Abstract
Amniotic fluid‐derived stem cells (AFSCs) are a unique stem cell source that may have great potential for use in tissue engineering (TE) due to their pluripotentiality. AFSCs have previously shown angiogenic potential and may present an alternative cell source for endothelial‐like cells that could be used in range of applications, including the pre‐vascularisation of TE constructs and the treatment of ischaemic diseases. This study investigated the ability of these cells to differentiate down an endothelial lineage with the aim of producing an endothelial‐like cell suitable for use in pre‐vascularisation. As hypoxia and the associated HIF‐1 pathway have been implicated in the induction of angiogenesis in a number of biological processes, it was hypothesised that culture in hypoxic conditions could enhance the endothelial differentiation of AFSCs. The cells were cultured in endothelial cell media supplemented with 50 ng mL−1 of VEGF, maintained in normoxia, intermittent hypoxia or continuous hypoxia and assessed for markers of endothelial differentiation at day 7 and 14. The results demonstrated that AFSCs subjected to these culture conditions display an endothelial gene expression profile and adopted functional endothelial cell characteristics indicative of early endothelial differentiation. Culture in continuous hypoxia enhanced endothelial gene expression but did not enhance functional endothelial cell characteristics. Overall, AFSCs subjected to endothelial stimuli demonstrated a less mature endothelial gene expression profile and phenotype when compared with HUVECs, the endothelial cell control. However, this study is the first time that the positive effect of an extended period of continuous hypoxic culture on endothelial differentiation in AFSCs has been demonstrated.
Keywords: amniotic fluid‐derived stem cells, endothelial cells, endothelial differentiation, hypoxia, intermittent hypoxia, pre‐vascularisation
Introduction
The absence of a sufficient vascular supply in tissue engineering (TE) constructs has been established as a major limiting factor in implant success in vivo (Ishaug‐Riley et al. 1998; Cheema et al. 2012), as cells are only capable of surviving approximately 150–200 μm from the nearest network of blood vessels (Folkman & Hochberg, 1973). The successful post‐implantation engraftment of these constructs relies on the rapid formation of stable and functional vasculature (Laschke et al. 2006; Unger et al. 2010), especially in those used in the repair of thick tissues such as bone. If the formation of vasculature does not occur, this can result in core degradation and necrosis of the implant (Ko et al. 2007).
Therapeutic strategies to enhance vascularisation within TE constructs have involved a number of different approaches, including the delivery of growth factors to induce angiogenesis (Ehrbar et al. 2004; Epstein, 2011; Murphy et al. 2014) and the use of gene‐activated matrices to enhance vessel development (Kyriakides et al. 2001; Geiger et al. 2005; Duffy et al. 2010). These strategies demonstrate varying degrees of success but are limited due to their lack of target‐specificity. An alternative approach involving the engineering of a vascular network within a TE construct, in vitro, prior to implantation, has emerged as a potential solution. This ‘pre‐vascularisation’ approach recently has been shown to have potential in the enhancement of construct engraftment post‐implantation (Unger et al. 2010; Duffy et al. 2011; McFadden et al. 2013; Roubelakis et al. 2013).
Primary endothelial cells are the most commonly used cell type in pre‐vascularisation due to their role in the formation and homeostasis of the vascular system. However, there is no consensus for the ideal source of primary endothelial cells (Finkenzeller et al. 2009). These cells must be used at an early passage number as they begin to lose angiogenic potential and show an increased apoptotic tendency over time (Prasad Chennazhy & Krishnan, 2005; Prigozhina et al. 2011). However, the use of multi‐ and pluripotent stem cell types for the generation of endothelial‐like cells is an area of TE that shows great potential (Levenberg et al. 2002; Oswald et al. 2004; Doan et al. 2014). Amniotic fluid‐derived stem cells (AFSCs) are a cell source that has attracted recent attention due to their pluripotentiality (De Coppi et al. 2007). AFSCs have previously shown angiogenic potential, as they have been successfully differentiated into endothelial‐like cells (Zhang et al. 2009; Benavides et al. 2012; Ginsberg et al. 2012). They do not display any telomere shortening within the first 250 passages, allowing them to be maintained in culture for long periods of time (Miranda‐Sayago et al. 2011), which, along with their easy isolation, culture and maintenance (Shaw et al. 2011), potentially makes them an alternative cell source to primary endothelial cells. Therefore, the ability of AFSCs to undergo endothelial differentiation was assessed in this study.
In stem cell biology, there is a growing appreciation of the use of external stimuli to replicate biologically relevant culture conditions. Hypoxia and the HIF‐1 pathway have been implicated in the induction of angiogenesis in a number of biological processes, including bone development (Liao & Johnson, 2007; Portal‐Nunez et al. 2012). A deficiency of HIF‐1α in knockout mice embryos has been shown to result in lethal disruptions to cardiac and vascular development (Iyer et al. 1998; Kotch et al. 1999). Upregulated HIF‐1α, however, has shown potential in the enhancement of endothelial differentiation in a number of stem cell types. For example, it been shown to restore the gene expression of pro‐angiogenic factors in aged adipose‐derived stem cells (Efimenko et al. 2011) and to enhance the endothelial differentiation of embryonic stem cells and human bone marrow stem cells (Ong et al. 2010; Prado‐Lopez et al. 2010; Kusuma et al. 2014). The angiogenic growth factor VEGF is also known to be a downstream target of the HIF‐1 pathway (Liu et al. 1995; Namiki et al. 1995; Forsythe et al. 1996). For these reasons, in this study, hypoxia was chosen as a stimulus to direct endothelial differentiation of AFSCs. Two forms of exposure to hypoxia were utilised in this study: intermittent and continuous. Intermittent hypoxia was used as alternative to continuous hypoxia in order to investigate whether shorter periods of hypoxic exposure could have a similar effect on endothelial differentiation. The use of intermittent hypoxia in this manner has never been investigated.
In this study, we hypothesised that culture in hypoxic conditions could enhance the endothelial differentiation of AFSCs to produce an endothelial‐like cell suitable for use in pre‐vascularisation. We also aimed to obtain more information on the ability of AFSCs to adopt endothelial cell‐like characteristics. With this in mind, the objective of this study was to investigate whether culture in either intermittent or continuous hypoxia could enhance the ability of AFSCs to differentiate down an endothelial lineage and enhance their potential for use in TE applications. Human umbilical vein endothelial cells (HUVECs) were used as a positive control to allow the endothelial potential of the AFSCS to be assessed.
Methods
Cell culture
All cell culture work was carried out under sterile conditions in a laminar flow hood (SterilGard 111, MSC, Ireland). All cells were maintained at 37 °C in a 5% CO2 atmosphere in an incubator.
Culture of AFSCs
Amniotic fluid‐derived stem cells were obtained from Professor Shay Soker's group, Wake Forest University, North Carolina. The AFSCs had previously been isolated from back‐up amniocentesis samples (obtained with consent) using magnetic‐activated cell sorting (MACS) separation prior to their delivery. Cells were isolated on the basis of expression for C‐kit (CD117) as previously described (De Coppi et al. 2007). AFSCs were expanded in basic amniotic fluid cell (BAFC) growth media, which consisted of alpha‐MEM medium (Invitrogen, California, USA) containing 15% embryonic stem cell qualified‐fetal bovine serum (ES‐FBS) (Invitrogen), 1% l‐glutamine (Sigma‐Aldrich, Arklow, Ireland) and 2% penicillin/streptomycin (PenStrep) (Invitrogen), and was supplemented with 18% Chang B and 2% Chang C (Irvine Scientific, California, USA). Upon reaching 70% confluency, cells were washed using phosphate‐buffered saline (PBS) (Sigma‐Aldrich) and detached using 0.25% trypsin ethylenediaminetetraacetic acid (EDTA) (Sigma‐Aldrich). Cells were plated at a density of 3.5 × 103 cm−2. All AFSCs used during the study were used prior to passage 24.
Induction of endothelial differentiation of AFSCs
To facilitate endothelial differentiation, AFSCs were cultured in EndoGro™‐ VEGF complete media supplemented with 50 ng mL−1 recombinant VEGF‐165 (R&D systems), 5 ng mL−1 human fibroblast growth factor‐b, 5 ng mL−1 epidermal growth factor, 15 ng mL−1 insulin‐like growth factor‐1, 1 μg mL−1 hydrocortisone hemisuccinate, 0.75 U mL−1 heparin sulphate, 50 μg mL−1 ascorbic acid, 1% l‐glutamine and 2% FBS (Millipore, Massachusetts, USA). Media were changed every 3 days. AFSCs cultured in growth media were used as a negative control.
AFSC hypoxic culture regime
To assess the effect of hypoxia on endothelial differentiation, AFSCs were cultured in three O2 conditions: normoxia, intermittent hypoxia and continuous hypoxia (Table 1).
Table 1.
Summary of culture conditions.
| Normoxia | 20% O2 for 14 days |
| Intermittent hypoxia | 2% O2 for 8 h, followed by 20% O2 for 16 h per day, for 14 days in total |
| Continuous hypoxia | 2% O2 for 14 days |
AFSCs cultured in normoxia were maintained in a standard incubator (20% O2, 5% CO2) at 37 °C for the duration of the 14‐day culture period.
AFSCs cultured in intermittent hypoxia were maintained in an 856‐HYPO/EXP model hypoxia chamber (Plas Labs, MI, USA) at 2% O2 (5% CO2) for 8 h every day. For the remaining 16 h, the cells were maintained in a standard incubator at 20% O2.
AFSCs cultured in continuous hypoxia were maintained in a hypoxia chamber at 2% O2 and 5% CO2 for the full 14‐day culture period.
AFSCs were assessed for markers of endothelial differentiation and endothelial functionality at day 7 and 14.
Culture of HUVECs
HUVECs were used as a positive control to allow assessment of the endothelial potential of the AFSCS. Pooled‐donor HUVECs were purchased from Lonza (Berkshire, UK) and cultured in EndoGro™ media supplemented with 5 ng mL−1 of VEGF (Millipore). Media was replaced every 3 days and the cells were cultured until 90% confluency. When confluency was reached, the cells were washed using PBS and detached using 0.25% trypsin EDTA. All HUVECs in this experiment were used prior to passage 9.
The effect of hypoxic culture on HIF‐1α protein expression
At day 3 of culture, AFSCs were lysed with RotiLoad‐1 loading buffer 1× (80 μL per well) (Carl Roth, Karlsruhe, Germany), removed using Nunc™ Cell Scrapers (Thermo Scientific, Massachusetts, USA) and stored at −20 °C. HIF‐1 α expression was analysed using Western blotting as described by Dohle et al. (2011). Briefly, protein extracts were separated by molecular weight using polyacrylamide gel (Laemmli, 1970). Protein extracts were incubated at 95 °C for 5 min to allow for denaturation before sample loading. Separation of proteins was performed at 25 mA in SDS‐running buffer (25 mm Tris, 192 mm glycine, 0.1% SDS) (Sigma‐Aldrich, Schnelldorf, Germany). Protein transfer from the gel onto a nitrocellulose membrane was performed at 65 V in 1× transfer buffer (25 mm Tris, 190 mm glycine and 20% methanol) for 30 min using Standard SD transfer protocol (25 V, 1.0 A) for a Trans‐Blot Turbo Blotting System (Bio‐Rad Laboratories, Munich, Germany). The membrane was blocked in 5% milk powder containing 0.2% Tween PBS (blocking solution) (Sigma‐Aldrich) for 1 h at room temperature and was subsequently incubated with a mouse anti‐human HIF‐1α primary antibody (BD Biosciences, Oxford, UK) at a dilution of 1 : 250 in blocking solution. Blots were rinsed three times in wash buffer (0.2% Tween 20/PBS) and then incubated with an anti‐mouse horseradish peroxidase (HRP)‐conjugated secondary antibody (BD Biosciences) diluted 1 : 1000 in 5% milk powder in PBS for 2 h at room temperature. To detect unequal protein loading, ERK2 was detected on the membrane using a rabbit anti‐human primary antibody (1 : 3000) (BD Biosciences) and an anti‐rabbit secondary antibody (1 : 3000) (BD Biosciences) and used as reference protein in analysis. For detection of antibody‐labelled target proteins, membranes were covered with Super Signal West Dura Extended Detection Substrate (Thermo Scientific) for 5 min, excess substrate was removed and chemifluorescence was recorded with a CHEMI‐SMART 5100 (Peqlab, Erlangen, Germany).
The effect of hypoxic culture on gene expression
At day 7 and 14 of culture, AFSCs were lysed using a buffer composed of 1 : 100 β‐mercaptoethanol (Sigma‐Aldrich) in RLT buffer (Qiagen, Ireland) and stored at −80 °C. RNA was extracted using an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. RNA quality and quantity were determined using an RNA nanodrop (Thermo Scientific). Following RNA isolation, reverse transcription PCR was carried out using 200 ng of RNA. Genomic DNA was removed, followed by reverse transcription of the RNA (Qiagen) according to the manufacturer's instructions. Quantitative real‐time PCR (qRT‐PCR) was carried out using the 7500 Real Time polymerase chain reaction system (Applied Biosystems, Paisley, UK). The QuantiTect SYBR Green PCR kit (Qiagen) was used for this process according to the manufacturer's instructions with the QuantiTect primers for Angiopoietin 1 (Hs_ANGPT1_1_SG), VEGFR2 (Hs_KDR_1_SG), PECAM‐1 (CD31) (Hs_PECAM1_1_SG) and von Willebrand factor (vWF) (Hs_VWF_1_SG). These genes were chosen due to their expression by endothelial cells and association with endothelial differentiation. All gene expression was normalised against 18s (Hs_RRN18S_1_SG), a housekeeping gene commonly used for this purpose. Expression of the endothelial marker genes studied was determined using the relative quantification ΔΔCt method (Livak & Schmittgen, 2001).
The effect of hypoxic culture on VEGF secretion
VEGF concentration within the cell media after 7 and 14 days of culture was quantified using a DuoSet ELISA kit (R&D systems) as per the manufacturer's instructions. Cells were cultured in EndoGro™ media without supplemented VEGF for 2 days before each time‐point to prevent interference with assay results. Samples were evaluated on a photometric plate reader (Varioskan Flash, Fisher Scientific, Dublin, Ireland) at 450 nm with corrections at 570 nm.
The effect of hypoxic culture on CD31 cell‐surface expression
The cell‐surface expression of CD31 in AFSCs was analysed, as CD31 is a well‐known marker protein expressed in all endothelial cells and is commonly used as a criterion for their identification and isolation (Baldwin et al. 1994; Dong et al. 1997). At day 7 and 14 of culture, AFSCs were fixed using 3.7% paraformaldehyde (PFA) (Sigma‐Aldrich) and stored in PBS at 4 °C. Fixed samples were permeabilised in 0.002% Triton/PBS (Sigma‐Aldrich) for 10 min. Samples were washed in PBS and then incubated in a 1 : 50 dilution of monoclonal mouse anti‐human CD31 antibody (Dako, Hamburg, Germany) in 1% bovine serum albumin (BSA)/PBS (Sigma‐Aldrich). Samples were washed in PBS again and subsequently incubated in a 1 : 1000 dilution of goat anti‐mouse 488 (Life Technologies, Darmstadt, Germany) in 1% BSA/PBS for 1 h. Samples were then washed twice in PBS and cell nuclei were stained using a 1 : 10 000 dilution of Hoechst stain solution in PBS (Sigma‐Aldrich) for 5 min.
Assessing the effect of hypoxic culture on endothelial‐like functionality
In order for AFSCs to be considered endothelial‐like cells, they must be able to demonstrate that they possess abilities that are characteristic of functional endothelial cells. With this in mind, the ability of AFSCs to adopt functional endothelial cell characteristics was assessed using two standard tests for endothelial functionality: the uptake of acetylated low‐density lipoprotein (ac‐LDL) and tubule formation on Matrigel™.
The effect of hypoxic culture on ac‐LDL uptake
The ability of AFSCs to take up fluorescently tagged ac‐LDL was analysed at day 7 and 14 of culture. Low‐density lipoprotein (LDL) refers to a class of lipoprotein particles which carry cholesterol in the blood and around the body for use by cells. Endothelial cells from various sources in the body have previously been shown to possess a higher affinity for ac‐LDL rather than for native LDL (Stein & Stein, 1980; Pitas et al. 1981). Therefore, uptake of ac‐LDL is a well recognised prerequisite for cells to be considered to be differentiating down an endothelial lineage, as it is known to be a functional characteristic of mature endothelial cells (Voyta et al. 1984). As a result, it is a well established method for the assessment and validation of endothelial differentiation (Silva et al. 2005; Wang et al. 2007; Zhang et al. 2011; Janeczek Portalska et al. 2012; Doan et al. 2014). At day 7 and 14 of culture, AFSCs were incubated in 10 ng mL−1 of Alexa Fluor 488‐conjugated ac‐LDL (Life Technologies) diluted in EndoGro™ media. The cells were incubated with ac‐LDL for 4 h, at which point they were fixed with 3.7% PFA (Sigma‐Aldrich) and stored in PBS at 4 °C. Uptake of ac‐LDL was subsequently visualised using fluorescent microscopy (4× objective, Keyence BZ‐9000, Keyence, Neu‐Isenburg, Germany) with BZ II viewer software (Keyence). Media was changed every 3 days of culture.
The effect of hypoxic culture on tubule formation on Matrigel™
The ability of AFSCs to form tubules on Matrigel™ was analysed at day 7 and 14 of culture. This is considered a functional characteristic of endothelial cells. Matrigel™ is generally used to study the effect of pro‐angiogenic and anti‐angiogenic factors on endothelial cell function and to define endothelial cell populations (Browning et al. 2008; Arnaoutova et al. 2009). As a result, it is a well established method for the assessment and validation of endothelial differentiation and has been used in a number of studies for this purpose (Thangarajah et al. 2009; Zhang et al. 2009; Roura et al. 2012; Portalska et al. 2013). Growth Factor Reduced Matrigel™ (BD Biosciences) 120 μL was evenly distributed on a 48‐well plate (Greiner, Frickenhausen, Germany). EndoGro™ media (50 ng mL−1 VEGF) 1 mL was gently added to the wells. Cells from each O2 group were seeded at a density of 3 × 104 in their assigned wells. Tubule formation by cells on Matrigel™ was imaged at 6, 8, 12 and 24 h post‐seeding with a Leica DMIL microscope (10× objective, DFC420C digital camera). Five images were taken per well at random positions. Total tubule length was calculated using imagej software (ImageJ, U.S. National Institutes of Health, Maryland, USA). Total tubule length was quantified using tubules over 30 μm in length. This was done to minimise the quantification of non‐tubule cell structures.
Statistical analysis
To assess statistical differences between O2 groups, a one‐way anova with Tukey post‐hoc analysis was performed. To calculate statistical difference in tubule formation on Matrigel™, a two‐way anova with Bonferroni post‐test was performed. Error is reported in figures as the standard deviation of the mean and significance was determined using a probability value of P < 0.05. All experiments were carried out with a sample size of 3 unless otherwise stated.
Results
Hypoxic culture enhances endothelial differentiation
Hypoxic culture upregulates HIF‐1α protein expression in AFSCs
Western blotting was performed to confirm that culture in hypoxic conditions was leading to an upregulation of HIF‐1α, a master regulator of hypoxic response. Protein expression of HIF‐1α was observed in both intermittent hypoxia and continuous hypoxia by day 3 of culture (Fig. 1). Expression of HIF‐1α in intermittent hypoxia indicates that even an 8 h period of hypoxic exposure every 24 h was enough to activate the HIF‐1α pathway. Little to no visible HIF‐1α expression was observed in the normoxic group. Expression of ERK2, the loading control protein, was found to be at a similar level in each of the O2 conditions studied.
Figure 1.
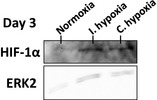
The effect of hypoxia on HIF‐1α protein expression in AFSCs after 3 days of culture as visualised using Western blotting. Bands indicating the expression of HIF‐1α protein were visible in intermittent hypoxia and continuous hypoxia. Little to no HIF‐1α was visible in normoxia. ERK2 was used as the housekeeping/reference protein.
Hypoxic culture enhances the endothelial gene expression profile of AFSCs
Endothelial gene expression levels were analysed using qRT‐PCR. Endothelial gene expression patterns of AFSCs in normoxia, intermittent hypoxia and continuous hypoxia were compared with those of AFSCs in growth media and HUVECs, the negative and positive controls, respectively, to assess the effect of hypoxia on endothelial differentiation.
By day 7, AFSCs in all three O2 conditions were adopting an early stage endothelial gene expression profile (Fig. 2). A significant (P < 0.001) four‐fold decrease in Angiopoietin 1 expression (Fig. 2A) was seen in AFSCs cultured in all O2 conditions relative to AFSCs in growth media (except for continuous hypoxia, which demonstrated a two‐fold decrease relative to AFSCs in growth media). HUVECs also displayed low expression levels of Angiopoietin 1 that were significantly (P < 0.001) decreased relative to AFSCs in growth media and continuous hypoxia. VEGFR2 and CD31 expression (Figs. 2B and C) was significantly enhanced (P < 0.001 and P < 0.01, respectively) 15‐ and 7‐fold, respectively, in continuous hypoxia relative to AFSCs in growth media and the other two O2 conditions. Non‐significant increases in the levels of expression of these two genes were also noted in normoxia and intermittent hypoxia in comparison with AFSCs in growth media. vWF expression (Figs. 2D) was non‐significantly increased in all three O2 conditions in comparison with AFSCs in growth media. VEGFR2, CD31 and vWF expression was significantly (P < 0.001) higher in HUVECs relative to all other groups.
Figure 2.
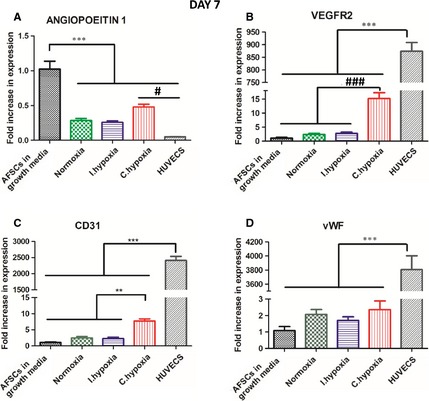
qRT‐PCR analysis investigating the expression of the endothelial markers (A) Angiopoietin 1, (B) VEGFR2, (C) CD31 and (D) vWF by AFSCs at day 7 of culture. AFSCs in growth media were used as a negative control and HUVECs were used as a positive control. AFSCs in all O2 conditions adopted an early stage endothelial gene expression profile. Significant increases in VEGFR2 and CD31 in continuous hypoxia indicated that continuous hypoxia enhanced the endothelial gene expression profile of AFSCs relative to AFSCs in growth media and the other O2 conditions. Endothelial gene expression in AFSCs in all three O2 conditions was significantly (P < 0.001) lower than the baseline expression of HUVECs. Values are expressed as mean ± SD, n = 4. ** P < 0.01 statistical significance differences relative to all other groups excluding HUVECs. *** P < 0.001 statistical significance differences relative to all other groups. # P < 0.001 statistical significance differences relative to HUVECs. ### P < 0.001 statistical significance differences relative to all other groups excluding HUVECs.
At day 14, an early stage endothelial gene expression profile similar to that of day 7 was observed (Fig. 3) in AFSCs in all three O2 conditions. Angiopoietin 1 expression (Fig. 3A) was significantly downregulated by approximately 50% in all groups relative to AFSCs in growth media (P < 0.01 relative to normoxia, P < 0.001 relative to all other groups). Angiopoietin 1 expression in HUVECs was approximately 10‐fold lower than in AFSCs in growth media (P < 0.001). Expression of VEGFR2 (Figs. 3B) was increased at least two‐fold in continuous hypoxia relative to normoxia (P < 0.05), intermittent hypoxia and AFSCs in growth media. CD31 (Figs. 3C) was significantly (P < 0.01) increased approximately five‐fold in continuous hypoxia relative to normoxia, intermittent hypoxia and AFSCs in growth media, demonstrating that continuous hypoxia was enhancing endothelial gene expression. vWF expression (Figs. 3D) was non‐significantly higher in normoxia and continuous hypoxia in comparison with AFSCs in growth media. VEGFR2, CD31 and vWF expression levels in HUVECs were significantly (P < 0.001) higher than in all other groups (Figs. 3B,C and D).
Figure 3.
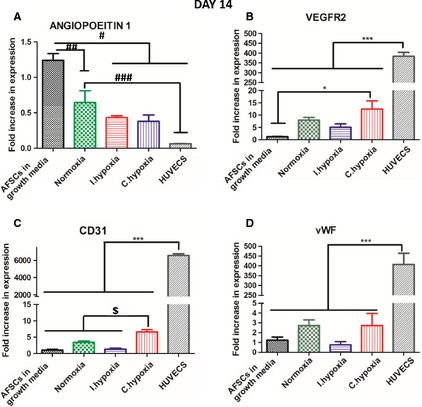
qRT‐PCR analysis investigating the expression of the endothelial markers (A) Angiopoietin 1, (B) VEGFR2, (C) CD31 and (D) vWF by AFSCs at day 14. AFSCs in growth media were used as a negative control and HUVECs were used as a positive control. AFSCs in all O2 conditions adopted an endothelial gene expression profile. Significant increases in CD31 (P < 0.01) and VEGFR2 (P < 0.05) expression in continuous hypoxia indicate that continuous hypoxia enhanced the endothelial gene expression profile of AFSCs. Endothelial gene expression was significantly (P < 0.001) lower in AFSCs subjected to endothelial stimuli than that of the baseline expression of HUVECs, indicating that differentiating AFSCs were not as mature as HUVECs. Values are expressed as mean ± SD, n = 4. * P < 0.05 statistical significance differences relative to AFSCs in growth media. *** P < 0.001 statistical significance relative to all other groups. # P < 0.001 statistical significance differences relative to intermittent hypoxia, continuous hypoxia and HUVECs. ## P < 0.01 statistical significance differences relative to normoxia. ### P < 0.01 statistical significance differences relative to HUVECs. $ P < 0.01 statistical significance differences relative to all other groups excluding HUVECs.
Table 2 shows the endothelial marker gene expression levels of the continuous hypoxia group from the previous two figures (Figs 2 and 3) together with those of the HUVEC control at day 14, in order to allow for a comparison of changes in gene expression between day 7 and 14. Continuous hypoxia was chosen as it was the O2 condition which induced the greatest change in endothelial gene expression (see Figs 2 and 3. Angiopoietin 1 expression decreased over the culture period, although the value observed in the HUVECs group was approximately seven‐fold lower relative to day 14. VEGFR2, CD31 and vWF expression remained at similar levels between day 7 and 14; however, as previously demonstrated in Figs 2 and 3, baseline expression in HUVECs was far higher at day 14, demonstrating that although continuous hypoxia may enhance endothelial gene expression relative to normoxia and intermittent hypoxia, it is still much lower than the baseline expression observed in the HUVEC control.
Table 2.
Comparison of expression levels of endothelial markers in continuous hypoxia at day 7 and 14 compared with HUVECs at day 14. Endothelial marker expression in continuous hypoxia was similar at day 7 and 14 but was still low when compared with the baseline expression of HUVECS, the endothelial cell control. The red arrow indicates whether gene expression in the HUVEC control was higher or lower than AFSCs at day 7 and day 14.
| Day 7 | Day 14 | HUVECs Day 14 | ||
|---|---|---|---|---|
| Angiopoietin 1 | 0.512 ± 0.05 | 0.395 ± 0.22 | 0.062 ± 0.005 |

|
| VEGFR2 | 13.24 ± 2.79 | 12.73 ± 5.46 | 384.2 ± 39.2 |

|
| CD31 | 7.75 ± 1.10 | 6.61 ± 1.21 | 6563.4 ± 364.06 |

|
| vWF | 2.36 ± 0.91 | 3.29 ± 2.70 | 407.8 ± 112.38 |

|
The endothelial gene expression profile of AFSCs over a 14‐day culture period indicates that differentiation is taking place in all three O2 conditions. Continuous hypoxia enhanced endothelial gene expression to the greatest extent; however, HUVECs displayed significantly higher baseline gene expression levels of VEGFR2, CD31 and vWF compared with AFSCs in any of the three O2 conditions.
Hypoxic culture enhances VEGF secretion by AFSCs
VEGF protein secretion by AFSCs during culture in normoxia, intermittent hypoxia or continuous hypoxia at day 7 and 14 was quantified using ELISA (Fig. 4). Low levels of VEGF secretion were observed in AFSCs in growth media and HUVECs after 14 days. Day 14 normoxia produced approximately five‐fold higher levels of VEGF in comparison with AFSCs in growth media and HUVECs, although this was non‐significant. Continuous hypoxia enhanced VEGF secretion at day 7 and 14 with levels that were significantly higher (P < 0.001) than all other groups. VEGF secretion in continuous hypoxia also significantly increased (P < 0.001) between day 7 and 14, with a two‐fold difference between the two time‐points. Intermittent hypoxia was significantly (P < 0.05) higher than AFSCs in growth media and normoxia at day 7. Overall, intermittent hypoxia produced the second highest levels of VEGF secretion after continuous hypoxia, implying that the length of exposure time to hypoxia could play a role in the enhancement of VEGF secretion by AFSCs. This is further corroborated by the two‐fold increase in VEGF between intermittent hypoxia groups at day 7 and 14.
Figure 4.
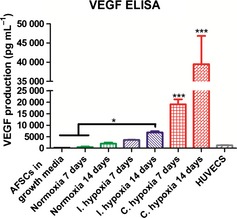
VEGF secretion by AFSCs over the course of 14 days in response to endothelial stimuli in various O2 conditions. VEGF secretion was significantly higher (P < 0.001) in continuous hypoxia at day 7 and 14. VEGF secretion in intermittent hypoxia was significantly (P < 0.05) higher relative to AFSCs in growth media and normoxia at day 7. AFSCs in growth media served as the negative control. HUVECs served as the positive control. Values are expressed as mean ± SD, n = 4. * P < 0.05 statistical significance differences relative to AFSCs in growth media and the normoxia group at day 7. *** P < 0.001 statistical significance differences relative to all other groups excluding continuous hypoxia at day 14.
Hypoxic culture does not affect CD31 cell‐surface expression in AFSCs
The ability of AFSCs to express the cell‐surface endothelial marker CD31 was assessed by fluorescent immunostaining at day 14 of culture (Fig. 5). No staining was detectable in AFSCs in growth media by day 14 of culture (Fig. 5i). Strong staining was visible in the HUVECs group (Fig. 5ii). AFSCs displayed weak staining at both day 14 of normoxia (Fig. 5A), intermittent hypoxia (Fig. 5B) and continuous hypoxia (Fig. 5C). This weak staining corroborates the CD31 gene expression seen using qRT‐PCR (Figs 2 and 3 respectively). There were no visible differences between AFSCs in any O2 condition in at either time‐point.
Figure 5.
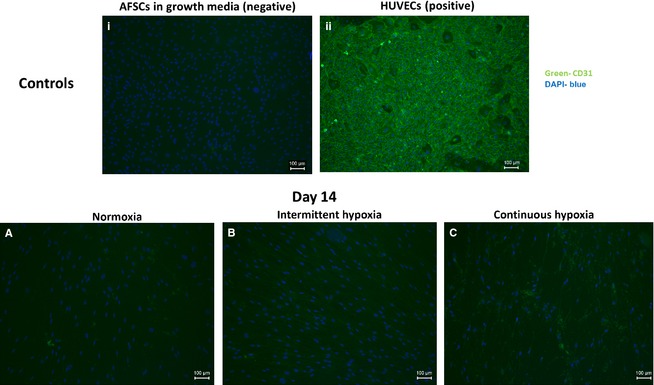
Fluorescent staining indicating CD31 expression by AFSCs in the presence of endothelial stimuli at day 14 of culture. No CD31 expression was visible in AFSC in growth media (i). Strong CD31 expression was visible in HUVECs (ii). AFSCs in normoxia (A), intermittent hypoxia (B) and continuous hypoxia (C) displayed weak expression of CD31 by day 14. AFSCs in growth media and HUVECs were used as the negative and positive controls, respectively. Images of AFSCs in growth media and of HUVECs were taken at day 14 of culture. Green represents CD31‐positive staining, blue represents DAPI nuclear stain. Scale bar: 100 μm.
Hypoxic culture does not enhance functional endothelial characteristics adopted by AFSCs
Hypoxic culture does not affect the uptake of ac‐LDL by AFSCs
The ability to take up ac‐LDL is known to be a functional characteristic of endothelial cells. For this reason, the ability of AFSCs to take up fluorescently tagged ac‐LDL was investigated. AFSCs in growth media were capable of little to no ac‐LDL uptake at any point during the culture period (Fig. 6i), as demonstrated by the weakness of green fluorescence in that group, therefore indicating that ac‐LDL uptake is not an innate characteristic of AFSCs. HUVECs showed effective ac‐LDL uptake, indicated by the visibility of strong green fluorescence within the boundaries of the cells. This was indicative of their mature endothelial cell type phenotype (Fig. 6ii). AFSCs showed effective ac‐LDL uptake in normoxia (Fig. 6A,B), intermittent hypoxia (Fig. 6C,D) and continuous hypoxia (Fig. 6E,F) at both day 7 and 14 of culture. There were no observable differences in ac‐LDL uptake between the O2 conditions at either time‐point. These data indicate that AFSCs in all of the three O2 conditions had acquired the ability to take up ac‐LDL.
Figure 6.
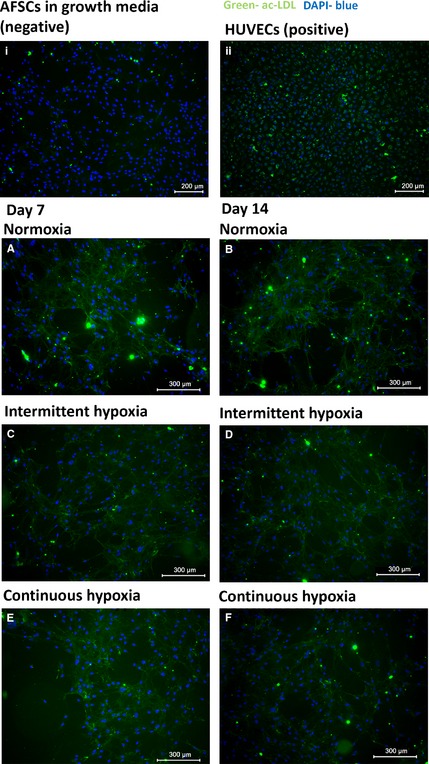
Florescent imaging demonstrating ac‐LDL uptake by AFSCs over the course of 14 days in response to endothelial stimuli in all three O2 conditions. AFSCs cultured in normoxia (A‐B), intermittent hypoxia (C‐D) and continuous hypoxia (E‐F) were all capable of ac‐LDL uptake at day 7 and 14 of culture. HUVECs were also capable of ac‐LDL uptake (ii). AFSCs in growth media were capable of little to no uptake of ac‐LDL at any point during the 14‐day culture period (i). AFSCs in growth media and HUVECs were used as the negative and positive controls, respectively. Images of AFSCs in growth media and of HUVECs were taken at day 14 of culture. Green represents ac‐LDL uptake, blue represents DAPI nuclear stain. Scale bar: 300 μm.
Hypoxic culture does not affect the ability of AFSCs to form tubules on Matrigel™
As hypoxia was found to enhance endothelial gene expression and VEGF protein secretion, its effect on tubule formation on Matrigel™ was investigated. The ability to form tubules on Matrigel™ is considered to be a functional characteristic of endothelial cells. At day 7 and 14 of culture, AFSCs were seeded on Matrigel™ for up to 24 h and it was found that AFSCs in normoxia, intermittent hypoxia and continuous hypoxia were all capable of tubule formation (Fig. 7). There were no significant differences between O2 conditions at any time‐point. HUVECs displayed significantly (P < 0.001) greater tubule length, approximately four‐fold higher than all other groups at every time‐point. This indicates that although the AFSCs are capable of tubule formation, they are not able to replicate the high levels of tubule formation seen by HUVECs.
Figure 7.
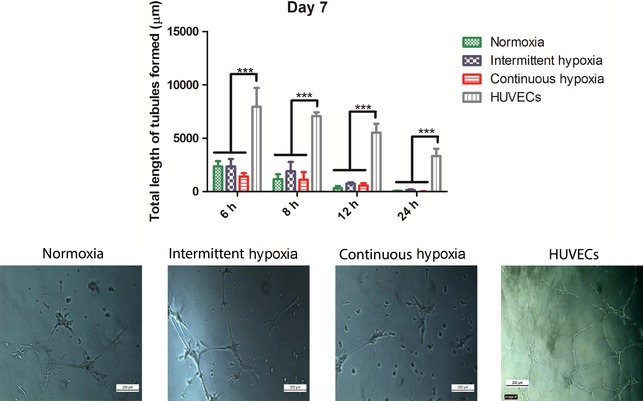
Quantification of tubule formation by AFSCs on Matrigel™ after 7 days of culture in normoxia, intermittent hypoxia or continuous hypoxia. No significant differences were observed between the three O2 conditions at any time‐point. However, HUVECs displayed significantly (P < 0.001) higher tubule length than all other groups at all time‐points. Quantitative analysis is accompanied by representative images of all three O2 conditions and the HUVEC control taken at the 6 h time‐point. Values are expressed as mean ± SD, n = 3. ***P < 0.001 statistical significance differences relative to all other groups. Scale bar: 200 μm.
Similar to the results seen at day 7, at day 14 there were no significant differences between O2 conditions at any time‐point (Fig. 8). HUVECs displayed significantly higher tubule formation at all time‐points (P < 0.001) relative to normoxia, intermittent hypoxia and continuous hypoxia.
Figure 8.
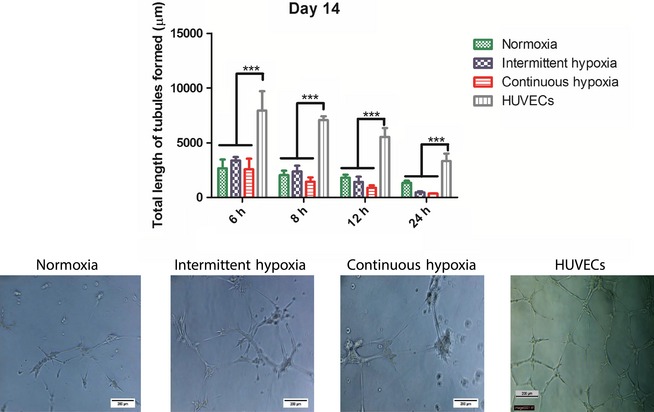
Quantification of tubule formation by AFSCs on Matrigel™ after 14 days of culture in normoxia, intermittent hypoxia or continuous hypoxia. No significant differences were observed between the three O2 conditions at any time‐point. HUVECs displayed significantly higher tubule length than all other groups at all time‐points (P < 0.001). Quantitative analysis is accompanied by representative images taken at the 6 h time‐point. Values are expressed as mean ± SD, n = 3. ***P < 0.001 statistical significance differences relative to all other groups. Scale bar: 200 μm.
These results demonstrate the ability of AFSCs exposed to endothelial stimuli to adopt the functional endothelial‐like characteristics of ac‐LDL uptake and tubule formation on Matrigel™ as early as day 7 of culture regardless of O2 culture conditions. However, HUVECs demonstrated significantly greater total tubule length at all time‐points, indicating tha, HUVECs still possess a superior tubule‐forming ability and therefore represent a more mature endothelial cell type in comparison with AFSCs.
Discussion
The objective of this study was to investigate whether culture in hypoxia could enhance the ability of AFSCs to differentiate down an endothelial lineage in order to produce an endothelial‐like cell type. The results demonstrated that AFSCs adopted an endothelial gene expression pattern as well as the functional endothelial cell characteristics of ac‐LDL uptake and tubule formation on Matrigel™. Culture in continuous hypoxia significantly enhanced both the endothelial gene expression profile and VEGF protein secretion relative to AFSCs in growth media, normoxia and intermittent hypoxia, although it had no beneficial effect on the adoption of functional endothelial characteristics. However, as expected, HUVECs presented a more advanced endothelial cell type, as evidenced by the more mature endothelial gene expression profile, stronger CD31 expression and superior tubule forming ability on Matrigel™. Thus, this study demonstrates the positive effect of continuous hypoxia on endothelial gene expression and VEGF secretion of AFSCs undergoing endothelial differentiation.
The 14‐day culture period caused the AFSCs in normoxia, intermittent hypoxia and continuous hypoxia to adopt a similar, albeit much less mature, endothelial gene expression profile to HUVECs. This endothelial expression profile manifested in the form of decreased Angiopoietin 1 expression and increased CD31, VEGFR2 and vWF expression in comparison with AFSCs in growth media. This increase in CD31 and vWF expression in AFSCs subjected to endothelial stimuli is consistent with the literature. For example, Zhang et al. (2009) demonstrated a three‐fold increase in CD31 expression and a four‐fold increase in vWF expression between week 1 and week 3 of a period of endothelial stimulation. Similar increases in CD31, vWF and VEGFR2 have been noted in other stem cell types differentiating down an endothelial lineage (Levenberg et al. 2002; Zhang et al. 2011). AFSCs in continuous hypoxia demonstrated significantly increased expression levels of CD31 and VEGFR2 relative to AFSCs in growth media, normoxia and intermittent hypoxia at both day 7 and 14. This indicates that long‐term continuous hypoxia may be a potent stimulus in the enhancement of endothelial gene expression of AFSCs, a result that has not been demonstrated previously. Zhang et al. (2009) have previously shown that pre‐culturing AFSCs in hypoxia for 48 h prior to endothelial stimulation could increase the upregulation of angiogenic genes such as placental and hepatocyte growth factor. However, that study did not investigate the effects of hypoxia on endothelial gene expression beyond a 48 h pre‐culture period. The role of hypoxia in endothelial differentiation has, however, been demonstrated in other stem cell types. For example, it has been shown that hypoxic preconditioning and culture can enhance angiogenic gene expression in both adipose‐derived stem cells and human pluripotent stem cells (Efimenko et al. 2011; Kusuma et al. 2014). However, this study is the first to demonstrate the positive effect of an extended period of continuous hypoxic culture on endothelial differentiation in AFSCs.
Amniotic fluid‐derived stem cells in continuous hypoxia were shown to have the highest levels of VEGF protein at day 7 and 14. VEGF secretion has previously been used as an indicator of endothelial differentiation in stem cells (Thangarajah et al. 2009; Zhang et al. 2009, 2011; Bassaneze et al. 2010). HUVECs displayed a low level of VEGF secretion, although this is probably due to the fact that as a mature endothelial cell, HUVECs are more likely to rely on the secretion of angiogenic growth factors from surrounding support cells for vessel formation (Yamagishi et al. 1999; Mayer et al. 2005). Therefore, the presence of elevated VEGF levels in the AFSC groups may indicate that they are less mature than HUVECs and may possibly better fulfil the role of an ‘attractor cell’ for endothelial cells in their current form. However, Benavides et al. (2012) have shown that AFSCs differentiating down an endothelial lineage undergo an increase in VEGF secretion. In this study, it was observed that AFSCs in continuous hypoxia demonstrated significantly higher levels of VEGF secretion relative to all other groups. AFSCs in intermittent hypoxia showed the second highest level of VEGF secretion at day 14, which implies that the length of exposure time to hypoxia affects the level of VEGF secretion. VEGF is known to be a downstream target of HIF‐1α (Neufeld et al. 1999) and the HIF‐1α pathway has previously been linked to an increase in VEGF secretion by a number of different cell types, including endothelial cells and bone marrow‐derived mesenchymal stem cells (MSCs) (Namiki et al. 1995; Potier et al. 2007; Thangarajah et al. 2009). We theorise that by using continuous hypoxia to increase VEGF expression, the AFSCs could be directed further down an endothelial cell lineage, as increased VEGF supplementation has been shown previously to enhance endothelial differentiation of AFSCs (Benavides et al. 2012). The increase in VEGF protein secretion by AFSCs in continuous hypoxia corresponds with increased CD31 and VEGFR2 gene expression seen in that group previously, indicating that the secreted VEGF potentially is acting upon the AFSCs to enhance differentiation.
Amniotic fluid‐derived stem cells cultured in each of the three O2 conditions displayed faint CD31 cell‐surface expression as evidenced by fluorescent immunostaining. This corroborates the data produced by qRT‐PCR that suggest AFSCs subjected to endothelial stimuli were expressing CD31 but that this expression was weak relative to HUVECs. There were no visible differences in CD31 expression between AFSCs in any O2 condition, which suggests that the increase in CD31 gene expression seen previously in continuous hypoxia was not high enough to translate into an enhanced CD31 cell‐surface expression. Oswald et al. (2004) demonstrated that differentiating MSCs do not express CD31 after a 7‐day differentiation period, and concluded that this marker was ‘later expressed in endothelial maturation’. In this study, AFSCs in growth media displayed no visible expression of CD31, which suggests that there has been an acquisition of an endothelial phenotype by AFSCs in each of the three O2 conditions over the 14‐day culture period. HUVECs displayed high levels of CD31 expression, with the highest expression rates at the gap junctions between cells, which is indicative of a healthy endothelial cell monolayer (Bazzoni & Dejana, 2004). This further suggests that the AFSCs, while acquiring an early endothelial phenotype, are still immature in comparison with HUVECs.
Having established that the AFSCs acquired an early stage endothelial gene expression profile and phenotype, the ability of these cells to adopt functional endothelial‐like characteristics was assessed. First, the ability of AFSCs to take up fluorescently tagged ac‐LDL was analysed at day 7 and 14 of culture. AFSCs were found to be capable of taking up ac‐LDL when cultured for up to 14 days in all three O2 conditions, similar to the HUVEC control. Uptake of ac‐LDL is a well recognised prerequisite for cells to be considered to be differentiated down an endothelial lineage, as it is known to be a functional characteristic of mature endothelial cells. As a result, it is a well established method for the assessment and validation of endothelial differentiation (Silva et al. 2005; Wang et al. 2007; Zhang et al. 2011; Janeczek Portalska et al. 2012; Doan et al. 2014). The uptake of ac‐LDL suggests that the AFSCs have acquired some functional endothelial characteristics as early as day 7, although functional characteristics by themselves are not indicative of full differentiation. Other studies have obtained similar results when attempting to differentiate AFSCs down an endothelial lineage, observing that AFSCs were capable of ac‐LDL uptake after 14 days when subjected to endothelial stimuli (Zhang et al. 2009; Benavides et al. 2012). Similar to the results of the CD31 fluorescent immunostaining, there were no visible differences in ac‐LDL uptake between AFSCs cultured in any of the three O2 conditions, which suggests that the increase in endothelial gene expression seen previously in continuous hypoxia was not high enough to translate into an enhanced uptake of ac‐LDL. AFSCs in growth media were incapable of ac‐LDL uptake. This indicates that AFSCs in growth media do not possess innate endothelial characteristics, which indicates that the AFSCs changed towards an endothelial phenotype over the course of the differentiation period.
Having now determined that the AFSCs subjected to endothelial stimuli were capable of ac‐LDL uptake and that continuous hypoxia did not affect this process, the formation of tubule networks by the AFSCs in all three O2 conditions was investigated at day 7 and 14. The ability to form tubules on Matrigel™, like ac‐LDL uptake, is considered a functional characteristic of endothelial cells. The results indicated that AFSCs in all three O2 conditions were capable of tubule formation, demonstrating that all groups had indeed begun to undergo endothelial differentiation. However, total tubule length in the HUVECs group was found to be significantly greater at all time‐points, indicating that although AFSCs in all three O2 conditions are capable of tubule formation, HUVECs still possess a superior tubule‐forming ability. These results demonstrate that AFSCs in all three O2 conditions are capable of tubule formation, indicating their progress towards differentiating down an endothelial lineage.
Overall, these results suggest that AFSCs subjected to culture for 14 days in all of the three O2 conditions displayed a gene expression profile, phenotype and functional characteristics associated with endothelial differentiation. This endothelial gene expression profile was enhanced by culture in continuous hypoxia. Despite this, AFSCs in all three O2 conditions were also found to possess a less mature endothelial gene expression profile and phenotype compared with HUVECs, the endothelial cell control. This was evidenced by the elevated levels of endothelial gene expression, CD31 cell‐surface expression and the superior tubule‐forming ability of HUVECs on Matrigel™. The results from this study suggest that differentiated AFSCs may simply not be mature enough to present a viable source of endothelial cells for the desired purpose of vascularising TE scaffolds and that this role is more suited to a primary endothelial cell type. In addition to this, Rouwkema et al. (2009) has shown previously that primary endothelial cells can fulfil the role of vessel‐producing cell more effectively than endothelial cells derived from differentiated stem cells.
Conclusion
The results of this study demonstrated that AFSCs subjected to endothelial stimuli over a 14‐day culture period display an early endothelial gene expression profile and acquired functional endothelial cell characteristics, indicating early endothelial differentiation had taken place. Culture in continuous hypoxia was found to enhance endothelial gene expression and VEGF protein secretion but did not enhance endothelial‐like functionality. AFSCs subjected to endothelial stimuli demonstrated a less mature endothelial gene expression profile and phenotype when compared with HUVECs, the endothelial cell control. It is possible that a second stimulus, such as shear stress applied via fluid flow, may be required fully to induce endothelial differentiation in AFSCs. It is also possible that AFSCs may present a more suitable source for pericytes, with a role supporting the formation of nascent vessels, rather than acting as an endothelial‐like cell. Overall, however, this study suggests that continuous hypoxia may enhance the differentiation of AFSCs into an endothelial‐like cell type.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions
Cai Lloyd‐Griffith: Study design and concept, experimental work, acquisition of data, data analysis, drafting of the manuscript. Garry P. Duffy: Contributions to study design and concept, critical revision of the manuscript and approval of the article. Fergal J. O'Brien: Contributions to design and concept, critical revision of the manuscript and approval of the article.
Acknowledgements
The authors gratefully acknowledge grant support from the Health Research Board in Ireland (PhD/2007/11) and the European Research Council (239685 ‐ CollRegen ‐ERC‐2009‐STG). The authors would also like to thank Drs Shay Soker, Julie Allickson and Anthony Atala of the Wake Forest Institute for Regenerative Medicine, North Carolina, USA, for kindly providing the AFSCs used in this study. The authors would also like to thank Dr Amos Matsiko for his help in proof‐reading this manuscript.
References
- Arnaoutova I, George J, Kleinman HK, et al. (2009) The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12, 267–274. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, et al. (1994) Platelet endothelial cell adhesion molecule‐1 (PECAM‐1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 120, 2539–2553. [DOI] [PubMed] [Google Scholar]
- Bassaneze V, Barauna VG, Lavini‐Ramos C, et al. (2010) Shear stress induces nitric oxide‐mediated vascular endothelial growth factor production in human adipose tissue mesenchymal stem cells. Stem Cells Dev 19, 371–378. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E (2004) Endothelial cell‐to‐cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84, 869–901. [DOI] [PubMed] [Google Scholar]
- Benavides OM, Petsche JJ, Moise KJ Jr, et al. (2012) Evaluation of endothelial cells differentiated from amniotic fluid‐derived stem cells. Tissue Eng Part A, 18, 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning AC, Dua HS, Amoaku WM (2008) The effects of growth factors on the proliferation and in vitro angiogenesis of human macular inner choroidal endothelial cells. Br J Ophthalmol 92, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Cheema U, Rong Z, Kirresh O, et al. (2012) Oxygen diffusion through collagen scaffolds at defined densities: implications for cell survival in tissue models. J Tissue Eng Regen Med 6, 77–84. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G Jr, Siddiqui MM, et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25, 100–106. [DOI] [PubMed] [Google Scholar]
- Doan CC, Le TL, Hoang NS, et al. (2014) Differentiation of umbilical cord lining membrane‐derived mesenchymal stem cells into endothelial‐like cells. Iran Biomed J 18, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle E, Fuchs S, Kolbe M, et al. (2011) Comparative study assessing effects of sonic hedgehog and VEGF in a human co‐culture model for bone vascularisation strategies. Eur Cell Mater 21, 144–156. [DOI] [PubMed] [Google Scholar]
- Dong QG, Bernasconi S, Lostaglio S, et al. (1997) A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol 17, 1599–1604. [DOI] [PubMed] [Google Scholar]
- Duffy GP, D'arcy S, Ahsan T, et al. (2010) Mesenchymal stem cells overexpressing ephrin‐b2 rapidly adopt an early endothelial phenotype with simultaneous reduction of osteogenic potential. Tissue Eng Part A 16, 2755–2768. [DOI] [PubMed] [Google Scholar]
- Duffy GP, McFadden TM, Byrne EM, et al. (2011) Towards in vitro vascularisation of collagen‐GAG scaffolds. Eur Cell Mater 21, 15–30. [DOI] [PubMed] [Google Scholar]
- Efimenko A, Starostina E, Kalinina N, et al. (2011) Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar M, Djonov VG, Schnell C, et al. (2004) Cell‐demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 94, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Epstein NE (2011) Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenzeller G, Graner S, Kirkpatrick CJ, et al. (2009) Impaired in vivo vasculogenic potential of endothelial progenitor cells in comparison to human umbilical vein endothelial cells in a spheroid‐based implantation model. Cell Prolif 42, 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Hochberg M (1973) Self‐regulation of growth in three dimensions. J Exp Med 138, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, et al. (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia‐inducible factor 1. Mol Cell Biol 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger F, Bertram H, Berger I, et al. (2005) Vascular endothelial growth factor gene‐activated matrix (VEGF165‐GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res 20, 2028–2035. [DOI] [PubMed] [Google Scholar]
- Ginsberg M, James D, Ding BS, et al. (2012) Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell 151, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaug‐Riley SL, Crane‐Kruger GM, Yaszemski MJ, et al. (1998) Three‐dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 19, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia‐inducible factor 1 alpha. Genes Dev 12, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeczek Portalska K, Leferink A, Groen N, et al. (2012) Endothelial differentiation of mesenchymal stromal cells. PLoS ONE, 7, e46842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HC, Milthorpe BK, McFarland CD (2007) Engineering thick tissues – the vascularisation problem. Eur Cell Mater 14, 1–18; discussion 18‐9. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Iyer NV, Laughner E, et al. (1999) Defective vascularization of HIF‐1alpha‐null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209, 254–267. [DOI] [PubMed] [Google Scholar]
- Kusuma S, Peijnenburg E, Patel P, et al. (2014) Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler Thromb Vasc Biol 34, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Hartzel T, Huynh G, et al. (2001) Regulation of angiogenesis and matrix remodeling by localized, matrix‐mediated antisense gene delivery. Mol Ther 3, 842–849. [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Harder Y, Amon M, et al. (2006) Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng 12, 2093–2104. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub JS, Amit M, et al. (2002) Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 99, 4391–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS (2007) Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 26, 281–290. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, et al. (1995) Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ Res 77, 638–643. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mayer H, Bertram H, Lindenmaier W, et al. (2005) Vascular endothelial growth factor (VEGF‐A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem 95, 827–839. [DOI] [PubMed] [Google Scholar]
- McFadden TM, Duffy GP, Allen AB, et al. (2013) The delayed addition of human MSCs to pre‐formed endothelial cell networks results in functional vascularisation of a collagen‐GAG scaffold in vivo. Acta Biomater 9, 9303–9316. [DOI] [PubMed] [Google Scholar]
- Miranda‐Sayago JM, Fernandez‐Arcas N, Benito C, et al. (2011) Lifespan of human amniotic fluid‐derived multipotent mesenchymal stromal cells. Cytotherapy 13, 572–581. [DOI] [PubMed] [Google Scholar]
- Murphy CM, Schindeler A, Gleeson JP, et al. (2014) A collagen‐hydroxyapatite scaffold allows for binding and co‐delivery of recombinant bone morphogenetic proteins and bisphosphonates. Acta Biomater 10, 2250–2258. [DOI] [PubMed] [Google Scholar]
- Namiki A, Brogi E, Kearney M, et al. (1995) Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem 270, 31189–31195. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, et al. (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13, 9–22. [PubMed] [Google Scholar]
- Ong LL, Li W, Oldigs JK, et al. (2010) Hypoxic/normoxic preconditioning increases endothelial differentiation potential of human bone marrow CD133+ cells. Tissue Eng Part C Methods 16, 1069–1081. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, et al. (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22, 377–384. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Innerarity TL, Weinstein JN, et al. (1981) Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis 1, 177–185. [DOI] [PubMed] [Google Scholar]
- Portal‐Nunez S, Lozano D, Esbrit P (2012) Role of angiogenesis on bone formation. Histol Histopathol 27, 559–566. [DOI] [PubMed] [Google Scholar]
- Portalska KJ, Groen N, Krenning G, et al. (2013) The effect of donor variation and senescence on endothelial differentiation of human mesenchymal stromal cells. Tissue Eng Part A 19, 2318–2329. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Andriamanalijaona R, et al. (2007) Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 40, 1078–1087. [DOI] [PubMed] [Google Scholar]
- Prado‐Lopez S, Conesa A, Arminan A, et al. (2010) Hypoxia promotes efficient differentiation of human embryonic stem cells to functional endothelium. Stem Cells 28, 407–418. [DOI] [PubMed] [Google Scholar]
- Prasad Chennazhy K, Krishnan LK (2005) Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials, 26, 5658–5667. [DOI] [PubMed] [Google Scholar]
- Prigozhina NL, Heisel A, Wei K, et al. (2011) Characterization of a novel angiogenic model based on stable, fluorescently labelled endothelial cell lines amenable to scale‐up for high content screening. Biol Cell 103, 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubelakis MG, Tsaknakis G, Pappa KI, et al. (2013) Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS ONE 8, e54747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S, Bago JR, Soler‐Botija C, et al. (2012) Human umbilical cord blood‐derived mesenchymal stem cells promote vascular growth in vivo. PLoS ONE 7, e49447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwkema J, Westerweel PE, De Boer J, et al. (2009) The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A 15, 2015–2027. [DOI] [PubMed] [Google Scholar]
- Shaw SW, David AL, De Coppi P (2011) Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol 23, 109–116. [DOI] [PubMed] [Google Scholar]
- Silva GV, Litovsky S, Assad JA, et al. (2005) Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111, 150–156. [DOI] [PubMed] [Google Scholar]
- Stein O, Stein Y (1980) Bovine aortic endothelial cells display macrophage‐like properties towards acetylated 125I‐labelled low density lipoprotein. Biochim Biophys Acta 620, 631–635. [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Vial IN, Chang E, et al. (2009) IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 27, 266–274. [DOI] [PubMed] [Google Scholar]
- Unger RE, Ghanaati S, Orth C, et al. (2010) The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials 31, 6959–6967. [DOI] [PubMed] [Google Scholar]
- Voyta JC, Via DP, Butterfield CE, et al. (1984) Identification and isolation of endothelial cells based on their increased uptake of acetylated‐low density lipoprotein. J Cell Biol 99, 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Au P, Chen T, et al. (2007) Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol 25, 317–318. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Yonekura H, Yamamoto Y, et al. (1999) Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab Invest 79, 501–509. [PubMed] [Google Scholar]
- Zhang P, Baxter J, Vinod K, et al. (2009) Endothelial differentiation of amniotic fluid‐derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev 18, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Moudgill N, Hager E, et al. (2011) Endothelial differentiation of adipose‐derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev 20, 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]


