Abstract
ICUs are experiencing an epidemic of patients with acute brain dysfunction (delirium) and weakness, both associated with increased mortality and long-term disability. These conditions are commonly acquired in the ICU and are often initiated or exacerbated by sedation and ventilation decisions and management. Despite > 10 years of evidence revealing the hazards of delirium, the quality chasm between current and ideal processes of care continues to exist. Monitoring of delirium and sedation levels remains inconsistent. In addition, sedation, ventilation, and physical therapy practices proven successful at reducing the frequency and severity of adverse outcomes are not routinely practiced. In this article, we advocate for the adoption and implementation of a standard bundle of ICU measures with great potential to reduce the burden of ICU-acquired delirium and weakness. Individual components of this bundle are evidence based and can help standardize communication, improve interdisciplinary care, reduce mortality, and improve cognitive and functional outcomes. We refer to this as the “ABCDE bundle,” for awakening and breathing coordination, delirium monitoring, and exercise/early mobility. This evidence-based bundle of practices will build a bridge across the current quality chasm from the “front end” to the “back end” of critical care and toward improved cognitive and functional outcomes for ICU survivors.
The ICU provides treatment of the sickest hospitalized patients. Advances in intensive care have rescued many who would have previously died. However, many survivors suffer from severe symptoms of disease processes acquired or accelerated during the ICU stay. These symptoms typically arise from two common and often unrecognized conditions that have a significant impact on the quality and quantity of life following critical illness: ICU delirium and ICU-acquired weakness and their chronic sequelae.
Delirium is an acute, fluctuating change in consciousness and cognition that develops over a brief time period.1 It can be hyperactive, characterized by agitation and emotional lability (less common), or hypoactive, characterized by apathy and diminished responsiveness (more common), or mixed.2,3 ICU delirium is a frequent complication of critical care, developing in approximately two-thirds of critically ill patients.4‐6 Despite the high prevalence, without active monitoring, it goes undiagnosed in up to 72% of cases.7‐9 Many patients have preexisting risk factors, including comorbidities (eg, dementia) and acute physiologic derangements present at ICU admission (eg, elevated creatinine, admission severity of illness). Importantly, a substantial proportion of patients acquire additional risk factors while in the ICU that independently predict delirium incidence or amplify preexisting risk factors. Some of these iatrogenic risk factors are modifiable, including both pharmacologic and nonpharmacologic factors. For example, multiple studies highlight the relationship between ICU delirium and the use and management of potent sedative and analgesic agents, with a notable increased risk of delirium with benzodiazepines.10‐12 Nonpharmacologic examples include immobility in the ICU13,14 and environmental factors (eg, lack of access to daylight) that are both amenable to intervention.6,15,16 For the purposes of this discussion we refer to newly acquired and potentially modifiable delirium in the critically ill as ICU-acquired delirium. This terminology is selected to bring attention to the potentially modifiable nature of this disorder and to draw parallel with terminology that is increasingly accepted for neuromuscular disease in the critically ill, or ICU-acquired weakness.17
ICU-acquired weakness is the acute onset of neuromuscular/functional impairment in the critically ill for which there is no plausible etiology other than critical illness.18,19 Generalized weakness impairs ventilator weaning and functional mobility. Acute morbidities (eg, acute physiology score, hyperglycemia) and medications (eg, corticosteroid use) are reported risk factors for this condition.20‐23 An additional key risk factor for ICU-acquired weakness is the duration of mechanical ventilation experienced by patients,24,25 with weakness occurring in up to 58% of patients who receive mechanical ventilation for at least 7 days.26,27
Although unique in their clinical presentation, these conditions share important risk factors, outcomes, and evidence-based preventative care processes that are widely available but currently underused. In this article, we review these disorders, propose a preventative bundle of care processes, highlight gaps in quality performance, and provide guiding principles to fill these gaps.
ICU-Acquired Delirium and Weakness: Precursors to Poor Patient Outcomes
ICU delirium is independently predictive of numerous adverse outcomes. Patients experiencing ICU delirium have a 49% increased risk of remaining in the hospital on any given day compared with those without delirium.28 Among hospital survivors, delirium influences poor long-term outcomes, including cognitive impairment,29,30 institutional placement,31 and mortality.32,33 Two prospective cohort studies similarly revealed that each additional day with delirium was independently associated with a 10% increased risk of death at 6 months (hazard ratio, 1.10; 95% CI, 1.0-1.3)32 and 1 year (hazard ratio, 1.10; 95% CI, 1.0-1.2),33 respectively.
ICU-acquired weakness is likewise predictive of poor outcomes. Among patients ventilated more than a week, those with ICU-acquired weakness require approximately 20 additional days of mechanical ventilation26,34 and have increased mortality (48% vs 19%, P = .03).27,35 Effects of ICU weakness persist well after hospital discharge, with 60% of patients experiencing continued muscle dysfunction up to 1 year after illness.25
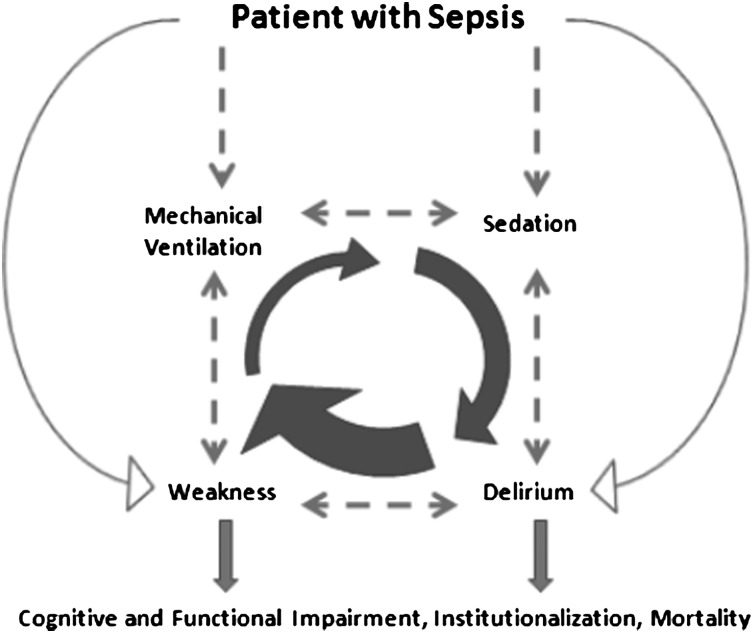
Delirium and weakness are conditions that are influenced by illness, aggravated by treatment modalities in the ICU, and that interact with each other. Sepsis is a diagnostic prototype that illustrates the synergistic relationship between ICU-acquired delirium and weakness36,37 and demonstrates the important role of predisposing as well as iatrogenic risk factors (Fig 1). Sepsis-induced endotoxin and cytokine release,38,39 hypoxemia,40,41 and endothelial dysfunction42 present examples of unique mechanisms that reach common final pathways toward weakness and delirium. Patients with sepsis are also exposed to overlapping risk factors, such as sedation and mechanical ventilation-care processes tightly linked with and potent risk factors for delirium and weakness, respectively.10,11,24
Figure 1.
Relationship between ICU-acquired delirium and weakness in a patient with sepsis.
Sedation without explicit targets extends mechanical ventilation needs, creating a dynamic feedback loop that aggravates the risks of delirium and weakness.43 For example, excessive sedation decreases the likelihood a patient can safely perform or pass a spontaneous breathing trial (SBT), leading to additional days of sedation and mechanical ventilation. Even when sedation stops, patients may continue to have delirium. Delirium commonly continues the cycle of prolonged ventilation, acting as a potent barrier to extubation following a successful SBT,44 once again resulting in additional days of sedation and ventilation.
Finally, emerging evidence suggests that physical performance and cognitive performance influence each other, further potentiating the feedback loop.14,45,46 A preventative strategy must take advantage of shared and reinforcing features outlined above (and in Fig 1) to be more efficient and effective in minimizing delirium, weakness, and additional adverse outcomes.
ABCDE: A Unifying Strategy to Mitigate ICU-Acquired Delirium and Weakness
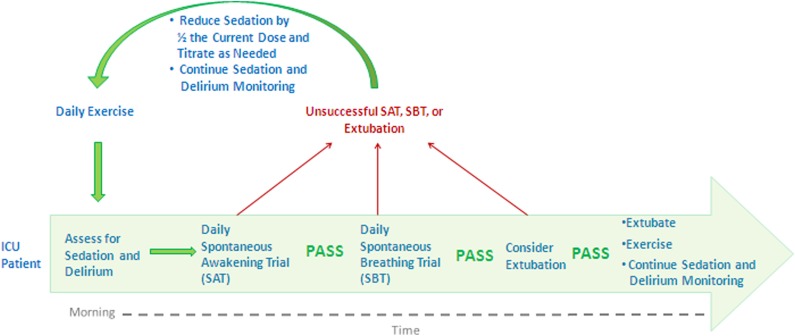
Improving outcomes in patients experiencing ICU-acquired delirium and weakness can be achieved by aligning and supporting the people, processes, and technology already existing in ICUs. Not only are advanced machines, expensive medications, and additional provider technical skills not required, but technology can be reduced, medications adjusted, and teamwork improved to minimize the burden of provider tasks. Such improvements may result from implementing a set of practices such as: awakening and breathing coordination, delirium monitoring, and exercise/early mobility—the ABCDE bundle (Fig 2). ABCDE is a multicomponent process that is intentionally interdependent and designed to: (1) improve collaboration among clinical team members, (2) standardize care processes, and (3) break the cycle of oversedation and prolonged ventilation, which appear causative to delirium and weakness.
Figure 2.
ABCDE is an ICU-acquired delirium and weakness mitigation strategy. This strategy is a protocolized bundle performed daily on mechanically ventilated and/or sedated patients in the ICU. This strategy is interdisciplinary by design and most effective when implemented by nursing, respiratory therapy, and physical therapy personnel working together as an ICU team. ABCDE = awakening and breathing coordination, delirium monitoring, and exercise/early mobility.
Awaken the Patient Daily: Sedation Cessation
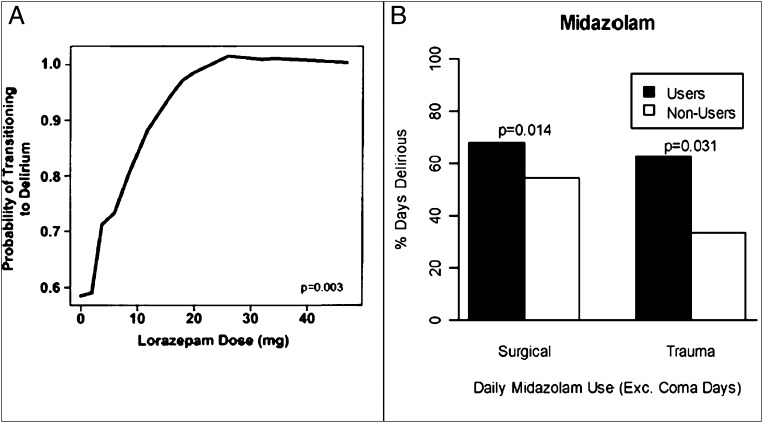
Sedative selection and management are leading modifiable risk factors for preventing delirium in the ICU.10,11 Sedative drugs, universally used in critically ill patients, reduce the work of breathing and alleviate agitation. Evidence suggests that benzodiazepines pose the highest risk. Although withholding benzodiazepines may not eliminate delirium, strong evidence suggests that increasing doses dramatically increases the risk of delirium in a dose-dependent fashion (Fig 3)10,12 and simultaneously prevents patient mobility.24 In multiple studies, daily spontaneous awakening trials (SATs) facilitate the transition from drug-induced coma to consciousness, reduce the duration of mechanical ventilation, and reduce ICU complications as well as costs.47‐49 Although the benefits of protocolized SATs have been reported among broad populations, the benefits may not be realized by some specific populations, most notably those experiencing alcohol withdrawal and delirium tremens, or those already maintained at a very light level of sedation.50,51
Figure 3.
A, Data from Pandharipande et al10 indicate that lorazepam dose in the preceding 24 h is an independent predictor for transitioning to delirium in the ICU. The effect rapidly increased up to doses of 20 mg/d, at which point the effect plateaued at near 100% probability of transition to delirium. B, Data from Pandharipande et al12 demonstrate that in both surgical and trauma ICU patients, users of midazolam have statistically increased number of days of delirium.
Breathing: Daily Interruptions of Mechanical Ventilation
Terminating mechanical ventilation requires objective assessment for patient readiness, best performed by daily SBTs in patients meeting established safety criteria, such as being on ≤ 50% Fio2 and positive end-expiratory pressure < 8 cm H2O. The first randomized trial of a spontaneous breathing protocol with a control group of which we are aware established decreased ventilator days and complications of mechanical ventilation.52 Later reports confirmed that nonphysician protocolized management (eg, using respiratory therapists) of SBTs resulted in no loss of safety, while simultaneously increasing nonphysician provider autonomy.53 In addition, these trials highlighted the importance of interdisciplinary coordination of sedation and ventilation between respiratory therapists and nurses.
Coordination: Daily Awakening and Daily Breathing
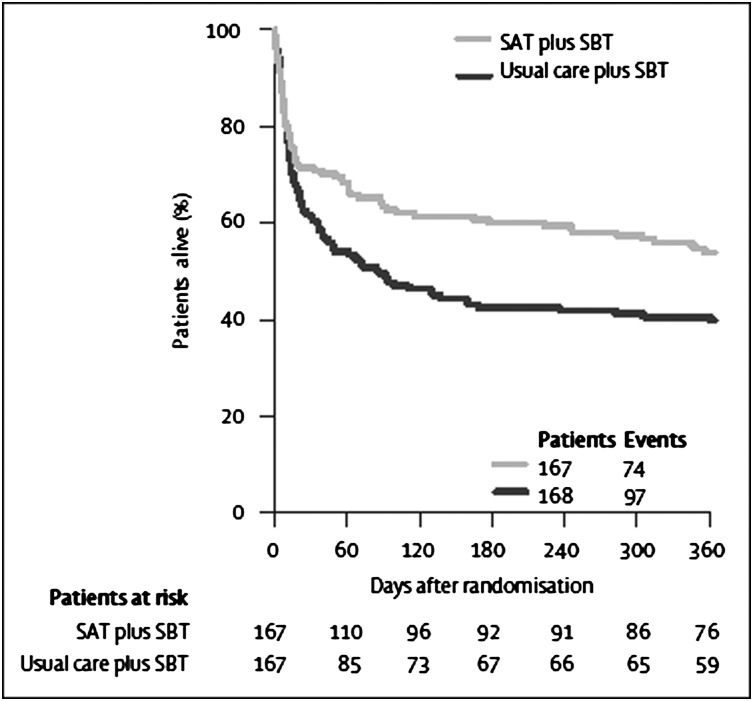
Recognizing the benefit of both protocolized interruptions of sedation and mechanical ventilation, Girard et al54 combined these approaches in what is known as the Awakening and Breathing Controlled trial, or the ABC trial. Daily SATs coordinated with daily SBTs decreased adverse cognitive outcomes, reduced hospital length of stay (LOS) by 4 days, and reduced death at 1 year by 14% (Fig 4). The processes were synergistic, yielding outcome reductions in excess of what has been shown when the protocols were delivered independently (ie, 4 days improvement in LOS vs 2 days when done independently).47,52,54
Figure 4.
One-year survival analysis of the Awakening and Breathing Controlled Trial from Girard et al.54 Survival was 14% higher at 1 year among the intervention group (spontaneous awakening trial coordinated with spontaneous breathing trial) vs the control group (usual care plus spontaneous breathing trial). SAT = spontaneous awakening trial; SBT = spontaneous breathing trial.
Delirium Monitoring
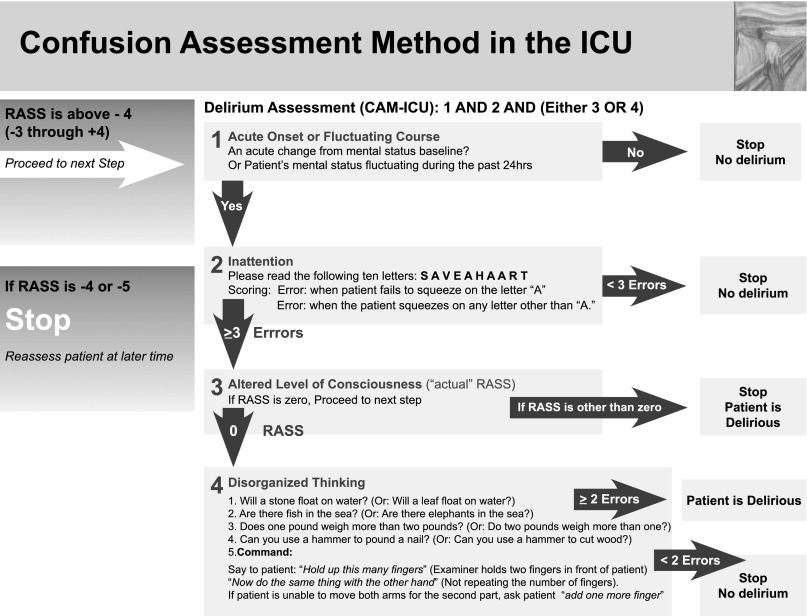
Delirium monitoring is a guideline-recommended practice.55 Instruments such as the Intensive Care Delirium Screening Checklist56 and the confusion assessment method for the ICU (CAM-ICU)5,57 offer highly sensitive and specific tools for delirium detection. Both tools have been validated across populations of mechanically ventilated and nonventilated patients and are in use in > 20 countries and languages. Of course, monitoring delirium alone may not be sufficient to change outcomes. Similar to other modes of physiologic monitoring in the ICU, it is what one does with the information that matters. Using such tools to identify delirium prompts providers to investigate and treat underlying conditions that may otherwise be delayed or missed altogether.
Sedation monitoring can likewise be achieved with simple instruments validated in the ICU. Sedation scales (eg, Sedation Agitation Scale,58 Ramsay Score,59 Richmond Agitation-Sedation Score,60 Minnesota Sedation Assessment Tool61) should be integrated with, and used complementary to, delirium instruments (eg, Intensive Care Delirium Screening Checklist,56 CAM-ICU5). Figure 5 illustrates an example of this.57 Use of sedation assessment tools provides a shared language to assess and communicate sedation across providers, thus clarifying sedation indications, goals, and outcomes. In the absence of reliable measures of sedation, clinicians risk both oversedation and undersedation. This can lead to severe adverse consequences of the potent medications, including prolonged mechanical ventilation, immobility, and delirium. In contrast, objective measures of sedation, rather than clinician judgment alone, can lead to reduced mechanical ventilation time and complications.62,63
Figure 5.
CAM-ICU. Available at www.icudelirium.org. This stepwise approach integrates information from the any sedation scale into the delirium assessment. CAM-ICU = confusion assessment method for the ICU5,56; RASS = Richmond Agitation-Sedation Score.60 Republished with permission from the American Medical Association.5
Exercise/Early Mobility
Early mobilization of ICU patients reduces acute cognitive and physical dysfunction. Multiple studies have demonstrated the feasibility of early mobilization among patients with respiratory failure.13,14,64,65 In addition, early physical therapy has been shown to independently reduce hospital LOS up to 3 days,13,65 reduce delirium incidence,13,14 and increase return to independent functioning.13,14 Although not yet a part of formal guidelines, the compelling body of evidence suggests that early physical exercise should be considered strongly as a routine part of ICU care.
The relationship between ICU-acquired delirium and long-term cognitive impairment, and subsequent risks to patient safety and quality of life, demands that cognitive rehabilitation also be considered.28,29 Cognitive rehabilitation improves neuropsychologic abilities, such as attention, memory, and executive function,66,67 for multiple conditions associated with acute brain injury, although these techniques of cognitive rehabilitation have not been formally tested in general medical/surgical ICU survivors. Patients at high risk for acquired and long-lasting cognitive dysfunction (ie, prolonged delirium, sepsis) or with measured cognitive deficits may be considered for referral to cognitive rehabilitation, especially with attention toward executive and memory deficits.
ABCDEs: Simple Does Not Mean Easy
The Institute of Medicine’s 2001 landmark report, Crossing the Quality Chasm: A New Health System for the 21st Century, stated that “health care today harms too frequently and routinely fails to deliver potential benefits.”68 Although great strides have been made in improving the quality and safety of critical care, adherence to recommended strategies of ICU care remains inadequate. The ABCDE intervention bundle is one important approach to cross this quality chasm.
Implementing Awakening and Breathing Coordination
Despite the benefit from SATs, only 40% of ICU providers reported using daily sedation interruptions, and among those, only 63% did so for all patients.69 A more recent survey of intensivists revealed < 50% reported using SATs.70 Use of SBTs is also lagging. Kahn et al71 found that among academic ICUs, the rates of SBTs ranged from 31% to 42%, despite high levels of intensivist staffing. In a recently published Canadian survey, only 20% of intensivists reported always performing an SBT (or assessing for readiness).72
Implementing Delirium Monitoring
Monitoring for delirium is an emerging component of modern ICUs over the past 10 years. A Canadian survey in 2001 found that only 4% of intensivists used a delirium scoring system,69 whereas 5 years later a US survey in 2006 found that 33% of intensivists use a validated delirium tool.70 The use of validated sedation monitoring scales is also inadequate. In one study, sedation scales were routinely used in approximately 50% of ICUs.69 For institutions using a scale, it was common to choose unvalidated scales over scales specifically validated for use in the ICU. More recent surveys revealed that 30% to 40% of intensivists continue to manage sedation without a specific monitoring instrument.70,73
Implementing Exercise/Early Mobility
Use of physical therapy targeted toward ICU patients is increasingly common, yet not uniform, and faces many barriers, including competing processes of care, lack of early mobilization care planning, and inadequate technology and/or collaboration.64 Morris et al,65 reported that among 165 patients in a “usual care” arm of a trial testing early ICU mobilization, only 47% of patients had any physical therapy. In another study of critically ill patients, 20% received no physical activity, and 15% more only received passive range of motion exercises during the ICU stay.74
Crossing the Quality Gap With ABCDE: Key Implementation Principles
Implementation of new discoveries into practice is commonly delayed. The gaps in process performance to prevent and/or mitigate ICU-acquired delirium and weakness are not unexpected, as the most appropriate care is often provided only half of the time.75 Similar to other conditions, reversing the epidemic of ICU-acquired delirium and weakness will not depend solely on new evidence. Application of current evidence into routine practice should yield great benefit. To do so, we provide seven guiding principles:
Cognitive and functional decline in the ICU must change from being viewed as “part of the inevitable consequences of critical illness” to a modifiable condition. Central line-associated bloodstream infections were once considered part of the unavoidable complications of ICU care. We now know this is not so. With rigorous adherence to infection control techniques, coupled with simulation-based training, such infections can be nearly eliminated.76‐78 An analogous transformation must occur in approaches to ICU-acquired delirium and weakness. Although these conditions may occur in the absence of specific modifiable risk factors (Fig 3), ICU provider teams must recognize the important role that modifiable risk factors play and use process improvement methods to achieve reductions believed unimaginable today. In fact, government stakeholders already view delirium through the lens of quality and safety, having recently proposed its inclusion as an avoidable hospital-acquired condition.79
Improvement requires evolution in critical care team roles. Repeated studies demonstrate that nurses and respiratory therapists can successfully use sedation and mechanical ventilation protocols53,54,63,65 and streamline the process of care from a minimum of three separate processes to one unifying process. Although physician expertise and judgment is critical in ICU management, mounting evidence suggests that a team-based, evidence-based, and systematic approach to care delivery is required.
Teams must shift from multidisciplinary to interdisciplinary care. Implementation of ABCDE cannot succeed with multidisciplinary silos. ABCDE requires each team member to provide and receive constant bidirectional feedback facilitated by common assessment tools that describe complex constructs of sedation and delirium. The very nature of ABCDE combines processes with intentional synergy, wherein the success of one depends and augments the success of others. For example, communication across physicians, nurses, respiratory therapists, and physical therapists is facilitated with standardized sedation and delirium measures. SBTs cannot begin until nurses perform and communicate safe completion of SATs. Safe extubation cannot occur without SAT and SBT results communicated to the physician. Participation in physical therapy requires appropriately targeted sedation.
ABCDE should become the default practice. Patients cannot afford to wait for physicians to make decisions regarding initiation of an SAT, SBT, or physical therapy, especially when nurses or respiratory therapists may be present at the bedside more often and have an even better ability to manage processes of care and monitor for subsequent improvements or decline. ABCDE practices must be structured as a daily part of care with clearly laid out safety guidelines. The components of this bundle are safe, reliable, and save lives.14,54
Patients will wake up, breath, and exercise if we allow them. First, although sedative administration is a common practice, it may not always be required. In a randomized, controlled trial comparing a standard sedation protocol vs an analgesia-sedation protocol, Strøm et al80 demonstrated that critically ill patients can safely tolerate an analgesia-sedation strategy and experience reductions in the duration of mechanical ventilation. Second, implementation of daily SATs and SBTs raises appropriate concerns about inadequate rest and potential psychologic trauma, yet, to date there is no strong evidence to suggest either occurs.79,81 Despite a documented increased risk of self-extubation with paired SATs and SBTs, there is no evidence of increased reintubations among the same patients.54 Finally, in the case of protocolized exercise, not only did patients walk 5 days earlier when prompted by a protocol, but they did so without excess unplanned extubation or equipment removal.14,65 Qualitative data from patient interviews highlight perceived benefits of early exercise without evidence of serious psychologic side effects (see patient interview at www.hopkinsmedicine.org/oacis).
Checklists and daily goals should be used; not elegant, but effective. Prior studies have demonstrated the benefits of using a checklist in patient safety.76,77 A five-step checklist reduced the central line-associated bloodstream infection rate to near zero across 108 Michigan hospitals.77 A similar approach applied to ABCDE interventions may yield analogous benefits in preventing ICU-acquired delirium and weakness.
Incorporate process and outcomes monitoring. ICUs must strive to monitor their adherence with SATs, SBTs, and exercise/early mobility protocols. Such monitoring must include rates of delirium, sedation levels in comparison with process targets, and important outcomes of care (eg, duration of mechanical ventilation, mortality). Tracking performance offers the only chance to assess current performance, understand improvement barriers, and generate new targets for improvement.
Conclusions
ICU-acquired delirium and weakness are disorders of epidemic proportions and should be viewed as potentially preventable and/or modifiable outcomes for ICU survivors. Currently available processes of care can dramatically reduce this burden of disease. We propose implementation of a bundle of processes— awakening and breathing coordination, delirium monitoring, and exercise/early mobility—or the ABCDE bundle, to achieve this goal. The bundle requires minimal additional resources yet mandates an important investment in changing the operational culture of the ICU. Delirium and weakness must now be viewed as urgent issues affecting the immediate and long-term quality and safety of our sickest patients. ABCDE processes should be protocolized and all ICU team members empowered, including physicians, nurses, respiratory therapists, and physical therapists. Through constant measurement, analysis, and process improvement cycles, the critical care team can understand quality gaps, identify best practices, and continue to work toward the liberation of patients from harmful effects of prolonged sedation, mechanical ventilation, and bed rest. Indeed, patients’ survival and long-term quality of life depend on it.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Ely has received grant support and honoraria from Eli Lilly, Pfizer, Hospira, Aspect Medical Systems, and GlaxoSmithKline. Ms Pun has received honoraria from Hospira. Drs Vasilevskis, Speroff, and Dittus and Ms Boehm have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
Other contributions: We thank Teresa Chipps, BS, Department of Medicine (General Internal Medicine and Public Health), Center for Health Services Research, Vanderbilt University, Nashville, TN, for her administrative and editorial assistance in the preparation of this manuscript. The work was performed at the Veterans Administration, Tennessee Valley Healthcare System in Nashville, TN and Vanderbilt University, Nashville, TN.
Abbreviations
- ABCDE
awakening and breathing coordination, delirium monitoring, and exercise/early mobility
- CAM-ICU
confusion assessment method for the ICU
- LOS
length of stay
- SAT
spontaneous awakening trial
- SBT
spontaneous breathing trial
Footnotes
For editorial comment see page 1034
Funding/Support: Dr Vasilevskis was supported by the Veterans Affairs Clinical Research Center of Excellence and the Geriatric Research Education and Clinical Center, Veterans Affairs, Tennessee Valley Healthcare, Nashville, TN.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 2.Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75–85. doi: 10.153/SCNP00500075. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 4.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 6.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167(15):1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 7.Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins N, Blanchard MR, Tookman A, Sampson EL. Detection of delirium in the acute hospital. Age Ageing. 2010;39(1):131–135. doi: 10.1093/ageing/afp201. [DOI] [PubMed] [Google Scholar]
- 9.van Eijk MMJ, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37(6):1881–1885. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pisani MA, Murphy TE, Araujo KLB, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 17.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131(5):1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 18.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10) suppl:S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 19.Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–1231. doi: 10.1136/jnnp.47.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22(9):849–855. doi: 10.1007/BF02044106. [DOI] [PubMed] [Google Scholar]
- 21.De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Groupe de Reflexion et d’Etude sur les Neuromyopathies En Reanimation. Intensive Care Med. 1998;24(12):1242–1250. doi: 10.1007/s001340050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Letter MA, Schmitz PIM, Visser LH, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–2286. doi: 10.1097/00003246-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 23.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 24.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 25.Bercker S, Weber-Carstens S, Deja M, et al. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(4):711–715. doi: 10.1097/01.ccm.0000157969.46388.a2. [DOI] [PubMed] [Google Scholar]
- 26.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 27.Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274(15):1221–1225. [PubMed] [Google Scholar]
- 28.Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42(8):809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 29.Girard TD, Jackson JC, Pandharipande PP, et al. Duration of delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 33.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garnacho-Montero J, Amaya-Villar R, García-Garmendía JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med. 2005;33(2):349–354. doi: 10.1097/01.ccm.0000153521.41848.7e. [DOI] [PubMed] [Google Scholar]
- 35.Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 36.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33(6):941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 37.Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99(1):176–184. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- 38.Arvin B, Neville LF, Barone FC, Feuerstein GZ. Brain injury and inflammation. A putative role of TNF alpha. Ann N Y Acad Sci. 1995;765:62–71. doi: 10.1111/j.1749-6632.1995.tb16561.x. [DOI] [PubMed] [Google Scholar]
- 39.Zamir O, Hasselgren PO, Kunkel SL, Frederick J, Higashiguchi T, Fischer JE. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg. 1992;127(2):170–174. doi: 10.1001/archsurg.1992.01420020052008. [DOI] [PubMed] [Google Scholar]
- 40.Z’Graggen WJ, Lin CS, Howard RS, Beale RJ, Bostock H. Nerve excitability changes in critical illness polyneuropathy. Brain. 2006;129(pt 9):2461–2470. doi: 10.1093/brain/awl191. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 42.Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol. 2003;106(1):75–82. doi: 10.1007/s00401-003-0704-3. [DOI] [PubMed] [Google Scholar]
- 43.Dunn WF, Adams SC, Adams RW. Iatrogenic delirium and coma: a “near miss.”. Chest. 2008;133(5):1217–1220. doi: 10.1378/chest.08-0471. [DOI] [PubMed] [Google Scholar]
- 44.Robertson TE, Mann HJ, Hyzy R, et al. Partnership for Excellence in Critical Care Multicenter implementation of a consensus-developed, evidence-based, spontaneous breathing trial protocol. Crit Care Med. 2008;36(10):2753–2762. doi: 10.1097/ccm.0b013e3181872833. [DOI] [PubMed] [Google Scholar]
- 45.Yang FM, Inouye SK, Fearing MA, Kiely DK, Marcantonio ER, Jones RN. Participation in activity and risk for incident delirium. J Am Geriatr Soc. 2008;56(8):1479–1484. doi: 10.1111/j.1532-5415.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170(2):186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 47.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 48.Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32(6):1272–1276. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 49.Brook ADM, Ahrens TSD, Schaiff RP, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 50.de Wit M, Gennings C, Jenvey WI, Epstein SK. Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Crit Care. 2008;12(3):R70. doi: 10.1186/cc6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treggiari MM, Romand J-A, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37(9):2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 52.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 53.Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999;159(2):439–446. doi: 10.1164/ajrccm.159.2.9805120. [DOI] [PubMed] [Google Scholar]
- 54.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 55.Jacobi J, Fraser GL, Coursin DB, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 57.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 59.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2(5920):656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 61.Weinert C, McFarland L. The state of intubated ICU patients: development of a two-dimensional sedation rating scale for critically ill adults. Chest. 2004;126(6):1883–1890. doi: 10.1378/chest.126.6.1883. [DOI] [PubMed] [Google Scholar]
- 62.Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 63.Quenot JPM, Ladoire SM, Devoucoux FM, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007;35(9):2031–2036. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 64.Bailey PP, Miller RR, III, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10) suppl:S429–S435. doi: 10.1097/CCM.0b013e3181b6e227. [DOI] [PubMed] [Google Scholar]
- 65.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 66.Ho MR, Bennett TL. Efficacy of neuropsychological rehabilitation for mild-moderate traumatic brain injury. Arch Clin Neuropsychol. 1997;12(1):1–11. [PubMed] [Google Scholar]
- 67.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86(8):1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 68.Corrigan JM, Donaldson MS, Kohn LT, et al. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The Institute of Medicine; 2001. [Google Scholar]
- 69.Mehta SM, Burry LP, Fischer SM, et al. Canadian Critical Care Trials Group Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34(2):374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 70.Patel RPS, Gambrell MB, Speroff TP, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahn JM, Brake H, Steinberg KP. Intensivist physician staffing and the process of care in academic medical centres. Qual Saf Health Care. 2007;16(5):329–333. doi: 10.1136/qshc.2007.022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burns KEA, Lellouche F, Loisel F, et al. Weaning critically ill adults from invasive mechanical ventilation: a national survey. Can J Anaesth. 2009;56(8):567–576. doi: 10.1007/s12630-009-9124-8. [DOI] [PubMed] [Google Scholar]
- 73.Tanios MA, de Wit M, Epstein SK, Devlin JW. Perceived barriers to the use of sedation protocols and daily sedation interruption: a multidisciplinary survey. J Crit Care. 2009;24(1):66–73. doi: 10.1016/j.jcrc.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 74.Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36(4):1119–1124. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- 75.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 76.Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295–302. doi: 10.1136/qshc.2004.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 78.Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423. doi: 10.1001/archinternmed.2009.215. [DOI] [PubMed] [Google Scholar]
- 79.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 81.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]