Psychosomatics. 2007;48(5):436-439. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11(1):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209-218. [DOI] [PubMed] [Google Scholar]
- 7.Weiss DS, Marmar CR.Wilson JP, Keane TM. The Impact of Event Scale-Revised.In.eds. Assessing Psychological Trauma and PTSD: a Practioner’s Handbook New York: Guilford Press; 1997;399-411. [Google Scholar]
- 8.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. [DOI] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003;49(1):1-6. [DOI] [PubMed] [Google Scholar]
- 10.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49(1):7-18. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818-824. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health Clinical Center. Evaluating health outcomes and quality of life after acute lung injury among participants of the ALTA, OMEGA, EDEN, and SAILS (ARDS Network) studies (The ALTOS Study). NCT00719446. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2008 http://www.clinicaltrials.gov/ct2/show/NCT00719446?term=NCT00719446&rank=1. Updated January 4, 2013
- 13.Needham DM, Dennison CR, Dowdy DW, et al. Study protocol: The Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2006;10(1):R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatr Pract. 2004;10(5):307-313. [DOI] [PubMed] [Google Scholar]
- 15.Weiss DS.Wilson JP, Keane TM. The Impact of Event Scale-Revised.In.eds. Assessing Psychological Trauma and PTSD: A Practitioner’s Handbook 2nd ed. New York: Guilford Press; 2004;168-189. [Google Scholar]
- 16.Rash CJ, Coffey SF, Baschnagel JS, Drobes DJ, Saladin ME. Psychometric properties of the IES-R in traumatized substance dependent individuals with and without PTSD. Addict Behav. 2008;33(8):1039-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asukai N, Kato H, Kawamura N, et al. Reliability and validity of the Japanese-language version of the impact of event scale-revised (IES-R-J): four studies of different traumatic events. J Nerv Ment Dis. 2002;190(3):175-182. [DOI] [PubMed] [Google Scholar]
- 18.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale-Revised. Behav Res Ther. 2003;41(12):1489-1496. [DOI] [PubMed] [Google Scholar]
- 19.Sveen J, Low A, Dyster-Aas J, Ekselius L, Willebrand M, Gerdin B. Validation of a Swedish version of the Impact of Event Scale-Revised (IES-R) in patients with burns. J Anxiety Disord. 2010;24(6):618-622. [DOI] [PubMed] [Google Scholar]
- 20.Adkins JW, Weathers FW, McDevitt-Murphy M, Daniels JB. Psychometric properties of seven self-report measures of posttraumatic stress disorder in college students with mixed civilian trauma exposure. J Anxiety Disord. 2008;22(8):1393-1402. [DOI] [PubMed] [Google Scholar]
- 21.Beck JG, Grant DM, Read JP, et al. The impact of event scale-revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22(2):187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helzer JE, Kraemer HC, Krueger RF, Wittchen HU, Sirovatka PJ, Regier DA. Dimensional Approaches in Diagnostic Classification: Refining the Research Agenda for DSM-V. Arlington, VA: American Psychiatric Association;2008 [Google Scholar]
- 23.Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. [Google Scholar]
- 24.Carlier IVE, Gersons BPR. Partial posttraumatic stress disorder (PTSD): the issue of psychological scars and the occurrence of PTSD symptoms. J Nerv Ment Dis. 1995;183(2):107-109. [PubMed] [Google Scholar]
- 25.Taylor S, Koch WJ. Anxiety disorders due to motor vehicle accidents: nature and treatment. Clin Psychol Rev. 1995;15(8):721-738. [Google Scholar]
- 26.Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: findings from a community survey. Am J Psychiatry. 1997;154(8):1114-1119. [DOI] [PubMed] [Google Scholar]
- 27.Lipschitz DS, Rasmusson AM, Anyan W, Cromwell P, Southwick SM. Clinical and functional correlates of posttraumatic stress disorder in urban adolescent girls at a primary care clinic. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1104-1111. [DOI] [PubMed] [Google Scholar]
- 28.Schnurr PP, Ford JD, Friedman MJ, Green BL, Dain BJ, Sengupta A. Predictors and outcomes of posttraumatic stress disorder in World War II veterans exposed to mustard gas. J Consult Clin Psychol. 2000;68(2):258-268. [PubMed] [Google Scholar]
- 29.Mylle J, Maes M. Partial posttraumatic stress disorder revisited. J Affect Disord. 2004;78(1):37-48. [DOI] [PubMed] [Google Scholar]
- 30.Zlotnick C, Rodriguez BF, Weisberg RB, et al. Chronicity in posttraumatic stress disorder and predictors of the course of posttraumatic stress disorder among primary care patients. J Nerv Ment Dis. 2004;192(2):153-159. [DOI] [PubMed] [Google Scholar]
- 31.Breslau N, Lucia VC, Davis GC. Partial PTSD versus full PTSD: an empirical examination of associated impairment. Psychol Med. 2004;34(7):1205-1214. [DOI] [PubMed] [Google Scholar]
- 32.Favaro A, Tenconi E, Colombo G, Santonastaso P. Full and partial post-traumatic stress disorder among World War II prisoners of war. Psychopathology. 2006;39(4):187-191. [DOI] [PubMed] [Google Scholar]
- 33.Maia DB, Marmar CR, Metzler T, et al. Post-traumatic stress symptoms in an elite unit of Brazilian police officers: prevalence and impact on psychosocial functioning and on physical and mental health. J Affect Disord. 2007;97(1-3):241-245. [DOI] [PubMed] [Google Scholar]
- 34.Bienvenu OJ, Wuyek LA, Stein MB. Anxiety disorders diagnosis: some history and controversies. Curr Top Behav Neurosci. 2010;2:3-19. [DOI] [PubMed] [Google Scholar]
- 35.Friedman MJ, Resick PA, Bryant RA, Brewin CR. Considering PTSD for DSM-5. Depress Anxiety. 2011;28(9):750-769. [DOI] [PubMed] [Google Scholar]
- 36.Kapfhammer HP, Rothenhäusler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45-52. [DOI] [PubMed] [Google Scholar]
- 37.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132-156. [DOI] [PubMed] [Google Scholar]
- 38.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11(2):124-133. [Google Scholar]
- 39.Gordis L. Epidemiology. 3rd ed Philadelphia, PA: Elsevier Saunders;2004. [Google Scholar]
- 40.Stoll C, Kapfhammer HP, Rothenhäusler HB, et al. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999;25(7):697-704. [DOI] [PubMed] [Google Scholar]
- 41.Twigg E, Humphris G, Jones C, Bramwell R, Griffiths RD. Use of a screening questionnaire for post-traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesthesiol Scand. 2008;52(2):202-208. [DOI] [PubMed] [Google Scholar]
- 42.Foa EB. Posttraumatic Stress Diagnostic Scale Manual. Minneapolis, MN: National Computer Systems Inc.;1995. [Google Scholar]
- 43.McDonald SD, Calhoun PS. The diagnostic accuracy of the PTSD checklist: a critical review. Clin Psychol Rev. 2010;30(8):976-987. [DOI] [PubMed] [Google Scholar]
- 44.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40(2):618-624. [DOI] [PubMed] [Google Scholar]
- 45.Jones C, Bäckman C, Capuzzo M, Flaatten H, Rylander C, Griffiths RD. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33(6):978-985. [DOI] [PubMed] [Google Scholar]
- 46.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DE Quervain DJ, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci. 2006;1071:46-53. [DOI] [PubMed] [Google Scholar]
- 47.Schelling G, Stoll C, Kapfhammer HP, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Crit Care Med. 1999;27(12):2678-2683. [DOI] [PubMed] [Google Scholar]
- 48.Jones C, Bäckman C, Capuzzo M, et al. ; RACHEL group. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deja M, Denke C, Weber-Carstens S, et al. Social support during intensive care unit stay might improve mental impairment and consequently health-related quality of life in survivors of severe acute respiratory distress syndrome. Crit Care. 2006;10(5):R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peris A, Bonizzoli M, Iozzelli D, et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Crit Care. 2011;15(1):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement