Abstract
Bone marrow (BM) stem cells may be an ideal source of cells for intervertebral disc (IVD) regeneration. However, the harsh biochemical microenvironment of the IVD may significantly influence the biological and metabolic vitality of injected stem cells and impair their repair potential. This study investigated the viability and production of key matrix proteins by nucleus pulposus (NP) and BM stem cells cultured in the typical biochemical microenvironment of the IVD consisting of altered oxygen and glucose concentrations. Culture‐expanded NP cells and BM stem cells were encapsulated in 1.5% alginate and ionically crosslinked to form cylindrical hydrogel constructs. Hydrogel constructs were maintained under different glucose concentrations (1, 5 and 25 mM) and external oxygen concentrations (5 and 20%). Cell viability was measured using the Live/Dead® assay and the production of sulphated glycosaminoglycans (sGAG), and collagen was quantified biochemically and histologically. For BM stem cells, IVD‐like micro‐environmental conditions (5 mM glucose and 5% oxygen) increased the accumulation of sGAG and collagen. In contrast, low glucose conditions (1 mM glucose) combined with 5% external oxygen concentration promoted cell death, inhibiting proliferation and the accumulation of sGAG and collagen. NP‐encapsulated alginate constructs were relatively insensitive to oxygen concentration or glucose condition in that they accumulated similar amounts of sGAG under all conditions. Under IVD‐like microenvironmental conditions, NP cells were found to have a lower glucose consumption rate compared with BM cells and may in fact be more suitable to adapt and sustain the harsh microenvironmental conditions. Considering the highly specialised microenvironment of the central NP, these results indicate that IVD‐like concentrations of low glucose and low oxygen are critical and influential for the survival and biological behaviour of stem cells. Such findings may promote and accelerate the translational research of stem cells for the treatment of IVD degeneration.
Keywords: bone marrow, glucose, intervertebral disc, metabolism, microenvironment, nucleus pulposus, oxygen, stem cells
Introduction
Low back pain (LBP) is a significant epidemiological problem and economic burden worldwide (Hoy et al. 2010). It is established that the primary cause of LBP is degeneration of the intervertebral disc (IVD), characterised by decreased extracellular matrix (ECM) synthesis and increased cell death (Deyo & Weinstein, 2001). IVD degeneration initiates within the nucleus pulposus (NP) and progresses with attrition of the annulus fibrosus (AF), which leads to eventual impairment of the IVD.
Healthy NP tissue contains randomly organised collagen types II and VI, embedded in a highly hydrated gel‐like matrix rich in proteoglycans (PGs), with aggrecan being predominantly abundant (Inoue, 1981). Other proteoglycans such as biglycan, decorin and fibromodulin are also present (Singh et al. 2009). The high osmotic pressure within the NP, provided by the proteoglycans, is important in maintaining tissue hydration and resisting compressive forces during normal motion and activities. As degeneration progresses, the proteoglycan content of the NP diminishes resulting in decreased osmotic pressure with a concomitant loss of hydration and reduction in disc height, thereby impairing the mechanical functionality of the IVD. The synthesis and composition of collagens also vary with progressive degeneration; with increased collagen type I produced in the NP, leading to a fibrotic transformation of the NP tissue and a progressive inability to identify a clear demarcation between the NP and AF tissues. Concomitant with matrix degradation and reduced disc height is often an in‐growth of blood vessels and nerves into the normally avascular and aneural tissue (Freemont et al. 1997).
Cell‐based therapies targeted to regenerate the NP region may prevent progressive degeneration. Autologous disc cell transplantation (ADCT) is a therapy that involves harvesting NP tissue from the patient, isolating and expanding cells to required numbers and injecting the expanded cells into the central NP region of an early‐stage degenerated IVD (Hohaus et al. 2008). However, limitations with this approach include the low yield of healthy NP cells obtainable from degenerated discs and the limited expansion capability of NP cells (Hiyama et al. 2008; Xia et al. 2013). This has motivated the exploration of stem cells due to their propensity to proliferate and their ability to form multiple tissue types (Caplan, 1991).
BM stem cells possess significant potential and perhaps provide a clinically feasible source of cells to promote the repair of NP tissue. The rationale and benefits to transplanting stem cells into the IVD are twofold; first, transplanted stem cells may stimulate endogenous NP cells, and secondly, the resident host NP cells may promote differentiation of the transplanted stem cells towards a nucleus pulposus phenotype (Richardson et al. 2006; Miyamoto et al. 2010). In vivo studies have shown that implantation of stem cells into experimentally induced degenerate animal discs leads to improved disc height and accumulation of proteoglycans (Sakai et al. 2003; Crevensten et al. 2004; Risbud et al. 2004). Furthermore, a human clinical study performed by Orozco et al. injected autologous bone marrow stem cells into the nucleus pulposus of 10 patients diagnosed with lumbar disc degeneration. Results indicated that pain, disability and quality of life improved over the 12‐month trial (Orozco et al. 2011).
However, the regenerative potential of BM stem cells may be limited by the harsh microenvironment within the disc, characterised by low oxygen, low glucose and low pH conditions (Bartels et al. 1998; Urban, 2002; Grunhagen et al. 2006). In the central nucleus pulposus the oxygen concentration ranges from 5% to as low as 1% (Mwale et al. 2011), the pH ranges from 7.1 to as low 6.5 (Urban, 2002), and the glucose concentration ranges from 5 mM to lower levels (Bibby et al. 2005) as the degeneration transgresses from mildly degenerated to a severely degenerated state. NP cells have been shown to be well adapted to this harsh microenvironment (Risbud et al. 2006) but this biochemical microenvironment may negatively influence the biological and metabolic vitality of stem cells and impair their regenerative potential. Therefore, understanding how stem cells respond to limited nutrient availability is a key factor for clinical translation.
Numerous studies have focused on cell growth and survival (Johnson et al. 2008; Stephan et al. 2011). Stephan et al. (2011) cultured bovine NP cells in alginate beads under zero glucose or high glucose conditions and demonstrated that NP cell proliferation and survival are influenced by the availability of glucose. The absence of glucose resulted in more apoptotic and senescent cells. Interestingly, Johnson et al. (2008) cultured bovine NP cells encapsulated in alginate gels under similar conditions and observed that glucose deprivation leads to a minimal increase in cell proliferation. Mwale et al. (2011) also cultured bovine NP cells encapsulated in alginate beads under different oxygen concentrations and found that low oxygen levels increased the expression of aggrecan mRNA levels but, interestingly, this was not reflected in GAG release. Also, Stoyanov et al. (2011) cultured BM stem cells in alginate beads under low and high oxygen concentrations and observed that hypoxia increased aggrecan and collagen gene expression. Although these studies describe the influence of glucose and oxygen on NP cell and BM stem cell growth and survival, little is known of the effect on the capacity of these cells to produce NP‐like matrix. Further experimentation is required to address ECM synthesis, which is of major importance to the functioning of the disc. Furthermore, the same studies have investigated the effects of oxygen (Risbud et al. 2006; Mwale et al. 2011; Stoyanov et al. 2011; Yang et al. 2013) or glucose (Li et al. 2007; Wuertz et al. 2008; Deorosan & Nauman, 2011; Stephan et al. 2011; Liang et al. 2012) independently, which has resulted in several contradictions in the literature and confirms the need to study the effect of a combination of environmental factors that more likely reflects the situation as it exists in vivo.
The objective of this study was to investigate how microenvironmental conditions may affect subsequent matrix production of porcine NP and BM stem cells encapsulated in 3D alginate hydrogels cultured in three different glucose (1, 5 and 25 mM) media at two different oxygen concentrations (5 and 20%).
Methods
Nucleus pulposus and bone marrow stem cell isolation and culture
NP cells were harvested from the intervertebral discs (IVDs) of porcine donors (n = 2, 3–4 months, 20–30 kg) within 3 h of sacrifice as previously described (Naqvi & Buckley, 2015). NP tissue was isolated and enzymatically digested in 2.5 mg mL−1 pronase solution for 1 h followed by 3 h in 0.5 mg mL−1 collagenase solution at 37 °C. Digested tissue/cell suspension was passed through a 100‐μm cell strainer to remove tissue debris followed by 70‐ and 40‐μm cell strainers to separate notochordal cells (NC) from the desired nucleus pulposus cells (NP) as previously described (Spillekom et al. 2014). Cells were washed three times by repeated centrifugation (650 g for 5 min), plated at a density of 5 × 103 cells cm−2 and cultured to passage 2 in T‐175 cm2 flasks with low‐glucose Dulbecco's modified Eagle's medium (LG‐DMEM, 1 mg mL−1 d‐glucose), supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 0.25 μg mL−1 amphotericin B, 5 ng mL−1 fibroblast growth factor‐2 (FGF‐2; PeproTech, UK).
Donor matched bone marrow (BM) was isolated from the femora and plated at 10 × 106 cells in T‐75 cm2 flasks to allow for colony formation (P0) in supplemented LG‐DMEM. After P0, cells were re‐plated at 5 × 103 cells cm−2 and expanded to P2 in a humidified atmosphere at 37 °C and 5% CO2. The differentiation capacity of BM cells from donors was assessed as previously described (Vinardell et al. 2011). In all cases, BM stem cells demonstrated successful differentiation towards the osteogenic, adipogenic and chondrogenic lineages.
Alginate hydrogel encapsulation and culture
Expanded cells (NP and BM) were encapsulated in 1.5% alginate (Pronova UP LVG, FMC NovaMatrix, Norway) at a seeding density of 4 × 106 cells mL−1 and ionically crosslinked with 100 mM calcium chloride (CaCl2) for 30 min to form cylindrical constructs (diameter = 5 mm; height = 3 mm). The geometric construct dimensions used in this study were based on previous work from our laboratory (Buckley et al. 2012). Constructs were maintained in 1, 5 or 25 mM glucose medium consisting of DMEM supplemented with penicillin (100 U mL−1)‐streptomycin (100 μg mL−1) (both from GIBCO, Invitrogen, Ireland), 0.25 μg mL−1 amphotericin B, 100 μg mL−1 sodium pyruvate, 40 μg mL−1 l‐proline, 1.5 mg mL−1 bovine serum albumin, 4.7 μg mL−1 linoleic acid, 1× insulin–transferrin–selenium, 50 μg mL−1 l‐ascorbic acid‐2‐phosphate, 100 nm dexamethasone (all Sigma‐Aldrich, Ireland) and 10 ng mL−1 transforming growth factor (TGF)‐β3 (PeproTech, UK). Constructs were cultured in standard 24‐well plates with one construct per well with 2 mL of supplemented medium in hypoxic (5% oxygen) or normoxic (20% oxygen) conditions. Constructs were assessed at days 0 and 21 in terms of cell viability (n = 1), biochemical content (DNA, sulphated‐glycosaminoglycan (sGAG) and collagen content) (n = 3), histologically and immunohistochemically (n = 2). This study was performed twice with independent donors in each case. Results were reproducible for all conditions investigated.
Cell viability
Cell viability was assessed using a LIVE/DEAD® Viability/Cytotoxicity Assay Kit (Invitrogen, Bio‐science, Ireland). Constructs were removed from culture, sectioned, rinsed with phosphate‐buffered saline (PBS) and incubated for 1 h at 37 °C in live/dead solution containing 2 μm calcein AM, 4 μm ethidium homodimer‐1 (EthD‐1). After incubation, segments were again washed with PBS and imaged with an Olympus FV‐1000 Point‐ Scanning Confocal Microscope (Southend‐on‐Sea, UK) at 515 and 615 nm channels and analysed using fv10‐asw 2.0 viewer software.
Quantitative biochemical analysis
Samples were digested with papain (125 μg mL−1) in 0.1 m sodium acetate, 5 mM l‐cysteine HCl, and 0.05 m EDTA (Sigma‐Aldrich, Ireland) at 60 °C under constant agitation for 18 h. DNA content was quantified using the Hoechst 33258 dye‐based DNA QF Kit (Sigma‐Aldrich, Ireland). Proteoglycan content was quantified using the dimethylmethylene blue (DMMB) dye‐binding assay (Blyscan, Biocolor Ltd, Northern Ireland) with a chondroitin sulphate standard. Total collagen content was determined by measuring the hydroxyproline content. Briefly, samples were hydrolysed at 110 °C for 18 h in concentrated hydrochloric acid (HCl) (38%) and assayed using a chloramine‐T assay (Kafienah & Sims, 2004), at a hydroxyproline‐to‐collagen ratio of 1 : 7.69 (Ignat'eva et al. 2007).
Glucose concentrations in media samples from day 18 to day 21 were quantitatively measured using a glucose meter (Accu‐Chek Aviva glucose meter, Roche Diagnostics Ltd, UK). Samples of culture media (1, 5 and 25 mM) served as controls. Cellular consumption rates were determined by normalising to cell number and time.
Histology and immunohistochemistry
Constructs were fixed in 4% paraformaldehyde (PFA) overnight, dehydrated in ethanol, embedded in paraffin wax, and sectioned at a thickness of 8 μm. Sections were stained for glycosaminoglycans (GAGs) using aldehyde fuchsin and 1% alcian blue 8GX (Sigma‐Aldrich, Ireland) in 0.1 m HCl (Simmons et al. 2004) and picro‐sirius red to assess for collagen deposition. The deposition of collagen types I and II was identified through immunohistochemistry. Briefly, sections were rinsed with PBS before treatment with chondroitinase ABC in a humidified environment at 37 °C. Slides were rinsed with PBS and non‐specific sites were blocked with goat serum. Sections were incubated for 1 h at 4 °C with the primary antibody; mouse monoclonal collagen type I antibody (1 : 200; 1 mg mL−1) or mouse monoclonal anti‐collagen type II (1 : 80; 1 mg mL−1). After washing in PBS, the peroxidase activity of the sections was quenched and the sections incubated for 1 h in the secondary antibody; anti‐mouse IgG biotin antibody produced in goats (1 : 133; 2.1 mg mL−1). Colour was developed using the Vectastain ABC reagent followed by exposure to peroxidase DAB substrate kit. Positive and negative controls of porcine ligament and cartilage were included for each batch.
Statistical analyses
Statistical analyses were performed using graphpad prism (version 4) software. Two‐way anova was used for analysis of variance with Bonferroni post‐tests to compare groups. Numerical and graphical results are displayed as mean ± standard deviation. Significance was accepted at a level of P < 0.05.
Results
Viability of nucleus pulposus and bone marrow stem cells in IVD‐like microenvironmental conditions
For BM constructs, DNA content increased from day 0 for both oxygen concentrations irrespective of glucose condition (Fig. 1A). A similar result was obtained for NP constructs maintained in 5 mM glucose under normoxic conditions (20% oxygen). These results were confirmed through confocal imaging of live and dead cells (Fig. 1B,C). Interestingly, a core of dead cells was observed in all BM constructs irrespective of the culture condition, although this core effect was more pronounced in the group maintained in 1 mM glucose under hypoxia. A similar core effect was observed in NP constructs maintained in 5 mM glucose in normoxia. The increase in DNA content in BM constructs from day 0 to day 21 may be due to increased cell proliferation in the periphery of constructs.
Figure 1.
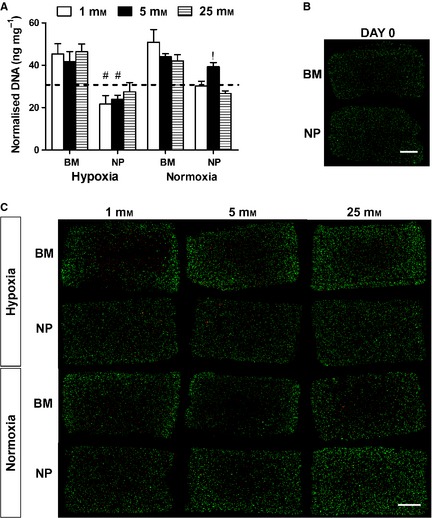
(A) DNA content normalised to wet weight at day 21 for nucleus pulposus (NP) and bone marrow (BM) stem cells maintained under different glucose conditions (1, 5, 25 mM) in hypoxia (5% O2) or normoxia (20% O2): #significance compared with normoxia for same cell type. !significance compared with 25 mM glucose condition for same cell type (P < 0.05), dashed line represents day 0 DNA content. (B) Cell viability for NP and BM constructs at day 0 (C) Cell viability for NP and BM constructs at day 21 Scale bar: 1 mm.
Bone marrow stem cells accumulated greater amounts of sGAG and collagen in NP‐like microenvironmental conditions
A differential response for total sGAG content was observed for NP and BM constructs depending on oxygen concentration (Fig. 2A). Under normoxic conditions, total sGAG content was significantly higher for NP than BM constructs. In contrast, hypoxia promoted the highest total sGAG accumulation for BM constructs maintained in IVD‐like microenvironmental conditions (5 mM glucose and 5% oxygen). When normalised to DNA content, NP constructs displayed similar amounts of sGAG content under all conditions, correlating highly with aldehyde fuchsin staining of the constructs, where NP constructs appeared to accumulate similar amounts of sGAG irrespective of glucose condition or oxygen concentration (Fig. 2B). Of note, BM constructs maintained in 1 mM glucose in hypoxia accumulated sGAG in the periphery of the gel, confirming the cell viability results where dead cells were located in the centre of the hydrogel construct. Also, BM constructs maintained in normoxia exhibited sGAG deposition in the pericellular region only.
Figure 2.
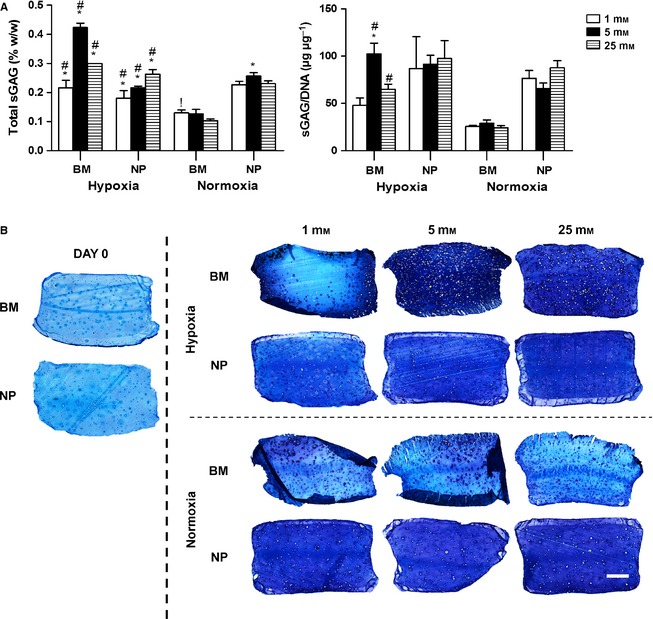
(A) Total sGAG normalised to wet weight and to DNA content at day 21 for nucleus pulposus (NP) and bone marrow (BM) stem cells maintained under different glucose conditions (1, 5, 25 mM) in hypoxia (5% O2) or normoxia (20% O2): #significance compared with normoxia for same cell type, !significance compared with 25 mM glucose condition for same cell type, *significance compared with the two other glucose conditions for the same cell type (P < 0.05). (B) Histological evaluation with aldehyde fuchsin and alcian blue to identify sGAG at day 0 and day 21; deep blue/purple staining indicates GAG accumulation and light blue staining indicates residual alginate. Scale bar: 1 mm.
In terms of collagen accumulation, NP constructs maintained in very low glucose conditions in hypoxia exhibited significantly less collagen content compared with those maintained in normoxia (Fig. 3A). BM constructs maintained in 1 mM glucose in normoxia demonstrated significantly higher collagen content compared with those maintained in hypoxia, and similar results were obtained when collagen content was normalised to DNA. These observations correlated highly with picro‐sirius red staining of constructs (Fig. 3B). NP constructs accumulated limited but similar amounts of collagen irrespective of oxygen or glucose condition. Of note, BM constructs maintained in 1 mM glucose and in hypoxia displayed limited collagen accumulation. Also, collagen staining of BM constructs maintained in normoxia demonstrated pericellular collagen deposition only.
Figure 3.
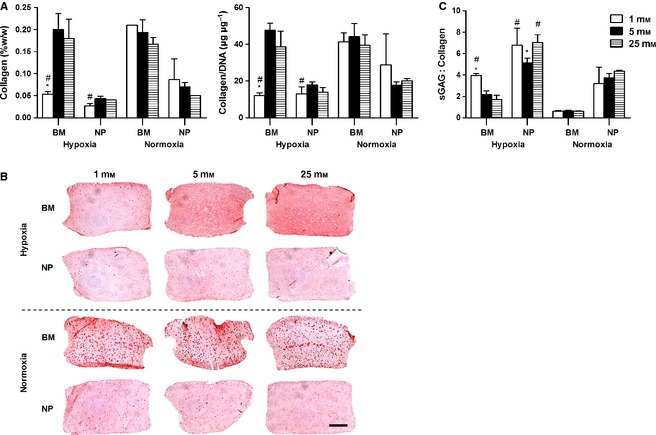
(A) Total collagen normalised to wet weight and to DNA at day 21 for nucleus pulposus (NP) and bone marrow (BM) stem cells maintained under different glucose conditions (1, 5, 25 mM) in hypoxia (5% O2) or normoxia (20% O2): #significance compared with normoxia for same cell type, *significance compared with the two other glucose conditions for the same cell type (P < 0.05). (B) Histological evaluation with picro‐sirius red to identify collagen at day 21. Scale bar: 1 mm. (C) sGAG : collagen ratio at day 21: #significance compared with normoxia for same cell type, *significance compared with the two other glucose conditions for same cell type (P < 0.05).
In terms of sGAG to collagen ratio, hypoxia resulted in higher ratios for both cell types, with the highest ratios observed for NP (Fig. 3C). Also, for BM constructs maintained in hypoxia, there was a decrease in the sGAG : collagen ratio with increasing glucose concentration.
Immunohistochemistry results revealed that BM constructs in 25 mM glucose in hypoxia resulted in increased accumulation of collagen type I compared with normoxia (Fig. 4A). In contrast, for NP constructs under the same glucose conditions, normoxia resulted in increased accumulation of collagen type I compared with hypoxia.
Figure 4.
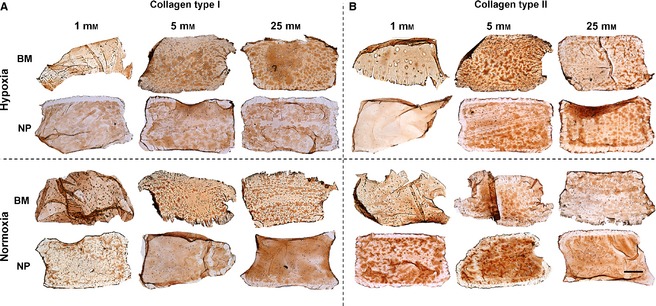
Immunohistochemical evaluation at day 21 for (A) collagen type I and (B) collagen type II for nucleus pulposus (NP) and bone marrow (BM) stem cells maintained under different glucose conditions (1, 5, 25 mM) in hypoxia (5% O2) or normoxia (20% O2). Scale bar: 1 mm.
In terms of collagen type II deposition, BM constructs deposited greater amounts when maintained in IVD‐like microenvironmental conditions (5 mM glucose and 5% oxygen) with limited deposition under ischaemic conditions (very low glucose and hypoxia) (Fig. 4B). Also, NP constructs maintained in ischaemic conditions (very low glucose and hypoxia) exhibited significantly less deposition of collagen type II compared with those maintained in normoxia (Fig. 4B).
Glucose consumption rate of bone marrow stem cells in IVD‐like microenvironmental conditions
BM constructs maintained in IVD‐like microenvironmental conditions (5 mM glucose and 5% oxygen) displayed a significantly higher glucose consumption rate (P < 0.01) compared with NP constructs maintained under the same conditions. In addition, under IVD‐like microenvironmental conditions, both cell types exhibited a significantly higher glucose consumption rate (P < 0.01) compared with constructs maintained in normoxic conditions (Fig. 5). Interestingly, under hypoxic conditions, BM constructs maintained in very low glucose (1 mM) exhibited a lower consumption rate compared with BM constructs under 5 mM and 25 mM glucose conditions (P < 0.01). Furthermore, under hypoxic conditions, BM constructs maintained in high glucose (25 mM) exhibited a significantly higher glucose consumption rate compared with those maintained in normoxia and NP constructs, irrespective of external oxygen concentration.
Figure 5.
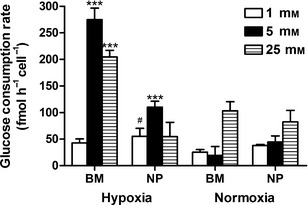
Glucose consumption rate (fmol h−1 cell−1) at day 21 for nucleus pulposus (NP) and bone marrow (BM) stem cells maintained under different glucose conditions (1, 5, 25 mM) in hypoxia (5% O2) or normoxia (20% O2): #significance compared with normoxia for same cell type ***significance compared with all other groups for the same glucose condition (P < 0.05).
Discussion
The IVD is an avascular organ relying on diffusion of essential nutrients such as oxygen and glucose through the endplate, thereby creating a challenging biochemical microenvironment. Translation of stem cell therapies into a multimodal protocol for IVD degeneration requires not only the survival of these cells but also their ability to function normally amid the harsh microenvironment of hypoxia, low nutrition, acidic pH, high mechanical loading, high osmolarity, and a complicated protease and cytokine network (Urban, 2002; Wuertz et al. 2008). In this study we investigated the influence of external oxygen concentration (5 and 20% O2) and three different glucose concentrations (1, 5 and 20 mM) on bone marrow (BM) stem cells and nucleus pulposus (NP) cells encapsulated in 3D alginate hydrogels.
We found that BM stem cells survive and synthesise appropriate matrix components such as sGAG and collagen in low external oxygen of 5% and low glucose concentration of 5 mM representative of IVD microenvironmental conditions. Under the same external oxygen concentration of 5% and a very low glucose concentration (1 mM), BM viability was reduced, particularly in the core region, where we also observed reduced accumulation of matrix components (sGAG and collagen). Importantly, the GAG : collagen ratio was relatively higher in BM constructs maintained in hypoxia than in normoxia. A high GAG : collagen ratio may provide an appropriate metric for identifying an NP‐like tissue type. The healthy NP contains randomly organised collagen type II (Inoue, 1981), embedded in a highly hydrated gel‐like matrix rich in proteoglycans (PGs), with aggrecan being predominantly abundant, resulting in a high GAG : collagen ratio. Conversely, the degenerated NP loses its proteoglycan content, with synthesis and composition of collagens also varying, resulting in an altered GAG : collagen ratio. If stem cells are to be differentiated towards a disc cell phenotype, it will be essential to verify that the ultimate matrix that they produce has an appropriate GAG to collagen ratio; for native NP, this is approximately 3.5 : 1 (Mwale et al. 2004). Although this ratio may not help in determining whether ultimate differentiation has occurred, it provides an indication of the correct composition of the tissue that the cells produce (Mwale et al. 2004).
Glucose is a source of energy that markedly affects viability, proliferation and differentiation of stem cells. It should be noted that cells were encapsulated in 3D alginate cylindrical constructs (diameter = 5 mm; height = 3 mm), thus for those gels maintained in hypoxia this 3D geometry has the effect of further reducing the oxygen concentration in the core due to cellular consumption (Buckley et al. 2012). This may explain the observed cell death due to inadequate oxygen combined with diminished glucose availability limiting homogeneous deposition of extracellular matrix that has been reported previously for bone marrow stem cells undergoing chondrogenesis (Farrell et al. 2012).
The results from this study illustrate that BM constructs exhibit an increased glucose consumption rate under hypoxic conditions compared with normoxic conditions. Interestingly, the same constructs exhibit an increased glucose consumption rate compared with NP constructs under the same external oxygen tension, demonstrating that NP cells consume less glucose than BM cells; this suggests that NP cells can more readily adapt to changes in microenvironmental conditions. These findings provide important insights in the development of clinical cell‐based therapies in determining the suitability of specific cell types for targeted regeneration.
Indeed, it has been reported previously that glucose uptake is increased during hypoxia and that stem cells are known to possess the ability to adapt their oxygen consumption rate to changes in the oxygen environment (Pattappa et al. 2013). Deschepper et al. (2010) previously demonstrated that stem cells can remain viable when maintained in severe hypoxic conditions (i.e. 0.2% O2) but not in the absence of glucose. This correlates well with the results from this study, where BM‐encapsulated gels maintained in hypoxia with sufficient glucose (5 and 25 mM) demonstrated higher cell viability, sGAG and collagen accumulation. Importantly, the reduced cell viability was not evident in BM‐encapsulated alginate hydrogels maintained under the same very low glucose concentration (1 mM glucose) and an external oxygen concentration of 20%. Under these conditions, cells appeared to remain viable but only deposited matrix pericellularly. Furthermore, this was observed for all BM‐encapsulated alginate hydrogels maintained under 20% oxygen conditions. Interestingly, the therapeutic potential of stem cells is commonly investigated under 20% O2 (normoxia) conditions in vitro, whereas the typical physiological oxygen concentration in human ranges from 4 to 7% (Packer & Fuehr, 1977; Kofoed et al. 1985) and falls to 1% in some pathological ischaemic tissues, as well as in the degenerated IVD (Bartels et al. 1998). Numerous studies have investigated the influence of hypoxia and have found that BM stem cells proliferated more rapidly, exhibited greater colony forming unit (CFU) formation ability (Grayson et al. 2006, 2007), and maintained better ‘stemness’ in hypoxia through the down‐regulation of E2A‐p21 by HIF‐1a‐Twist pathway (Tsai et al. 2011). Furthermore, previous studies have demonstrated that glucose is a significant factor in the metabolic response of mesenchymal stem cells (Deorosan & Nauman, 2011) and that a low oxygen environment enhances GAG synthesis in pellets and hydrogels (Sheehy et al. 2012). Risbud et al. (2004) found that 2% O2 and 10 ng mL−1 TGF‐β could stimulate rat BM stem cell differentiation to acquire phenotypes similar to that of NP cells.
In contrast, NP‐encapsulated alginate hydrogels maintained under the same very low glucose concentration (1 mM glucose) and an external oxygen concentration of 5% did not exhibit reduced cell viability. In fact, NP cells remained relatively insensitive to external microenvironmental conditions such that similar amounts of sGAG and collagen were deposited homogeneously throughout. This may be due to reduced glucose and oxygen consumption rates, as NP cells naturally reside in a microenvironment with limited nutrient availability. The cell‐specific response observed in this study may thus be a function of metabolic activity. It is plausible that at a particular oxygen concentration and glucose concentration, NP cells and stem cells possess altering metabolic demands. Agrawal et al. (2007) indicated that oxygen‐independent stabilisation of HIF‐1α, a transcription factor that regulates oxidative metabolism, in NP cells is a metabolic adaptation to a unique microenvironment. Furthermore, it should be noted that these experiments were performed using a cell density of 4 × 106 cells mL−1, which is the typical cell density of native nucleus pulposus tissue. Higher seeding densities that are typically used in tissue engineering investigations would exacerbate the nutrient demands, resulting in limited matrix formation. This is an important consideration for IVD regeneration strategies regarding the optimal number of cells that can be injected into the intervertebral disc to elicit a therapeutic response and formation of new tissue. The success of any cell‐based strategy will therefore be dependent on the state of degeneration and more importantly the microenvironment of the disc that can maintain the viability and support the function of injected cells.
Among several studies that have investigated the effects of IVD‐like culture conditions on stem cell survival and differentiation, Wuertz et al. (2008) demonstrated that combining low glucose with high osmolarity (485 mOsm) and low pH (6.8) is detrimental to the differentiation of stem cells, with decreased cellular proliferation and collagen and sGAG expression suggesting that the beneficial effects of IVD‐like low‐glucose culture are not sufficient for promotion of stem cell differentiation when other environmental factors are considered. Of note, Wuertz et al. (2008) did not examine the effect of hypoxia, which is known to be a potent regulator of matrix production. Furthermore, it is crucial to determine the response of stem cells to pro‐inflammatory cytokines to elucidate fully how these cells may respond post implantation in a degenerate IVD niche. Culture of stem cells in the presence of interleukin (IL)‐1b significantly decreases culture pellet size, and cells produce an ECM with atypical mechanical strength and decreased expression of matrix molecules (Felka et al. 2009).
Considering the highly specialised microenvironment of the central NP, these results indicate that IVD‐like low glucose and low oxygen are critical and influential for the survival and biological behaviour of BM stem cells. In this study, for BM constructs, glucose effects were only evident under hypoxic conditions, suggesting that low oxygen is an important regulator of matrix production. Furthermore, NP cells and BM stem cells respond differentially to varying environmental conditions due to altered metabolic activity. Under IVD‐like microenvironmental conditions, NP cells were found to have a lower glucose consumption rate compared with BM cells and may in fact be more suitable to survive the harsh microenvironment that exists within the IVD. Such findings may promote and accelerate the development of clinical therapies in demonstrating the suitability of different cell types for targeted regeneration of the IVD.
Acknowledgements
This work was supported by the Graduate Research Education Programme in Engineering (GREP‐Eng), PRTLI Cycle 5 funded programme. PRTLI is 50% co‐funded under the European Regional Development Fund. The authors have no conflict of interest to declare.
References
- Agrawal A, Guttapalli A, Narayan S, et al. (2007) Normoxic stabilization of HIF‐1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol 293, C621–C631. [DOI] [PubMed] [Google Scholar]
- Bartels EM, Fairbank JC, Winlove CP, et al. (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976) 23, 1–7; discussion 8. [DOI] [PubMed] [Google Scholar]
- Bibby SR, Jones DA, Ripley RM, et al. (2005) Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976), 30, 487–496. [DOI] [PubMed] [Google Scholar]
- Buckley CT, Meyer EG, Kelly DJ (2012) The influence of construct scale on the composition and functional properties of cartilaginous tissues engineered using bone marrow‐derived mesenchymal stem cells. Tissue Eng Part A 18, 382–396. [DOI] [PubMed] [Google Scholar]
- Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9, 641–650. [DOI] [PubMed] [Google Scholar]
- Crevensten G, Walsh AJ, Ananthakrishnan D, et al. (2004) Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 32, 430–434. [DOI] [PubMed] [Google Scholar]
- Deorosan B, Nauman EA (2011) The role of glucose, serum, and three‐dimensional cell culture on the metabolism of bone marrow‐derived mesenchymal stem cells. Stem Cells Int 2011, 429187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschepper M, Oudina K, David B, et al. (2010) Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long‐term, severe and continuous hypoxia. J Cell Mol Med 15, 1505–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Weinstein JN (2001) Low back pain. N Engl J Med 344, 363–370. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Comeau ES, Mauck RL (2012) Mesenchymal stem cells produce functional cartilage matrix in three‐dimensional culture in regions of optimal nutrient supply. Eur Cell Mater 23, 425–440. [DOI] [PubMed] [Google Scholar]
- Felka T, Schafer R, Schewe B, et al. (2009) Hypoxia reduces the inhibitory effect of IL‐1beta on chondrogenic differentiation of FCS‐free expanded MSC. Osteoarthritis Cartilage 17, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Peacock TE, Goupille P, et al. (1997) Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 350, 178–181. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Izadpanah R, et al. (2006) Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 207, 331–339. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, et al. (2007) Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun 358, 948–953. [DOI] [PubMed] [Google Scholar]
- Grunhagen T, Wilde G, Soukane DM, et al. (2006) Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am 88(Suppl 2), 30–35. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Mochida J, Iwashina T, et al. (2008) Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 26, 589–600. [DOI] [PubMed] [Google Scholar]
- Hohaus C, Ganey TM, Minkus Y, et al. (2008) Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 17(Suppl 4), 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D, Brooks P, Blyth F, et al. (2010) The epidemiology of low back pain. Best Pract Res Clin Rheumatol 24, 769–781. [DOI] [PubMed] [Google Scholar]
- Ignat'eva NY, Danilov NA, Averkiev SV, et al. (2007) Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem 62, 51–57. [Google Scholar]
- Inoue H (1981) Three‐dimensional architecture of lumbar intervertebral discs. Spine (Phila Pa 1976), 6, 139–146. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Stephan S, Roberts S (2008) The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: implications for degenerative disc disease. Arthritis Res Ther 10, R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafienah W, Sims TJ (2004) Biochemical methods for the analysis of tissue‐engineered cartilage. Methods Mol Biol 238, 217–230. [DOI] [PubMed] [Google Scholar]
- Kofoed H, Sjontoft E, Siemssen SO, et al. (1985) Bone marrow circulation after osteotomy. Blood flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop Scand 56, 400–403. [DOI] [PubMed] [Google Scholar]
- Li YM, Schilling T, Benisch P, et al. (2007) Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun 363, 209–215. [DOI] [PubMed] [Google Scholar]
- Liang C, Li H, Tao Y, et al. (2012) Responses of human adipose‐derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med 10, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Muneta T, Tabuchi T, et al. (2010) Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase‐related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther 12, R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwale F, Roughley P, Antoniou J (2004) Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 8, 58–63. [DOI] [PubMed] [Google Scholar]
- Mwale F, Ciobanu I, Giannitsios D, et al. (2011) Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine (Phila Pa 1976), 36, E131–E138. [DOI] [PubMed] [Google Scholar]
- Naqvi SM, Buckley CT (2015) Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels. Tissue Eng Part A, 21, 288–299. [DOI] [PubMed] [Google Scholar]
- Orozco L, Soler R, Morera C, et al. (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92, 822–828. [DOI] [PubMed] [Google Scholar]
- Packer L, Fuehr K (1977) Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 267, 423–425. [DOI] [PubMed] [Google Scholar]
- Pattappa G, Thorpe SD, Jegard NC, et al. (2013) Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods 19, 68–79. [DOI] [PubMed] [Google Scholar]
- Richardson SM, Walker RV, Parker S, et al. (2006) Intervertebral disc cell‐mediated mesenchymal stem cell differentiation. Stem Cells 24, 707–716. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Albert TJ, Guttapalli A, et al. (2004) Differentiation of mesenchymal stem cells towards a nucleus pulposus‐like phenotype in vitro: implications for cell‐based transplantation therapy. Spine (Phila Pa 1976) 29, 2627–2632. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Stokes DG, et al. (2006) Nucleus pulposus cells express HIF‐1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem 98, 152–159. [DOI] [PubMed] [Google Scholar]
- Sakai D, Mochida J, Yamamoto Y, et al. (2003) Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 24, 3531–3541. [DOI] [PubMed] [Google Scholar]
- Sheehy EJ, Buckley CT, Kelly DJ (2012) Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun 417, 305–310. [DOI] [PubMed] [Google Scholar]
- Simmons CA, Alsberg E, Hsiong S, et al. (2004) Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562–569. [DOI] [PubMed] [Google Scholar]
- Singh K, Masuda K, Thonar EJ, et al. (2009) Age‐related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976), 34, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillekom S, Smolders LA, Grinwis GC, et al. (2014) Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture. Tissue Eng Part C Methods 20, 8. [DOI] [PubMed] [Google Scholar]
- Stephan S, Johnson WE, Roberts S (2011) The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur Cell Mater 22, 97–108. [DOI] [PubMed] [Google Scholar]
- Stoyanov JV, Gantenbein‐Ritter B, Bertolo A, et al. (2011) Role of hypoxia and growth and differentiation factor‐5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus‐like cells. Eur Cell Mater 21, 533–547. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chen YJ, Yew TL, et al. (2011) Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down‐regulation of E2A‐p21 by HIF‐TWIST. Blood 117, 459–469. [DOI] [PubMed] [Google Scholar]
- Urban JP (2002) The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans 30, 858–864. [DOI] [PubMed] [Google Scholar]
- Vinardell T, Buckley CT, Thorpe SD, et al. (2011) Composition‐function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med 5, 673–683. [DOI] [PubMed] [Google Scholar]
- Wuertz K, Godburn K, Neidlinger‐Wilke C, et al. (2008) Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976), 33, 1843–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XP, Chen HL, Cheng HB (2013) Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta‐analysis. Spine (Phila Pa 1976), 38, 597–608. [DOI] [PubMed] [Google Scholar]
- Yang SH, Hu MH, Sun YH, et al. (2013) Differential phenotypic behaviors of human degenerative nucleus pulposus cells under normoxic and hypoxic conditions: influence of oxygen concentration during isolation, expansion, and cultivation. Spine J 13, 1590–1596. [DOI] [PubMed] [Google Scholar]


