Abstract
Biophysical cues play a key role in directing the lineage commitment of mesenchymal stem cells or multipotent stromal cells (MSCs), but the mechanotransductive mechanisms at play are still not fully understood. This review article first describes the roles of both substrate mechanics (e.g. stiffness and topography) and extrinsic mechanical cues (e.g. fluid flow, compression, hydrostatic pressure, tension) on the differentiation of MSCs. A specific focus is placed on the role of such factors in regulating the osteogenic, chondrogenic, myogenic and adipogenic differentiation of MSCs. Next, the article focuses on the cellular components, specifically integrins, ion channels, focal adhesions and the cytoskeleton, hypothesized to be involved in MSC mechanotransduction. This review aims to illustrate the strides that have been made in elucidating how MSCs sense and respond to their mechanical environment, and also to identify areas where further research is needed.
Keywords: differentiation, mechanobiology, mesenchymal stem cells, multipotent stromal cells, substrate
Introduction
How cells respond to mechanical signals is thought to play a key role in embryonic development, as well as in tissue healing and repair. Understanding how mesenchymal stem cells or multipotent stromal cells (MSCs) derived from adult tissues such as bone marrow and adipose tissue respond to mechanical signals is a major area of research and has important implications for tissue engineering and regenerative medicine. While progress has been made in understanding how mechanical signals are sensed by MSCs and then transduced to affect their behaviour (such as proliferation and differentiation), there is still much that is not fully understood. Extra‐ and intra‐cellular molecules, membrane proteins (e.g. integrins, ion channels, etc.) and numerous cytoskeletal components are all believed to play key roles in determining how MSCs sense and transmit mechanical signals, making mechanotransduction mechanisms extremely complex. This review aims to summarize our current understanding of how mechanical cues direct MSCs towards various musculoskeletal lineages. The paper will begin by describing the role of substrate mechanics (e.g. stiffness and topography) in regulating MSC differentiation. Next, the effects that various extrinsic mechanical signals (e.g. fluid flow, compression, hydrostatic pressure (HP) and tension) have on MSC fate will be summarized. Finally, the role of various cellular components believed to play a role in the mechanotransduction of these different mechanical cues will be described (Fig. 1).
Figure 1.
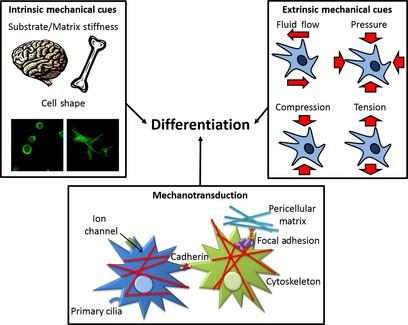
This review describes the intrinsic and extrinsic mechanical cues that regulate the differentiation of MSCs and the specific cellular components hypothesized to be involved in MSCs mechanotransduction.
Role of substrate stiffness and cell shape
Cells exist within tissues of vastly differing stiffness, from soft brain tissue to stiff cortical bone. In vitro, matrix or substrate stiffness has been shown to play a role in regulating the differentiation of MSCs towards specific lineages (Table 1; Engler et al. 2006; Park et al. 2011). When cultured on 2D substrates that mimicked the stiffness of physiological neurogenic, myogenic and osteogenic environments, MSCs adopted a phenotype corresponding to the tissue stiffness, as demonstrated by cellular morphology, transcript markers and protein production (Engler et al. 2006). In a similar experiment, MSCs seeded onto soft substrates were shown to have a greater adipogenic and chondrogenic potential, while those on stiffer substrates had a stronger myogenic potential (Park et al. 2011). In such 2D culture systems, substrate stiffness is generally found to affect cellular morphology, while in certain 3D hydrogels MSCs have been shown to retain a spherical morphology irrespective of the hydrogel stiffness (Huebsch et al. 2010; Parekh et al. 2011). In spite of this, the fate of encapsulated MSCs is still generally dependent on the stiffness of the hydrogel, with stiffer gels supporting osteogenesis and softer gels supporting adipogenesis (Huebsch et al. 2010). Integrin binding has been shown to be necessary in order for osteogenic differentiation to occur on stiff substrates, while an absence of integrin binding has little to no effect on MSC differentiation down adipogenic or neurogenic lineages when cultured on soft substrates (Huebsch et al. 2010; Pek et al. 2010; Parekh et al. 2011). In both 2D and 3D it has been demonstrated that with increasing matrix stiffness, the number of integrins bound to the matrix forms a bell curve distribution, with the hydrogel stiffness that facilitates peak bond formation also providing the optimal stiffness for osteogenic differentiation (Engler et al. 2004; Huebsch et al. 2010). Inhibition of myosin contractile machinery eliminated the dependence of bond formation on matrix stiffness, suggesting that traction‐mediated forces are necessary for the cell to properly sense its mechanical environment (Huebsch et al. 2010). Inhibition of bond formation decreased the osteogenic potential of the cells, suggesting that matrix stiffness‐mediated integrin binding has a direct effect on MSC lineage commitment (Huebsch et al. 2010). Myosin‐generated contraction is also required for MSCs to respond to substrate stiffness in 2D (Pelham & Wang, 1997; Discher et al. 2005; Engler et al. 2006).
Table 1.
Effects of intrinsic mechanical cues on MSC differentiation
| Study | Cell source | Culture conditions | Intrinsic cues | Key findings |
|---|---|---|---|---|
| McBeath et al. (2004) | Human marrow | 2D Fibronectin‐coated PDMS substrate | Micropatterned small (rounded) and large (spread) islands | Mixed media: spread = osteogenic, round = adipogenic; regulated by RhoA and ROCK |
| Engler et al. (2006) | Human marrow | 2D Col‐coated polyacrylamide | Substrate elasticity = 0.1–40 kPa | Phenotype of MSCs mimics tissue‐level elasticity; dependent on NMM II |
| Gao et al. (2010) | Human marrow | 2D Fibronectin‐coated PDMS substrate | Micropatterned small (rounded) and large (spread) islands | TGF‐β3 media: spread = myogenic, round = chondrogenic; regulated by Rac1 and N‐cadherin |
| Huebsch et al. (2010) | Human marrow | 3D Alginate and agarose hydrogels | Matrix stiffness = 2.5–110 kPa | Stiff = osteogenic, soft = adipogenic; integrin binding forms bell curve with stiffness; myosin contractility required to sense matrix stiffness |
| Pek et al. (2010) | Human marrow | 3D PEG‐silica composite gel | Matrix stiffness = 7–100 Pa | Integrin binding had greater impact on MSC lineage commitment in stiff matrices |
| Parekh et al. (2011) | Human marrow | 3D PEG hydrogels | Matrix stiffness = 0.2–59 kPa | Increased stiffness increased osteogenesis; dependent on integrin binding, but not actin polymerization, NMM II or ROCK signalling |
| Park et al. (2011) | Human marrow | 2D Col‐coated polyacrylamide or Col gel | Substrate stiffness = 1–15 kPa and coated plastic dish | Stiff = myogenic, soft = adipo/chondrogenic; cell adhesion strength lower and decreased stress fibre formation in soft gels |
| Khetan et al. (2013) | Human marrow | 3D Hyaluronic acid hydrogels | Matrix stiffness = 4.4–91.6 kPa | Cell–matrix interactions and traction‐mediate forces regulate MSC fate decisions |
| Steward et al. (2013) | Porcine marrow | 3D Agarose gels | Matrix stiffness = 1–25 kPa | Osteogenesis increased and chondrogenesis decreased with increased stiffness; alterations in vinculin, actin and vimentin with increasing stiffness |
MSC, multipotent stromal cell; NMM II, non‐muscle myosin II; PDMS, polydimethylsiloxane; PEG, polyethylene glycol; RhoA, RhoA GTPase; ROCK, Rho kinase; TGF, transforming growth factor.
As already described, matrix stiffness appears to regulate cell shape for multiple cell types, particularly in 2D culture systems, due perhaps to substrate stiffness‐mediated changes in integrin binding, adhesion strength and cellular stiffness/contractility (Choquet et al. 1997; Solon et al. 2007; Chowdhury et al. 2010; Park et al. 2011). Cell shape is determined by both the internal configuration of the cytoskeleton and external interactions with the extracellular matrix (ECM) and adjacent cells. Cell shape has been shown to be a key regulator of MSC differentiation (McBeath et al. 2004). It has been possible to directly determine the role of cell shape on MSC differentiation by seeding cells on micropatterned fibronectin‐coated islands of differing size and then stimulating the cells with a mixed media that potentially permits differentiation along multiple lineages. On small islands where MSCs adopted a rounded morphology, adipogenesis was predominant, while on larger islands where MSCs adopted a spread morphology, osteogenesis was favoured (McBeath et al. 2004). This study also demonstrated that cell shape regulates RhoA GTPase (RhoA) and Rho kinase (ROCK) activity. RhoA is a key regulator of contractility, while ROCK is a Rho effector involved in myosin contraction. Inhibition of ROCK switched lineage commitment of cells from an osteogenic to an adipogenic phenotype, while activation of RhoA in cells exposed to adipogenic media promoted an osteogenic phenotype, indicating that cellular contractility controls MSC lineage commitment down either an osteogenic or adipogenic lineage (McBeath et al. 2004). In a similar study, MSCs stimulated with the transforming growth factor (TGF)‐β3 were either allowed to flatten and spread, or to maintain a rounded cell morphology. MSCs allowed to spread proceeded down a myogenic lineage, while those kept rounded committed to a chondrogenic lineage (Gao et al. 2010). RhoA was not upregulated; however, Rac1 (a member of the Rho GTPase family) was upregulated in the spread cells, and was sufficient to induce myogenesis and inhibit chondrogenesis. Together, Rac1 and TGF‐β3 were found to upregulate N‐cadherin (a molecule associated with cell–cell adhesions), indicating that structural changes to the cytoskeleton play key roles in determining MSC lineage commitment down multiple different pathways (McBeath et al. 2004; Gao et al. 2010).
Interestingly, although cell shape has been shown to control adipogenic/osteogenic lineage commitment through the Rho and ROCK pathways, uncertainty still exists in the literature over the roles of Rho and cytoskeletal tension on MSC response to substrate stiffness. In 2D, non‐muscle myosin II (NMM II), which acts to put the actin cytoskeleton into tension, was observed to be necessary in order for modulus‐driven differentiation to occur (Engler et al. 2006). NMM II is also correlated with an increase in the number of focal adhesions (Conti et al. 2004). Another 2D study found cell adhesion strength to be weaker on soft substrates, which led to a decrease in stress fibre formation (Park et al. 2011). Together these studies suggest the following hypothesis: integrin binding allows MSCs to probe the stiffness of its surrounding matrix and then cytoskeletal tension, generated by NMM II, adapts to its surrounding substrate (Pelham & Wang, 1997). These changes in cytoskeletal tension lead to changes in a myriad of cell signalling cascades that control cell behaviour. However, a study in 3D found that modulus‐driven differentiation of MSCs was independent of actin polymerization, ROCK signalling and NMM II (Parekh et al. 2011).
Recent developments in materials science have enabled the development of tuneable in vitro 3D model systems to help further decouple the role of cell shape, substrate stiffness and cytoskeletal tension in regulating MSC fate. In one such study, MSCs were encapsulated in covalently crosslinked 3D hydrogels with MMP‐degradable peptides to enable the development of cell‐generated traction forces as the hydrogels underwent controlled degradation (Khetan et al. 2013). Cells in the degradable hydrogels were found to be more spread, have more focal adhesions and cytoskeletal assemblies, and express more osteogenic markers, while cells in non‐degradable hydrogels (irrespective of their stiffness) were found to be more rounded, have less focal adhesions and cytoskeletal assemblies, and express more adipogenic markers, suggesting that cell‐mediated traction forces are important in guiding MSC differentiation (Khetan et al. 2013). In order to decouple cell shape from the effects of matrix degradation, MSCs were seeded in a degradable matrix, but then exposed to secondary crosslinkers after 1 week, arresting the degradation of the hydrogel while maintaining a spread morphology. These MSCs displayed a switch from an osteogenic to adipogenic lineage, indicating that cell–matrix interactions, and not cell shape, drive fate decisions. Therefore, an appreciation of cell–matrix interactions and traction‐mediated forces is necessary to improve current understanding of the mechanotransduction of substrate‐mediated mechanical cues (Khetan et al. 2013).
Role of external mechanical signals
Multipotent stromal cells respond not only to the biological and mechanical properties of the surrounding matrix, but also to external mechanical signals such as fluid flow, HP, and compressive and tensile loading (Table 2; Kelly & Jacobs, 2010). The type, frequency, magnitude and duration of such cues have all been shown to affect MSC differentiation. The following subsections will examine the response of MSCs to different forms of mechanical signals, and comment on obvious differences or similarities in cellular response to them.
Table 2.
Effects of extrinsic mechanical cues on MSC differentiation
| Study | Cell source | Culture conditions | Loading conditions | Key findings |
|---|---|---|---|---|
| Fluid flow | ||||
| Bancroft et al. (2002) | Rat marrow | 3D Ti mesh | Perfusion @ 0.3–3 mL min−2, 16 day | Increased calcium content and matrix distribution |
| Li et al. (2004) | Human marrow | 2D Glass slide | Parallel plate, 2 h, 1 Hz, peak shear of 10 dyn cm−2 | Increased osteopontin and osteocalcin mRNA, no change in Col 1 and CBF‐1 mRNA |
| Knippenberg et al. (2005) | Goat adipose | 2D Glass slide | Parallel plate, 1 h, 5 Hz, mean shear of 0.6 Pa | Increased NO and COX‐2, decreased ALP, no change in osteopontin or Col 1 mrNA |
| Datta et al. (2006) | Rat marrrow | 3D Ti or Ti/ECM mesh | Perfusion @ 1 mL min−1, 16 day | Larger increase in mineralization in Ti/ECM |
| Riddle et al. (2006) | Human marrow | 2D Glass slide | Parallel plate, ≤ 120 min, 1 Hz, shears of 5–20 dyn cm−2 | Increased ERK 1/2 activation and calcineurin activity |
| Arnsdorf et al. (2009b) | C3H/10T1/2 | 2D Fibronectin‐coated glass slide | Parallel plate, 1 h, 1 Hz, peak shear of 10 dyn cm−2 | Increased Runx2, Sox9 and PPARγ, RhoA and ROCKII regulates OFF‐induced osteogenesis |
| Huang et al. (2010) | Rat marrow | 2D Glass slide | Parallel plate, 24 h, shears of 5–20 dyn cm−2 | Increased cardiomyogenic mRNA and protein markers |
| Hydrostatic Pressure | ||||
| Angele et al. (2003) | Human marrow | Aggregate | 4 h day−1, 1–7 day, 1 Hz, 5.03 MPa | Increased proteoglycan and collagen content |
| Elder et al. (2005) | C3H/10T1/2 | Aggregate | 1800 or 7200 cycles day−1, 3 day, 1 Hz, 5 MPa (10 min on, 10 min off) |
1800 cycles: no differences 7200 cycles: increased sGAG content and collagen synthesis |
| Miyanishi et al. (2006a) | Human marrow | Pellet | 4 h day−1, 3–14 day, 1 Hz, 10 MPa | Increased Sox9, Col II and Agc mRNA; Col II and Agc matrix synthesis |
| Miyanishi et al. (2006b) | Human marrow | Pellet | 4 h day−1, 3–14 day, 1 Hz, 0.1–10 MPa | Increased Sox9, Agc, Col II mRNA; dose/time‐dependent increase in sGAG and collagen synthesis |
| Finger et al. (2007) | Human marrow | 2% Agarose | 4 h day−1, 14 day, 1 Hz, 7.5 MPa | Increased Sox9 mRNA, no change in Col II and Agc mRNA, time‐dependent increase in Col I |
| Luo & Seedhom (2007) | Ovine marrow | Polyester | 30 min day−1, 10 day, 0.25 Hz, 0.1 MPa | Increased sGAG and collagen content |
| Wagner et al. (2008) | Human marrow | Collagen I Sponge | 4 h day−1, 10 day, 1 Hz, 1 MPa | Increased proteoglycan content and Sox9, Agc and Col II mRNa; no change in Runx2 or TGF‐β1 mRNA |
| Li et al. (2009) | Rat marrow | 1.5% Alginate | 1 h day−1, 7 day, 0.25 Hz, 36 kPa | Increased Sox9, Runx2, Ihh, Agc and Col II mRNA |
| Ogawa et al. (2009) | Human adipose | Collagen I Sponge | 1 week, 0.5 Hz, 0.5 MPa | Increased Sox9, Agc, Col II and Col X mRNA, |
| Zeiter et al. (2009) | Bovine marrow | Aggregate | 3 h day−1,14 day, 1 Hz, 3 MPa | No change in Sox9, Agc, Col I, Col II mRNA or sGAG synthesis |
| Meyer et al. (2011) | Porcine marrow | 2% Agarose | 1 h day−1, 42 day, 1 Hz, 10 MPa | Increased sGAG and collagen content in donor‐dependent manner |
| Steward et al. (2012) | Porcine marrow | 2% Agarose or fibrin | 4 h day−1, 21 day, 1 Hz, 10 MPa | Increase in sGAG synthesis in fibrin gels only |
| Vinardell et al. (2012) | Porcine synovial membrane and fat pad | Pellet | 4 h day−1, 14 day, 1 Hz, 10 MPa | Increased Sox9 mRNA @ 1 ng mL−1 TGF‐β3 but not at 10 ng mL−1; decreased Ihh and Col X in synovial membrane cells |
| Steward et al. (2013) | Porcine marrow | 1 or 4% Agarose | 4 h day−1, 21 day, 1 Hz, 10 MPa | Increased sGAG synthesis, Sox9, Agc, Col II mRNA and vimentin organization in 4% gels only |
| Carroll et al. (2014) | Porcine marrow and fat pad | 2% Agarose | 4 h day−1, 35 day, 1 Hz, 10 MPa | Increased sGAG synthesis in both cell types, suppressed mineralization in bone marrow |
| Compression | ||||
| Takahashi et al. (1998) | Limb bud | Collagen gel | 20–30% strain | Increased Sox9, Agc and Col II mRNA; suppressed IL‐1β |
| Huang et al. (2004) | Rabbit marrow | 2% Agarose | 4 h day−1, 14 day, 1 Hz, 10% strain | Increased Agc, Col II and TGF‐β1 mRNA |
| Campbell et al. (2006) | Human marrow | 3% Alginate | 1.5 h on/4.5 h off, 8 day, 1 Hz, 15% strain | Increased Sox9, Agc, Col II and Col X mRNA; complex interplay between DC and TGF‐β1 supplementation |
| Mauck et al. (2006) | Bovine marrow | 2% Agarose | 3 h day−1, 5 day, 1 Hz, 10% strain | Increased Agc mRNA and sGAG synthesis |
| Mouw et al. (2007) | Bovine marrow | 3% Agarose | 3 h day−1, on day 8 or 16, 1 Hz, 10% strain | Increased sGAG synthesis, and Agc, Col I and Col II mRNA on day 16; stronger with addition of TGF‐β1 |
| Thorpe et al. (2008) | Porcine marrow | 2% Agarose | 1 h day−1, 42 day, 0.5 Hz, 10% strain | Decreased, sGAG and Col II synthesis |
| Kisiday et al. (2009) | Equine marrow | 2% Agarose | 45 min on/45 min off day−1, 15 or 21 day, 0.3 Hz, 7.5–10% strain | DC w/o TGF‐β1 increased sGAG synthesis, DC w/TGF‐β1 decreased sGAG synthesis |
| Pelaez et al. (2009) | Human marrow | Fibrin gels | ||
| Kupcsik et al. (2010) | Human marrow | Fibrin‐polyurethane gels | 1 h day−1, 7 day, 1 Hz, 10–20% strain | Increased sGAG synthesis and Agc, Col II, Col X, TGFB1 and TGFB3 mRNA; effects stronger at lower TGF‐β1 concentrations |
| Li et al. (2010a) | Human marrow | Fibrin‐polyurethane gels | 1 h day−1, 7 day, 1 Hz, 10–20% strain | Increased sGAG, TGF‐β1 and TGF‐β3 synthesis, and Agc, Col II and Col X mRNA; effects stronger at lower TGF‐β1 concentrations |
| Li et al. (2010b) | Human marrow | Fibrin‐polyurethane gels | 1 h day−1, 7 day, 0.1 or 1 Hz, 10–15, 20 or 30% strain | Higher frequency and amplitude = increased sGAG synthesis and chondrogenic gene expression |
| Haugh et al. (2011b) | Porcine marrow | 2% Agaorse | 1 h day−1, on day 7, 14 or 21, 1 Hz, 10% strain | Increased Agc, Col I and Col II mRNA in temporal and spatial‐dependent manner |
| Thorpe et al. (2012) | Porcine marrow | 2% Agarose or fibrin | 3 h day−1, 1 Hz, 21 or 42 days |
Day 21: decreased markers of chondro/myogenesis in both gels Day 42: increased chondrogenic markers in fibrin gels |
| Steward et al. (2014) | Porcine marrow | 1% or 4% Agarose | 1 h day−1, on day 7, 14 or 21, 1 Hz, 10% strain | Increased Sox9, Agc and Col II in 4% gels relative to 1%; inhibition of integrins abrogated this response |
| Tension | ||||
| Sumanasinghe et al. (2006) | Human marrow | Collagen matrix | 4 h day−1, 7 or 14 day, 1 Hz, 0,10, or 12% strain | Increased BMP‐2 mRNA when exposed to 10% (day 7/day 14) and 12% strain (day 14) |
| Ward et al. (2007) | Human marrow | Collagen matrix | 3–5% strain | Increased mineralization, osteogenic mRNA, ERK1/2 activation; decreased chondrogenic, adipogenic, neurogenic expression |
| McMahon et al. (2007) | Rat marrow | Col‐GAG scaffold | 7 day, 1 Hz, 10% strain | Increased sGAG synthesis rate |
| Byrne et al. (2008) | Rat marrow | Col‐GAG scaffold | 4 h day−1, day 5–7, 1 Hz, 5% styrain | Increased osteopontin mRNA |
| McMahon et al. (2008) | Rat marrow | Col‐GAG scaffold | 7 day, 1 Hz, 10% strain | Increased sGAG synthesis rate; dependent on stretch‐activated calcium channels |
| Qi et al. (2008) | Rat marrow | Plastic strip | 40 min, 0.5 Hz, 2000 με | Increased ALP activity, and Cbfa1 and Ets‐1 mRNA |
| Hanson et al. (2009) | Human adipose | Col‐coated BioFlex™ dish | 4 h day−1, 14 day, 1 Hz, 10% strain | Increased amount and rate of calcium deposition |
| Haudenschild et al. (2009) | Human marrow | 2% Alginate | 24 h, 0.2 Hz, 10% strain | Increased osteogenic gene expression and decreased chondrogenic gene expresson |
| Sumanasinghe et al. (2009) | Human marrow | Collagen matrix | 4 h day−1, 7 or 14 day, 1 Hz, 0, 10 or 12% strain | Increased IL‐6 and IL‐8 expression in 10/12% strain |
| Kearney et al. (2010) | Rat marrow | Col‐coated silicone | 14 day, 0.17 Hz, 2.5% strain | Increased Cbfa1, Col I, osteocalcin, BMP2 mRNA; stretch‐activated ion channels needed for Col I increase; ERK, p38 and PI3 needed for BMP increase |
| Jang et al. (2011) | Rabbit marrow | Silicone wafer | 3 day, 0.26 Hz, 3 and 10% strain | Increased ALP and α‐SMA |
| Rui et al. (2011) | Rat tendon | Col‐coated silicone | 0.5 Hz, 0, 4 or 8% strain | Increased BMP‐2 expression and mRNA, ALP activity and calcium deposition |
DC, dynamic compression; ECM, extracellular matrix; ERK, extracellular signal‐regulated kinase; HP, hydrostatic pressure; RhoA, RhoA GTPase; ROCK, Rho kinase; TGF, transforming growth factor.
Fluid flow
Oscillatory fluid flow (OFF) is known to be mechanically induced in bones in vivo (Weinbaum et al. 1994), and has been shown to regulate stem cell fate (Arnsdorf et al. 2009b). OFF has been shown to increase actin fibril density and Rho and ROCK signalling, and to upregulate Runx2, Sox9 and PPARγ expression in murine C3H10T1/2 progenitor cells (Arnsdorf et al. 2009b). With the addition of inhibitors of ROCK, NMM II and actin polymerization there was no pro‐osteogenic response to fluid flow. With regards to adipogenesis and chondrogenesis, however, inhibiting cytoskeletal tension was found to increase Sox9 and PPARγ expression, but abrogate any effects due to fluid flow (Arnsdorf et al. 2009b). Overall, an intact, dynamic actin cytoskeleton is needed to transduce OFF, which in turn influences multiple MSC lineage pathways. Other studies have also demonstrated that fluid flow stimulation increases the expression of osteogenic markers in adipose‐derived MSCs (Knippenberg et al. 2005) and bone marrow‐derived MSCs (Li et al. 2004; Riddle et al. 2006). OFF has been shown to increase intracellular calcium ions and activate mitogen‐activated protein (MAP) kinases [specifically extracellular signal‐regulated kinase (ERK) 1/2; Li et al. 2004; Riddle et al. 2006], which have been shown to influence MSC differentiation (Hardingham & Bading, 1999; Jaiswal et al. 2000). In addition, fluid flow has also been found to promote cardiomyogenic differentiation of bone marrow‐derived MSCs (Huang et al. 2010). A mounting body of work also exists pointing to a key role for the primary cilia in the mechanotransduction of OFF (Hoey et al. 2011, 2012b).
A number of studies have also explored how fluid flow regulates MSC fate within 3D constructs. Perfusion systems have consistently been found to promote osteogenesis of MSCs (Bancroft et al. 2002; Datta et al. 2006). The effect of different types of fluid flow on osteogenesis in a variety of scaffolds has been summarized previously (Meinel et al. 2004). Oftentimes, perfusion flow not only induces mechanical stress across the cell but also increases nutrient and gas transfer through the gel, making interpretation of the results difficult. Computational modelling has also begun to be utilized to investigate the complex relationships between fluid flow, the cell, and the surrounding matrix or substrate (Prendergast et al. 1997; McMahon et al. 2007; Zhao et al. 2007; Jungreuthmayer et al. 2008, 2009; Olivares et al. 2009; Sandino & Lacroix, 2011).
Hydrostatic Pressure
Hydrostatic pressure (HP) has been implicated as a key regulator of MSC differentiation. Numerous studies have demonstrated that cyclic HP leads to increases in chondrogenic gene expression (Sox9, aggrecan, collagen type II) and/or proteoglycan and collagen synthesis in MSCs (Angele et al. 2003; Elder et al. 2005; Miyanishi et al. 2006a,b; Luo & Seedhom, 2007; Wagner et al. 2008; Li et al. 2009; Ogawa et al. 2009; Meyer et al. 2011; Liu et al. 2012, 2014; Steward et al. 2012, 2013; Vinardell et al. 2012). In contrast, other studies have demonstrated that HP has no significant effect on chondrogenesis of MSCs (Finger et al. 2007; Zeiter et al. 2009), which might be explained, at least in part, by the finding that the response to HP may depend on the material within which the cells are encapsulated (Elder et al. 2006; Steward et al. 2012). Therefore, cell–substrate interactions may determine the response of MSCs to extrinsic mechanical cues such as HP and ultimately determine their fate.
Hydrostatic pressure is a non‐deforming mechanical stimulus, which makes putative mechanotransduction pathways less obvious than other loading regimes. Similar to OFF, changes in intracellular calcium concentrations have been suggested as a possible mechanotransductive cue for chondrocytes subjected to HP (Wright et al. 1992; Browning et al. 1999, 2004; Hall, 1999; Mizuno, 2005). In chondrocytes, static HP inhibits the Na/K and Na/K/2Cl pump (Hall, 1999), but enhances Na/H exchange (Browning et al. 1999). Also, 30 s of static HP has been shown to triple intracellular calcium concentration in chondrocytes (Browning et al. 2004), mainly by promoting release from intracellular calcium stores (Browning et al. 2004; Mizuno, 2005). Addition of gadolinium was also found to inhibit the increase in intracellular calcium in MSCs, indicating stretch‐activated ion channels as a pathway for calcium influx and subsequent calcium‐induced calcium release (Mizuno, 2005). However, less is known about the role of calcium signalling in the mechanotransduction of HP in MSCs.
Hydrostatic pressure has also been shown to effect components of the cytoskeleton (Bourns et al. 1988; Parkkinen et al. 1995; Jortikka et al. 2000; Shim et al. 2008). High‐magnitude static HP was observed to inhibit microtubule and actin fibre formation in epithelial cells, leading to cell rounding (Bourns et al. 1988). Disruption of microtubules was correlated with a decrease in matrix synthesis in chondrocytes in response to high‐magnitude static HP; however, intact, dynamic microtubules were needed for mechanotransduction of cyclic HP by chondrocytes (Jortikka et al. 2000). When comparing the response of MSCs seeded in either agarose (spherical MSC morphology) or fibrin (spread MSC morphology with clear stress fibre formation) hydrogels to the application of HP, it was demonstrated that while agarose provided a stronger pro‐chondrogenic environment, a more robust response to the application of HP was observed in fibrin hydrogels (Steward et al. 2012). In a subsequent study, MSCs were seeded in either soft or stiff agarose hydrogels. The pericellular matrix, cytoskeletal organization and focal adhesion formation were observed to be altered in the stiff gels relative to the soft gels, with MSCs in the soft gels exhibiting a stronger chondrogenic phenotype, but with a pro‐chondrogenic response to the application of HP only observed in the stiffer gels (Fig. 2A; Steward et al. 2013). Together, this suggests that while cell attachment and stress fibre formation may decrease chondrogenesis, they are required for the mechanotransduction of HP in MSCs (Steward et al. 2012, 2013). Pharmacological inhibition of actin and microtubule polymerization has separately been shown not to abrogate the pressure‐stimulated increases in chondrogenic gene expression (Shim et al. 2008); however, HP has been found to alter vimentin organization, suggesting a novel role for intermediate filaments in the mechanotransduction of HP (Steward et al. 2013). Recently, decoupling vimentin from focal adhesions in fibroblasts was found to attenuate the activity of FAK and its downstream targets (Gregor et al. 2014). Therefore, investigating vimentin and its interactions with focal adhesions may elucidate further insights into the mechanotransduction of HP by MSCs.
Figure 2.
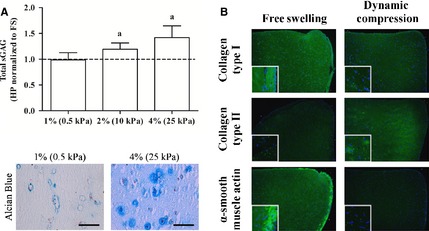
(A) Matrix stiffness alters the pericellular matrix and subsequently the chondrogenic response of MSCs to HP (Steward et al. 2013). Reproduced with kind permission from eCM journal (www.ecmjournal.org). (B) DC can override local substrate cues to switch MSCs from a myogenic to chondrogenic state (FS = free swelling; Thorpe et al. 2012). Reproduced with kind permission from Elsevier.
Endochondral ossification is the process by which cartilage is calcified and turns into bone. Understanding how joint‐specific factors, such as mechanical cues like cyclic HP, regulate the endochondral phenotype will be central to realising the potential of stem cell‐based therapies for articular cartilage repair (Sheehy et al. 2012). HP has been found to increase chondrogenic gene expression while having no significant effect on osteogenic genes (Wagner et al. 2008). There is also strong evidence to suggest that HP regulates the hypertrophy and endochondral ossification of chondrogenically primed MSCs. HP has been found to decrease the hypertrophic markers Indian Hedgehog (Ihh) and collagen type X in synovial‐derived MSCs in pellet culture, and to reduce alkaline phosphatase activity in bone marrow‐derived MSCs embedded in agarose hydrogels (Steward et al. 2012; Vinardell et al. 2012). Subsequently, HP was also found to decrease calcification of bone marrow‐derived MSCs in long‐term agarose culture (Carroll et al. 2014).
Compression
Similar to HP, direct compression of MSCs encapsulated in 3D hydrogels has been found to be a strong pro‐chondrogenic stimulus (Huang et al. 2004). Dynamic compression (DC) has been shown to increase chondrogenic gene expression in MSCs in the absence of exogenous growth factor stimulation, suggesting that compression alone is sufficient to induce chondrogenesis (Takahashi et al. 1998; Campbell et al. 2006; Mauck et al. 2006; Kisiday et al. 2009; Pelaez et al. 2009; Kupcsik et al. 2010; Li et al. 2010a). Further, it has been found that compression increases TGF‐β1 gene expression, suggesting that compression and exogenous TGF‐β stimulation activate similar pathways (Huang et al. 2004). There is some uncertainty as to the impact of simultaneously stimulating MSCs with both soluble TGF‐β and DC. Both the application of TGF‐β3 and DC have been found to increase chondrogenesis; however, some studies have demonstrated that the simultaneous application of TGF‐β3 and mechanical stimulation inhibits chondrogenesis [19 139]. Delaying the application of DC (i.e. initiating loading after prolonged exposure to TGF‐β) has been shown to enhance chondrogenesis of MSCs (Mouw et al. 2007; Li et al. 2010b; Thorpe et al. 2010; Haugh et al. 2011a).
Cell–matrix interactions are also important in determining the response of MSCs to extrinsic mechanical cues such as DC. When maintained in free swelling conditions and stimulated with TGF‐β3, MSCs seeded into fibrin hydrogels appeared to differentiate along a myogenic pathway in long‐term culture. The application of DC to these MSC‐seeded fibrin hydrogels led to an increase in chondrogenesis and a suppression of myogenesis (Fig. 2B; Thorpe et al. 2012). In another recent study, MSCs were seeded into either ‘soft’ or ‘stiff’ hydrogels that differentially support pericellular matrix formation. MSCs seeded in the stiffer hydrogels displayed a more pro‐chondrogenic response to the application of DC compared with those seeded in softer hydrogels (Steward et al. 2014). Furthermore, inhibition of integrin binding suppressed the beneficial response to DC in the stiff hydrogels (Steward et al. 2014). Overall, differences in scaffold type, cell type and loading regime (Maul et al. 2011) could all help to explain differences in these results; however, formation of a pericellular matrix seems to be an important factor in determining MSC response to DC.
Tension
Tensile strain has been shown to enhance the expression of ligamentous/fibrogenic (Altman et al. 2002; Baker et al. 2011; Subramony et al. 2013), osteogenic (Sumanasinghe et al. 2006; Ward et al. 2007; Byrne et al. 2008; Qi et al. 2008; Hanson et al. 2009; Kearney et al. 2010; Rui et al. 2011) and chondrogenic (McMahon et al. 2007, 2008) markers in MSCs. Applying cyclic tensile strain to MSCs has been shown to promote endogenous BMP‐2 expression, osteogenic gene expression and calcium deposition (Sumanasinghe et al. 2006; Byrne et al. 2008; Qi et al. 2008; Hanson et al. 2009; Rui et al. 2011). Similar to OFF, the MAP kinase pathway was found to be upregulated in MSCs exposed to cyclic tensile strain, suggesting it as an important mechanotransductive pathway in osteogenic differentiation (Ward et al. 2007). Another study found ERK and p38 to be involved in mechanotransduction of cyclic tension, and further implicated stretch‐activated cation channels in mediating increases in collagen I gene expression in response to tension (Kearney et al. 2010). In a study directly comparing cyclic compression and tension, tension was found to regulate many osteogenic and fibroblastic genes, while compression enhanced many chondrogenic‐related genes. Dynamic tension was found to upregulate β‐catenin (Haudenschild et al. 2009), which stabilizes cell–cell interactions and is known to inhibit chondrogenesis (Lee et al. 2000; Hwang et al. 2005), therefore implicating cell–cell interactions as key regulators of the osteogenic response of MSCs to dynamic tension. However, MSCs seeded in collagen‐GAG scaffolds that underwent cyclic tensile strain synthesized more proteoglycans than constrained unstrained controls, implying that the application of tensile stimuli can also lead to a pro‐chondrogenic response (McMahon et al. 2007, 2008). In addition, the magnitude of cyclic tension may regulate MSC fate decisions, with myogenesis favoured at high tensile strains while low tensile strains were more beneficial for osteogenesis of rabbit MSCs in the absence of growth factors (Jang et al. 2011). Cyclic tensile strain has also been shown to induce the expression of proinflammatory cytokines known to inhibit bone resorption, suggesting that mechanical stimulation not only induces osteogenesis but helps to maintain bone formation (Sumanasinghe et al. 2009).
Mechanotransduction
There is clearly strong evidence that mechanical signals, including substrate stiffness, cell shape, fluid flow, HP, compression and tension are key regulators of MSC differentiation. Understanding how MSCs sense and respond to these signals is currently a highly researched area. MSCs have the ability to take such ‘outside‐in’ signals, transmit the signal to the nucleus, and to then alter gene expression and protein activity. The response may alter the cells’ surrounding matrix, therefore causing ‘inside‐out’ signals. The cell membrane and numerous intracellular components all play key roles in this ‘outside‐inside‐out’ signalling, and will be reviewed in more detail in the following sections.
Cell membrane components
Numerous cell membrane proteins have been implicated in mechanotransduction, but this review will focus on ion channels, integrins and cadherins. Other putative mechanosensors on the cell membrane, such as the primary cilia, are reviewed in detail elsewhere (Hoey et al. 2012a). Briefly, primary cilia are membrane‐encased microtubular structures present on nearly every cell in the body. They are thought to act as ‘multifunctional antenna’ that sense both chemical and mechanical signals (Singla & Reiter, 2006; Hoey et al. 2012a). Due to their dual functionality as both chemo‐ and mechanosensors, the specific roles of primary cilia in mechanotransduction are unclear. Further research is needed in order to help decouple the chemosensing and mechanical sensing capabilities of primary cilia in relation to MSC differentiation.
Ionic concentrations within a cell control many cellular functions; ions act as second messengers in many signalling pathways, regulate osmosis and therefore cell volume, and a variety of other homeostatic functions. The cellular membrane is impermeable to most ions, and therefore ion channel proteins in the membrane are necessary for ion transport into and out of the cell. Some of these ion channels have been found to either activate or deactivate in response to mechanical stretch (Campbell et al. 2008). Ion channels are very complex, and there is much in the literature describing the mechanotransductive capabilities of stretch‐activated ion channels (Naruse & Sokabe, 1993; Wright et al. 1997; Martinac, 2004; Yoshimura & Sokabe, 2010). Stretch‐activated ion channels have also been proposed to transduce mechanical signals and effect the differentiation of MSCs. Induction of stress fibre formation was found to transmit a mechanical tension to the plasma membrane and activate ion channels (Formigli et al. 2005). In a follow‐up study, inhibition of ROCK was found to significantly decrease the sensitivity of the ion channels, suggesting that Rho‐dependent actin remodelling regulates ion channel sensitivity (Formigli et al. 2007). Blocking of the ion channels alone was also found to suppress myogenesis. Together, these data imply that ion concentrations affect myogenic differentiation of MSCs, and control of these concentrations is dependent on stress fibre‐generated tension and ion channel sensitivity (Formigli et al. 2007). Ion channels have also been shown to play an important role in the mechanotransduction of extrinsic mechanical cues. Cyclic tensile loading of MSCs has been shown to increase proteoglycan production, but this response was inhibited when ion channel activity was blocked, further indicating ion channels as important mechanotransducers in MSCs (McMahon et al. 2008).
While mechanosensitive ion channels play important roles in mechanotransduction, not all cellular responses to mechanical stimuli require them (Malek & Izumo, 1996). This suggests that other membrane proteins are also involved in mechanotransduction. The primary linkage between the extracellular environment and the interior of the cell occurs through integrin molecules in the plasma membrane (Hynes, 1992). Integrins are made up of α and β subunits, with the specific combination of the various available α and β subunits determining the specific ligands the integrin binds to (Van der Flier & Sonnenberg, 2001). A single integrin can bind to a variety of ECM components, and a single type of matrix component can be bound by a variety of integrins (Hynes, 1992). Integrins themselves may have little direct control of cellular behaviour, rather when mechanical signals are transmitted to integrins from the ECM, large protein complexes form that triggers signalling cascades within the cell (Giancotti & Ruoslahti, 1999).
While integrins allow a cell to bind with the ECM, cadherins are one type of membrane protein that allows a cell to bind with other cells. Cadherins are calcium‐dependent molecules that bind in a homophilic manner with cadherins on other cells. Calcium is thought to induce a conformational change in cadherins that allow them to bind with other cadherins (Takeichi, 1990). While cadherins bind to other cadherins extracellularly, they also interact with the actin cytoskeleton intracellularly. Cadherins anchor themselves intracellularly by forming complexes with catenins, which are known to bind with the cytoskeleton (Aberle et al. 1996). β‐Catenin specifically is known to be also involved in other signalling pathways (Nelson & Nusse, 2004). For example, it has been suggested that OFF acts to disassemble cadherin–catenin complexes, allowing β‐catenin to act as a signalling molecule that leads to an increased osteogenic response in MSCs (Arnsdorf et al. 2009a). Cellular condensation of MSCs during development through cadherin binding is required for chondrogenic differentiation to occur (DeLise et al. 2000). Also, N‐cadherins, a subclass of cadherins, have been shown to be necessary for myogenesis of MSCs (Gao et al. 2010). Overall, cadherins are critical regulators of stem cell lineage commitment and also appear to play an important role in mechanotransduction.
Intracellular components
As mentioned previously, integrins are a link between the extracellular and intracellular environment; extracellularly integrins binds with the ECM, but intracellularly integrins help to form large protein complexes known as focal adhesions (Burridge et al. 1988). Focal adhesion proteins are involved in numerous signalling pathways and are also an anchorage site for the actin cytoskeleton (Geiger et al. 2001; Hynes, 2002; Zaidel‐Bar et al. 2007; Ramage et al. 2009). They are composed of many structural proteins including, but not limited to, β‐subunits of integrins, vinculin, talin and the actin cytoskeleton. The assembly of focal adhesions helps to stabilize integrin binding, which in turn can regulate cell shape, which (as previously discussed) is a key regulator of MSC differentiation (McBeath et al. 2004). Focal adhesion assemblies can also provide a platform for numerous other proteins involved in signalling cascades to bind and transmit signals to the nucleus (Clark & Brugge, 1995). Tyrosine kinases such as FAK and paxillin (Burridge et al. 1992), serine‐threonine kinases such as MAPK (Chen et al. 1994), GTPases such as Rho (Hall, 1994), and intracellular calcium concentration (Juliano & Haskill, 1993; Kawano et al. 2006; Riddle et al. 2006) are all activated by the formation of focal adhesions, and they, along with their downstream signals, have all been implicated in MSC differentiation (McBeath et al. 2004; Lu et al. 2008; Pala et al. 2008; Kundu et al. 2009; Sen et al. 2011). As mentioned previously, the activity of such proteins is also affected by extrinsic mechanical signalling. Clearly, formation of focal adhesions, or lack thereof, plays a critical role in mechanotransduction and regulation of MSC differentiation.
Focal adhesions, along with similar cell–cell junctions, form platforms from which many downstream signalling cascades take place, but the effect of each of these signals can be extremely complex. In addition to their signalling capabilities, focal adhesion proteins also act as actin–integrin anchorage points for the cytoskeleton (Geiger et al. 2009). The cytoskeleton is comprised of filaments that provide structure and support to the cell. The cytoskeleton actively generates isometric tension within the cell by an actomyosin filament sliding mechanism similar to muscle (Harris et al. 1980; Burridge, 1981). Therefore, cells are pre‐stressed, and because the cytoskeleton anchors at integrin‐binding sites, mechanical loads can be transferred through the cell (Ingber, 1997). The tension generated by the cytoskeleton depends on substrate stiffness, ligand type and density, and intracellular signals (Burridge & Chrzanowska‐Wodnicka, 1996). This cytoskeletal tension can also determine cell morphology and affect the activity of focal adhesions and cell–cell junctions (Liu et al. 2010; Wolfenson et al. 2011); therefore, the mechanical state of the cytoskeleton plays a prominent role in MSC differentiation (Woods et al. 2007). RhoA and ROCK have been mentioned frequently throughout this review as key signals during mechanotransduction and differentiation. The main function of RhoA is to regulate focal adhesions and stress fibres through downstream phosphorylation cascades that effects myosin contractility (Chrzanowska‐Wodnicka & Burridge, 1996). Although it is well established that RhoA affects focal adhesions and stress fibres, and plays a key role in determining osteogenic–adipogenic fate decisions (McBeath et al. 2004), the downstream effects of such factors on other MSC differentiation pathways are less clear. For instance, RhoA/ROCK signalling has been shown to both enhance and inhibit chondrogenesis by regulating Sox9 expression depending upon the cell culture model used (Woods et al. 2005; Woods & Beier, 2006).
Recently, there has been a growing interest in the nucleus as a mechanosensor in of itself. When the nucleus deforms it alters chromatin architecture, which in turn can directly affect transcription (Dahl et al. 2008). Nuclei in MSCs have been found to deform more than fibroblast nuclei in response to both intrinsic and extrinsic mechanical signals; given that both cell types exhibit similar cytoskeletal architecture, this implys that differences in nuclear stiffness are due to different differentiation states (Pajerowski et al. 2007; Nathan et al. 2011). The nucleus is known to interact with actin and microtubules through binding proteins on the nuclear envelope known collectively as the LINC (linker of nucleus and cytoskeleton) complex (Crisp et al. 2006; Dahl et al. 2008). These proteins then interact with lamins inside the nucleus, which in turn provide support for several nuclear proteins involved in DNA replication, transcription and gene expression (Parnaik & Manju, 2006; Meaburn & Misteli, 2007; Dahl et al. 2008). How forces are transmitted from the matrix to the nucleus, and how such cues might regulate MSC differentiation, is an area requiring further research.
Future directions
The mechanical environment of MSCs is determined by the stiffness, composition and configuration of the local ECM and any extrinsic mechanical loading applied to this matrix. MSCs have the ability to both sense and respond to their mechanical environment, with numerous membrane proteins, cytoskeletal components and the nucleus itself all acting as putative mechanosensors in MSCs. The complex interactions between these diverse actors have been heavily researched, but are still not fully understood. Further research is needed to elucidate how MSCs sense and respond to the complex sets of mechanical stimuli they experience in vivo during developmental processes, following trauma or disease and in tissue regeneration.
This review has aimed to explain the response of MSCs exposed to various isolated stimuli; however, in vivo MSCs will be exposed to several cues simultaneously. While in vivo observations have formed the basis for the hypothesis that mechanical factors regulate the development and repair of musculoskeletal tissues (Glucksmann, 1942; Estes et al. 2004; Guilak et al. 2009; Kelly & Jacobs, 2010), the majority of studies exploring the mechanobiology of MSCs have been performed using in vitro systems. While such models have improved our understanding for how MSCs respond to specific stimuli, the in vivo environment is inherently more complex. Not only does an interplay between intrinsic and extrinsic mechanical cues exist, but there is also an interplay between the biophysical and biochemical environment to consider. While isolating single variables has aided in determining and understanding several mechanotransductive mechanisms, future research should focus on understanding how MSCs sense and integrate complex arrays of biophysical and biochemical signals and respond accordingly. Computational models are also gaining complexity and serve as useful tools to investigate the interplay of several different factors simultaneously and, furthermore, can potentially be used to help better elucidate how various environmental factors might regulate MSC fate in vivo (Burke & Kelly, 2012; Burke et al. 2013). Discovering how soluble signals and the diverse mechanical cues interact to determine MSC fate will ultimately help more translational efforts attempting to engineer complex tissues and organs for regenerative medicine applications.
Acknowledgements
D.J.K. receives funding from Science Foundation Ireland (08/Y15/B1336, 12/IA/1554) and the European Research Council (StemRepair‐258463). A.J.S. receives funding from a Naughton Graduate Fellowship.
References
- Aberle H, Schwartz H, Kemler R (1996) Cadherin‐catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 61, 514–523. [DOI] [PubMed] [Google Scholar]
- Altman GH, Lu HH, Horan RL, et al. (2002) Advanced bioreactor with controlled application of multi‐dimensional strain for tissue engineering. J Biomech Eng 124, 742–749. [DOI] [PubMed] [Google Scholar]
- Angele P, Yoo JU, Smith C, et al. (2003) Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro . J Orthop Res 21, 451–457. [DOI] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Jacobs CR (2009a) Non‐canonical wnt signaling and N‐cadherin related β‐catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4, e5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Kwon RY, et al. (2009b) Mechanically induced osteogenic differentiation – the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 122, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Shah RP, Huang AH, et al. (2011) Dynamic tensile loading improves the functional properties of mesenchymal stem cell‐laden nanofiber‐based fibrocartilage. Tissue Eng Part A 17, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft GN, Sikavitsas VI, van den Dolder J, et al. (2002) Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose‐dependent manner. PNAS 99, 12 600–12 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourns B, Franklin S, Cassimeris L, et al. (1988) High hydrostatic pressure effects in vivo: changes in cell morphology, microtubule assembly, and actin organization. Cell Motil Cytoskelet 10, 380–390. [DOI] [PubMed] [Google Scholar]
- Browning JA, Walker RE, Hall AC, et al. (1999) Modulation of Na+ × H+ exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta Physiol Scand 166, 39–45. [DOI] [PubMed] [Google Scholar]
- Browning JA, Saunders K, Urban JPG, et al. (2004) The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology 41, 299–308. [PubMed] [Google Scholar]
- Burke DP, Kelly DJ (2012) Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model. PLoS One 7, e40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Dishowitz M, Sweetwyne M, et al. (2013) The role of oxygen as a regulator of stem cell fate during fracture repair in TSP2‐null mice. J Orthop Res 31, 1585–1596. [DOI] [PubMed] [Google Scholar]
- Burridge K (1981) Are stress fibres contractile? Nature 294, 691–692. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska‐Wodnicka M (1996) Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12, 463–519. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, et al. (1988) Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol 4, 487–525. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH (1992) Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol 119, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne EM, Farrell E, McMahon LA, et al. (2008) Gene expression by marrow stromal cells in a porous collagen–glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J Mater Sci Mater Med 19, 3455–3463. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Lee DA, Bader DL (2006) Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology 43, 455–470. [PubMed] [Google Scholar]
- Campbell J, Bader D, Lee D (2008) Mechanical loading modulates intracellular calcium signaling in human mesenchymal stem cells. J Appl Biomater Biomech 6, 9–15. [PubMed] [Google Scholar]
- Carroll SF, Buckley CT, Kelly DJ (2014) Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad. J Biomech 47, 2115–2121. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, et al. (1994) Integrin‐mediated cell adhesion activates mitogen‐activated protein kinases. J Biol Chem 269, 26 602. [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP (1997) Extracellular matrix rigidity causes strengthening of integrin‐cytoskeleton linkages. Cell 88, 39–48. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Na S, Li D, et al. (2010) Material properties of the cell dictate stress‐induced spreading and differentiation in embryonic stem cells. Nat Mater 9, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska‐Wodnicka M, Burridge K (1996) Rho‐stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133, 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E, Brugge J (1995) Integrins and signal transduction pathways: the road taken. Science 268, 233–239. [DOI] [PubMed] [Google Scholar]
- Conti MA, Even‐Ram S, Liu C, et al. (2004) Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II‐A in mice. J Biol Chem 279, 41 263–41 266. [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, et al. (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJS, Lammerding J (2008) Nuclear shape, mechanics, and mechanotransduction. Circ Res 102, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N, Pham QP, Sharma U, et al. (2006) In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. PNAS 103, 2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLise AM, Fischer L, Tuan RS (2000) Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang Y (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143. [DOI] [PubMed] [Google Scholar]
- Elder S, Fulzele K, McCulley W (2005) Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomech Model Mechanobiol 3, 141–146. [DOI] [PubMed] [Google Scholar]
- Elder SH, Sanders SW, McCulley WR, et al. (2006) Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J Orthop Res 24, 740–747. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, et al. (2004) Substrate compliance versus ligand density in cell on gel responses. Biophys J 86, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, et al. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. [DOI] [PubMed] [Google Scholar]
- Estes BT, Gimble JM, Guilak F (2004) Mechanical signals as regulators of stem cell fate In: Current Topics in Developmental Biology, Stem Cells in Development and Disease. (ed. Schatten Gerald P.), pp. 91–126. Waltham, Massachusetts: Academic Press. [DOI] [PubMed] [Google Scholar]
- Finger AR, Sargent CY, Dulaney KO, et al. (2007) Differential effects on messenger ribonucleic acid expression by bone marrow‐derived human mesenchymal stem cells seeded in agarose constructs due to ramped and steady applications of cyclic hydrostatic pressure. Tissue Eng 13, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Formigli L, Meacci E, Sassoli C, et al. (2005) Sphingosine 1‐phosphate induces cytoskeletal reorganization in C2C12 myoblasts: physiological relevance for stress fibres in the modulation of ion current through stretch‐activated channels. J Cell Sci 118, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Formigli L, Meacci E, Sassoli C, et al. (2007) Cytoskeleton/stretch‐activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J Cell Physiol 211, 296–306. [DOI] [PubMed] [Google Scholar]
- Gao L, McBeath R, Chen CS (2010) Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N‐cadherin. Stem Cells 28, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, et al. (2001) Extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2, 793–805. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10, 21–33. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285, 1028. [DOI] [PubMed] [Google Scholar]
- Glucksmann A (1942) The role of mechanical stresses in bone formation in vitro . J Anat 76, 231–239. [PMC free article] [PubMed] [Google Scholar]
- Gregor M, Osmanagic‐Myers S, Burgstaller G, et al. (2014) Mechanosensing through focal adhesion‐anchored intermediate filaments. FASEB J 28, 715–729. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, et al. (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A (1994) Small GTP‐binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol 10, 31–54. [DOI] [PubMed] [Google Scholar]
- Hall AC (1999) Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol 178, 197–204. [DOI] [PubMed] [Google Scholar]
- Hanson AD, Marvel SW, Bernacki SH, et al. (2009) Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng 37, 955–965. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H (1999) Calcium as a versatile second messenger in the control of gene expression. Microsc Res Tech 46, 348–355. [DOI] [PubMed] [Google Scholar]
- Harris A, Wild P, Stopak D (1980) Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208, 177–179. [DOI] [PubMed] [Google Scholar]
- Haudenschild AK, Hsieh AH, Kapila S, et al. (2009) Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 37, 492–502. [DOI] [PubMed] [Google Scholar]
- Haugh MG, Meyer EG, Thorpe SD, et al. (2011a) Temporal and spatial changes in cartilage‐matrix‐specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A 17, 3085–3093, doi: 10.1089/ten.tea.2011.0198. [DOI] [PubMed] [Google Scholar]
- Haugh MG, Meyer EG, Thorpe SD, et al. (2011b) Temporal and spatial changes in cartilage‐matrix‐specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A 17, 3085–3093. [DOI] [PubMed] [Google Scholar]
- Hoey DA, Kelly DJ, Jacobs CR (2011) A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun 412, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Downs ME, Jacobs CR (2012a) The mechanics of the primary cilium: an intricate structure with complex function. J Biomech 45, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Tormey S, Ramcharan S, et al. (2012b) Primary cilia‐mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 30, 2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hagar KL, Frost LE, et al. (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone‐marrow derived mesenchymal stem cells. Stem Cells 22, 313–323. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jia X, Bai K, et al. (2010) Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch Med Res 41, 497–505. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, et al. (2010) Harnessing traction‐mediated manipulation of the cell/matrix interface to control stem‐cell fate. Nat Mater 9, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S‐G, Yu S‐S, Ryu J‐H, et al. (2005) Regulation of β‐catenin signaling and maintenance of chondrocyte differentiation by ubiquitin‐independent proteasomal degradation of α‐catenin. J Biol Chem 280, 12 758–12 765. [DOI] [PubMed] [Google Scholar]
- Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59, 575. [DOI] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, et al. (2000) Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J Biol Chem 275, 9645–9652. [DOI] [PubMed] [Google Scholar]
- Jang J‐Y, Lee SW, Park SH, et al. (2011) Combined effects of surface morphology and mechanical straining magnitudes on the differentiation of mesenchymal stem cells without using biochemical reagents. J Biomed Biotechnol 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortikka MO, Parkkinen JJ, Inkinen RI, et al. (2000) The role of microtubules in the regulation of proteoglycan synthesis in chondrocytes under hydrostatic pressure. Arch Biochem Biophys 374, 172–180. [DOI] [PubMed] [Google Scholar]
- Juliano R, Haskill S (1993) Signal transduction from the extracellular matrix. J Cell Biol 120, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungreuthmayer C, Donahue SW, Jaasma MJ, et al. (2008) A comparative study of shear stresses in collagen‐glycosaminoglycan and calcium phosphate scaffolds in bone tissue‐engineering bioreactors. Tissue Eng Part A 15, 1141–1149. [DOI] [PubMed] [Google Scholar]
- Jungreuthmayer C, Jaasma MJ, Al‐Munajjed AA, et al. (2009) Deformation simulation of cells seeded on a collagen‐GAG scaffold in a flow perfusion bioreactor using a sequential 3D CFD‐elastostatics model. Med Eng Phys 31, 420–427. [DOI] [PubMed] [Google Scholar]
- Kawano S, Otsu K, Kuruma A, et al. (2006) ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39, 313–324. [DOI] [PubMed] [Google Scholar]
- Kearney EM, Farrell E, Prendergast PJ, et al. (2010) Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng 38, 1767–1779. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Jacobs CR (2010) The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today 90, 75–85. [DOI] [PubMed] [Google Scholar]
- Khetan S, Guvendiren M, Legant WR, et al. (2013) Degradation‐mediated cellular traction directs stem cell fate in covalently crosslinked three‐dimensional hydrogels. Nat Mater 12, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiday JD, Frisbie DD, McIlwraith CW, et al. (2009) Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A 15, 2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippenberg M, Helder MN, Zandieh Doulabi B, et al. (2005) Adipose tissue‐derived mesenchymal stem cells acquire bone cell‐like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng 11, 1780–1788. [DOI] [PubMed] [Google Scholar]
- Kundu AK, Khatiwala CB, Putnam AJ (2009) Extracellular matrix remodeling, integrin expression, and downstream signaling pathways influence the osteogenic differentiation of mesenchymal stem cells on Poly(Lactide‐Co‐Glycolide) substrates. Tissue Eng Part A 15, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupcsik L, Stoddart MJ, Li Z, et al. (2010) Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A 16, 1845–1855. [DOI] [PubMed] [Google Scholar]
- Lee HS, Millward‐Sadler SJ, Wright MO, et al. (2000) Integrin and mechanosensitive ion channel‐dependent tyrosine phosphorylation of focal adhesion proteins and β‐catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res 15, 1501–1509. [DOI] [PubMed] [Google Scholar]
- Li YJ, Batra NN, You L, et al. (2004) Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res 22, 1283–1289. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao Z, Yang J, et al. (2009) p38 MAPK mediated in compressive stress‐induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds. J Cell Physiol 221, 609–617. [DOI] [PubMed] [Google Scholar]
- Li Z, Kupcsik L, Yao S, et al. (2010a) Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF‐β pathway. J Cell Mol Med 14, 1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yao S‐J, Alini M, et al. (2010b) Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin‐polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A 16, 575–584. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, et al. (2010) Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci USA 107, 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Buckley CT, Downey R, et al. (2012) The role of environmental factors in regulating the development of cartilaginous grafts engineered using osteoarthritic human infrapatellar fat pad‐derived stem cells. Tissue Eng Part A 18, 1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Buckley CT, Almeida HV, et al. (2014) Infrapatellar fat pad‐derived stem cells maintain their chondrogenic capacity in disease and can be used to engineer cartilaginous grafts of clinically relevant dimensions. Tissue Eng Part A. doi:10.1089/ten.tea.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZF, Doulabi BZ, Huang CL, et al. (2008) β1 integrins regulate chondrogenesis and rock signaling in adipose stem cells. Biochem Biophys Res Commun 372, 547–552. [DOI] [PubMed] [Google Scholar]
- Luo Z‐J, Seedhom BB (2007) Light and low‐frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: an in‐vitro study with special reference to cartilage repair. Proc Inst Mech Eng H 221, 499–507. [DOI] [PubMed] [Google Scholar]
- Malek AM, Izumo S (1996) Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci 109, 713–726. [DOI] [PubMed] [Google Scholar]
- Martinac B (2004) Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 117, 2449–2460. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Byers BA, Yuan X, et al. (2006) Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol 6, 113–125. [DOI] [PubMed] [Google Scholar]
- Maul TM, Chew DW, Nieponice A, et al. (2011) Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol 10, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, et al. (2004) Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev Cell 6, 483–495. [DOI] [PubMed] [Google Scholar]
- McMahon LA, Reid AJ, Campbell VA, et al. (2007) Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen‐gag scaffold: experimental and computational analysis. Ann Biomed Eng 36, 185–194. [DOI] [PubMed] [Google Scholar]
- McMahon LA, Campbell VA, Prendergast PJ (2008) Involvement of stretch‐activated ion channels in strain‐regulated glycosaminoglycan synthesis in mesenchymal stem cell‐seeded 3D scaffolds. J Biomech 41, 2055–2059. [DOI] [PubMed] [Google Scholar]
- Meaburn KJ, Misteli T (2007) Cell biology: chromosome territories. Nature 445, 379–381. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, et al. (2004) Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32, 112–122. [DOI] [PubMed] [Google Scholar]
- Meyer EG, Buckley CT, Steward AJ, et al. (2011) The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater 4, 1257–1265. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Trindade MCD, Lindsey DP, et al. (2006a) Dose‐ and time‐dependent effects of cyclic hydrostatic pressure on transforming growth factor‐beta3‐induced chondrogenesis by adult human mesenchymal stem cells in vitro . Tissue Eng 12, 2253–2262. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Trindade MCD, Lindsey DP, et al. (2006b) Effects of hydrostatic pressure and transforming growth factor‐beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro . Tissue Eng 12, 1419–1428. [DOI] [PubMed] [Google Scholar]
- Mizuno S (2005) A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am J Physiol Cell Physiol 288, C329–C337. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Connelly JT, Wilson CG, et al. (2007) Dynamic compression regulates the expression and synthesis of chondrocyte‐specific matrix molecules in bone marrow stromal cells. Stem Cells 25, 655–663. [DOI] [PubMed] [Google Scholar]
- Naruse K, Sokabe M (1993) Involvement of stretch‐activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol Cell Physiol 264, C1037–C1044. [DOI] [PubMed] [Google Scholar]
- Nathan AS, Baker BM, Nerurkar NL, et al. (2011) Mechano‐topographic modulation of stem cell nuclear shape on nanofibrous scaffolds. Acta Biomater 7, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R (2004) Convergence of Wnt, beta‐catenin, and cadherin pathways. Science 303, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R, Mizuno S, Murphy GF, et al. (2009) The effect of hydrostatic pressure on three‐dimensional chondroinduction of human adipose‐derived stem cells. Tissue Eng Part A 15, 2937–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares AL, Marsal È, Planell JA, et al. (2009) Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 30, 6142–6149. [DOI] [PubMed] [Google Scholar]
- Pajerowski JD, Dahl KN, Zhong FL, et al. (2007) Physical plasticity of the nucleus in stem cell differentiation. PNAS 104, 15 619–15 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala D, Kapoor M, Woods A, et al. (2008) Focal adhesion kinase/src suppresses early chondrogenesis. J Biol Chem 283, 9239–9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh SH, Chatterjee K, Lin‐Gibson S, et al. (2011) Modulus‐driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin‐based cytoskeletal tension. Biomaterials 32, 2256–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Chu JS, Tsou AD, et al. (2011) The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF‐[beta]. Biomaterials 32, 3921–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen JJ, Lammi MJ, Inkinen R, et al. (1995) Influence of short‐term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. J Orthop Res 13, 495–502. [DOI] [PubMed] [Google Scholar]
- Parnaik VK, Manju K (2006) Laminopathies: multiple disorders arising from defects in nuclear architecture. J Biosci 31, 405–421. [DOI] [PubMed] [Google Scholar]
- Pek YS, Wan ACA, Ying JY (2010) The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 31, 385–391. [DOI] [PubMed] [Google Scholar]
- Pelaez D, Charles Huang C‐Y, Cheung HS (2009) Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev 18, 93–102. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94, 13 661–13 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast PJ, Huiskes R, Søballe K (1997) Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech 30, 539–548. [DOI] [PubMed] [Google Scholar]
- Qi M‐C, Hu J, Zou S‐J, et al. (2008) Mechanical strain induces osteogenic differentiation: Cbfa1 and Ets‐1 expression in stretched rat mesenchymal stem cells. Int J Oral Maxillofac Surg 37, 453–458. [DOI] [PubMed] [Google Scholar]
- Ramage L, Nuki G, Salter DM (2009) Signalling cascades in mechanotransduction: cell–matrix interactions and mechanical loading. Scand J Med Sci Sports 19, 457–469. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Genetos DC, et al. (2006) MAP kinase and calcium signaling mediate fluid flow‐induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol 290, C776–C784. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Ni M, et al. (2011) Mechanical loading increased BMP‐2 expression which promoted osteogenic differentiation of tendon‐derived stem cells. J Orthop Res 29, 390–396. [DOI] [PubMed] [Google Scholar]
- Sandino C, Lacroix D (2011) A dynamical study of the mechanical stimuli and tissue differentiation within a CaP scaffold based on micro‐CT finite element models. Biomech Model Mechanobiol 10, 565–576. [DOI] [PubMed] [Google Scholar]
- Sen B, Guilluy C, Xie Z, et al. (2011) Mechanically induced focal adhesion assembly amplifies anti‐adipogenic pathways in mesenchymal stem cells. Stem Cells 29, 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy EJ, Buckley CT, Kelly DJ (2012) Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun 417, 305–310. [DOI] [PubMed] [Google Scholar]
- Shim JW, Wise DA, Elder SH (2008) Effect of cytoskeletal disruption on mechanotransduction of hydrostatic pressure by C3H10T1/2 murine fibroblasts. Open Orthop J 2, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF (2006) The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313, 629–633. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, et al. (2007) Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93, 4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward AJ, Thorpe SD, Vinardell T, et al. (2012) Cell–matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater 8, 2153–2159. [DOI] [PubMed] [Google Scholar]
- Steward AJ, Wagner DR, Kelly DJ (2013) The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. Eur Cell Mater 25, 167–178. [DOI] [PubMed] [Google Scholar]
- Steward AJ, Wagner DR, Kelly DJ (2014) Exploring the roles of integrin binding and cytoskeletal reorganization during mesenchymal stem cell mechanotransduction in soft and stiff hydrogels subjected to dynamic compression. J Mech Behav Biomed Mater 38, 174–182. doi:10.1016/j.jmbbm.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Subramony SD, Dargis BR, Castillo M, et al. (2013) The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34, 1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanasinghe RD, Bernacki SH, Loboa EG (2006) Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP‐2) mRNA expression. Tissue Eng 12, 3459–3465. [DOI] [PubMed] [Google Scholar]
- Sumanasinghe RD, Pfeiler TW, Monteiro‐Riviere NA, et al. (2009) Expression of proinflammatory cytokines by human mesenchymal stem cells in response to cyclic tensile strain. J Cell Physiol 219, 77–83. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Nuckolls GH, Takahashi K, et al. (1998) Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL‐1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci 111, 2067–2076. [DOI] [PubMed] [Google Scholar]
- Takeichi M (1990) Cadherins: a molecular family important in selective cell‐cell adhesion. Annu Rev Biochem 59, 237–252. [DOI] [PubMed] [Google Scholar]
- Thorpe SD, Buckley CT, Vinardell T, et al. (2008) Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem Biophys Res Commun 377, 458–462. [DOI] [PubMed] [Google Scholar]
- Thorpe SD, Buckley CT, Vinardell T, et al. (2010) The response of bone marrow‐derived mesenchymal stem cells to dynamic compression following TGF‐β3 induced chondrogenic differentiation. Ann Biomed Eng 38, 2896–2909. [DOI] [PubMed] [Google Scholar]
- Thorpe SD, Buckley CT, Steward AJ, et al. (2012) European Society of Biomechanics S.M. Perren Award 2012: the external mechanical environment can override the influence of local substrate in determining stem cell fate. J Biomech 45, 2483–2492. [DOI] [PubMed] [Google Scholar]
- Van der Flier A, Sonnenberg A (2001) Function and interactions of integrins. Cell Tissue Res 305, 285–298. [DOI] [PubMed] [Google Scholar]
- Vinardell T, Rolfe RA, Buckley CT, et al. (2012) Hydrostatic pressure acts to stabilise a chondrogenic phenotype in porcine joint tissue derived stem cells. Eur Cell Mater 23, 121–134. [DOI] [PubMed] [Google Scholar]
- Wagner DR, Lindsey DP, Li KW, et al. (2008) Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng 36, 813–820. [DOI] [PubMed] [Google Scholar]
- Ward DF Jr, Salasznyk RM, Klees RF, et al. (2007) Mechanical strain enhances extracellular matrix‐induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular‐related kinase‐dependent pathway. Stem Cells Dev 16, 467–480. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading‐induced bone fluid shear stresses. J Biomech 27, 339–360. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Bershadsky A, Henis YI, et al. (2011) Actomyosin‐generated tension controls the molecular kinetics of focal adhesions. J Cell Sci 124, 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Beier F (2006) RhoA/ROCK signaling regulates chondrogenesis in a context‐dependent manner. J Biol Chem 281, 13 134–13 140. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F (2005) RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem 280, 11 626–11 634. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F (2007) Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol 213, 1–8. [DOI] [PubMed] [Google Scholar]
- Wright MO, Stockwell RA, Nuki G (1992) Response of plasma membrane to applied hydrostatic pressure in chondrocytes and fibroblasts. Connect Tissue Res 28, 49–70. [DOI] [PubMed] [Google Scholar]
- Wright MO, Nishida K, Bavington C, et al. (1997) Hyperpolarisation of cultured human chondrocytes following cyclical pressure‐induced strain: evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J Orthop Res 15, 742–747. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Sokabe M (2010) Mechanosensitivity of ion channels based on protein–lipid interactions. J R Soc Interface 7, S307–S320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel‐Bar R, Itzkovitz S, Ma'ayan A, et al. (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiter S, Lezuo P, Ito K (2009) Effect of TGF β1, BMP‐2 and hydraulic pressure on chondrogenic differentiation of bovine bone marrow mesenchymal stromal cells. Biorheology 46, 45–55. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chella R, Ma T (2007) Effects of shear stress on 3‐D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol Bioeng 96, 584–595. [DOI] [PubMed] [Google Scholar]


