Abstract
Background:
Concomitant tricuspid valve repair (TVR) and double lung transplantation (DLTx) has been a surgical option at our institution since 2004 in an attempt to improve the outcome of DLTx for end-stage pulmonary hypertension, severe tricuspid regurgitation, and right ventricle (RV) dysfunction. This study is a review of that single institutional experience.
Methods:
Consecutive cases of concomitant TVR and DLTx performed between 2004 and 2009 (TVR group, n = 20) were retrospectively compared with cases of DLTx alone for severe pulmonary hypertension without TVR (non-TVR group, n = 58).
Results:
There was one in-hospital death in the TVR group. The 90-day and 1- and 3-year survival rates for the TVR group were 90%, 75%, and 65%, respectively, which were not significantly different from those for the non-TVR group. The TVR group required less inotropic support and less prolonged mechanical ventilation in the ICU. Follow-up echocardiography demonstrated immediate elimination of both volume and pressure overload in the RV and tricuspid regurgitation in the TVR group. Notably, there was a significantly lower incidence of primary graft dysfunction following transplantation in the TVR group (P < .05). Pulmonary functional improvement shown by an FEV1 increase after 6 months was also significantly better in the TVR group (40% vs 20%, P < .05).
Conclusions:
Combined TVR and DLTx procedures were successfully performed without an increase in morbidity or mortality and contributed to decreased primary graft dysfunction. In our experience, this combined operative approach achieves clinical outcomes equal or superior to the outcomes seen in DLTx patients without RV dysfunction and severe tricuspid regurgitation.
The “26th Official Adult Lung and Heart-Lung Transplantation Report” from the International Society for Heart and Lung Transplantation1 noted that patients with idiopathic pulmonary arterial hypertension (IPAH) have the lowest survival rate at 3 months among all patients receiving a lung transplantation (LTx) for end-stage lung disease most likely because of the high number of early complications, including primary graft dysfunction (PGD). We previously reported significant improvements in recent years in our institutional long-term survival outcomes of patients who underwent LTx and heart-lung transplantation for IPAH, which may be a result of our current refined protection and immunosuppressive techniques.2
In addition to these refinements, beginning in 2004, tricuspid valve repair (TVR) concomitant to double lung transplantation (DLTx) was used as a new surgical option for patients with IPAH and other secondary pulmonary arterial hypertension (PAH) with severe tricuspid regurgitation and right ventricle (RV) dysfunction. Functional tricuspid regurgitation is found in most patients with severe PAH, and annular dilatation and altered RV geometry are important factors in the pathogenesis of the development of tricuspid regurgitation resulting in RV dysfunction.3 Importantly, RV dysfunction (moderate to severe hypokinesis) is an independent risk factor for PGD after DLTx for severe PAH.4,5 Given this notable evidence, it occurred to us that in patients with severe tricuspid regurgitation and RV dysfunction, TVR concomitant to DLTx might accelerate cardiac adaptation and recovery from severe RV dysfunction, leading to prevention of PGD and other early major complications and improved overall survival outcomes. Our initial experience with combining TVR with DLTx for patients with severe pulmonary hypertension, tricuspid regurgitation, and RV dysfunction is detailed in this article.
Materials and Methods
Patients
Human subject approval for this study was obtained from the University of Pittsburgh Medical Center prior to obtaining data (IRB approval number 000511). From January 2004 to April 2009, we performed primary LTx in 558 patients with end-stage lung disease at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, exclusive of heart-lung transplantation cases. Of these, 82 recipients with severe pulmonary hypertension underwent DLTx. The diagnosis of severe pulmonary hypertension was based on right-sided heart catheterization findings showing transpulmonary gradient pressure > 30 mm Hg. The primary indication for LTx was IPAH in 18 patients, scleroderma (restricted) in 15 patients, idiopathic pulmonary fibrosis in 42 patients, pulmonary emphysema in three patients, connective tissue-related fibrosis in two patients, and silicosis in two patients.
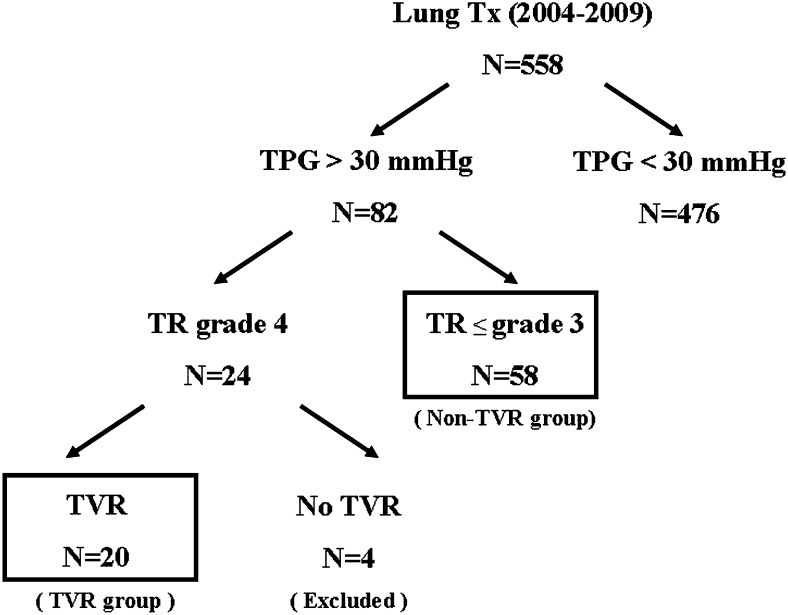
Of the 82 patients with severe pulmonary hypertension who underwent DLTx, 24 showed severe tricuspid regurgitation preoperatively by transthoracic echocardiogram, and 20 of the 24 patients underwent concomitant TVR surgery at the time of DLTx. Our indications for the concomitant procedure are shown in Table 1. When indicated, the possible procedures were discussed and approved by our multidisciplinary committee for LTx, and the final decision was based on intraoperative transesophageal echocardiography findings. Based on the criteria in Table 1, of the 24 patients with moderate or severe tricuspid regurgitation, the committee declined two for the combined procedure because these patients were aged > 70 years with marginal renal function, and two patients had only mild tricuspid regurgitation by intraoperative transesophageal echocardiogram. The 20 patients who underwent concomitant TVR and DLTx were compared with the patients who underwent DLTx without TVR (non-TVR group, n = 58) for severe pulmonary hypertension (Fig 1). The preoperative characteristics of all 82 patients, including those who were ultimately excluded from the TVR group, and all lung donors are shown in Table 2.
Table 1.
—Indications for Combining TVR With DLx
| Indications | |
| 1 | Severe pulmonary hypertension (TPG > 30 mm Hg) |
| 2 | Severe TR through TEE (TR grade 4) |
| 3 | Severe or moderate RV dysfunction |
| 4 | No contraindication or high risk for using cardiopulmonary bypass |
DLx = double lung transplantation; RV = right ventricle; TEE = transesophageal echocardiogram; TPG = transpulmonary pressure gradient; TR = tricuspid regurgitation; TVR = tricuspid valve repair.
Figure 1.
Study algorithm. TPG = transpulmonary pressure gradient; TR = tricuspid regurgitation; TVR = tricuspid valve repair; Tx = transplantation.
Table 2.
—Characteristics of Recipients and Donors, Comparing Patients Who Underwent Concomitant TVR and DLTx (TVR Group) With Those Who Underwent DLTx Alone (Non-TVR Group)
| Characteristic | TVR (n = 20) | Non-TVR (n = 58) | P Value | Excluded (n = 4) |
| Recipient | ||||
| Age, y | 50 ± 8.9 | 57 ± 12.5 | .07 | 62 ± 4.5 |
| Women, % | 40 | 57 | .06 | 25 |
| Original disease | .15 | |||
| IPAH | 7 | 11 | 0 | |
| Scleroderma | 4 | 11 | 0 | |
| IPF | 5 | 34 | 3 | |
| COPD | 2 | 0 | 1 | |
| Others | 2 | 2 | 0 | |
| History of smoking | 40 | 55 | .26 | 50 |
| 6MWT pretransplantation, ft | 543 ± 158 | 619 ± 161 | .10 | 582 ± 103 |
| FVC, % predicted | 61 ± 21 | 54 ± 29 | .50 | 59 ± 12 |
| FEV1, % predicted | 53 ± 19 | 58 ± 34 | .42 | 51 ± 23 |
| Diabetes mellitus, % | 35 | 31 | .29 | 25 |
| Hypertension, % | 30 | 21 | .19 | 50 |
| Chronic renal failure, % | 10 | 6 | .18 | 50 |
| Preoperative LVEF, % | 54.8 ± 8.3 | 58.2 ± 9.1 | .15 | 60.2 ± 10.9 |
| TPG, mm Hg | 49 ± 22 | 36 ± 16 | < .05 | 42 ± 29 |
| PCWP, mm Hg | 10 ± 5 | 11 ± 6 | .24 | 9 ± 5 |
| Cardiac index, L/min/m2 | 2.7 ± 0.6 | 2.6 ± 0.7 | .32 | 2.4 ± 0.3 |
| Donor | ||||
| Age, y | 44 ± 15 | 49 ± 13 | .07 | 53 ± 10 |
| Women, % | 45 | 52 | .06 | 50 |
| Pao2/Fio2, mm Hg | 452 ± 98 | 439 ± 89 | .15 | 385 ± 102 |
| CMV mismatch, %a | 4 | 5 | .24 | 0 |
Data are presented as counts or mean ± SD. 6MWT = 6-min walk test; CMV = cytomegalovirus; IPAH = idiopathic pulmonary arterial hypertension; IPF = idiopathic pulmonary fibrosis; LVEF = left ventricular ejection fraction (on transthoracic echocardiogram); PA = pulmonary artery; PCWP = pulmonary capillary wedge pressure. See Table 1 legend for expansion of other abbreviations.
Donor +; recipient −.
Assessment of Cardiac Function by Echocardiography
Transthoracic two-dimensional echocardiography as well as spectral Doppler and color Doppler echocardiography examinations were performed before and 1 month after transplantation. At the time of recipient surgery, transesophageal echocardiography also was performed routinely in all LTx cases. Left ventricle (LV) and RV dimensions and functions were assessed in standard parasternal long-axis and apical four-chamber views. The severity of tricuspid regurgitation was graded in a semiquantitative manner by using the width and length of the regurgitation jet in the right atrium (grade 0-4) with color flow Doppler echocardiography. Grades of tricuspid regurgitation were as follows: 0, 1 + (jet-to-area ratio, < 10% [trace]), 2 + (ratio, 10%-24% [mild]), 3 + (ratio, 25%-49% [moderate]), and 4 + (ratio, > 50% [severe]). Peak systolic pulmonary artery pressure was calculated from the tricuspid velocity profile, which was obtained with continuous-wave Doppler echocardiography in the four-chamber view. In addition, subjective assessments of RV function were routinely performed on all patients with an adequately visible RV. Visually characterizing the RV on the basis of wall motion, septal motion, and tricuspid annular motion, a subjective characterization of RV function as normal, mild, moderate, or severe was made. This was performed by reviewing the RV contraction in multiple views, including parasternal short axis for septal motion, apical four chamber, and subcostal. Aspects used in the determination of the visual assessment of RV function are summarized in recent guidelines.6
Surgical Procedure
TVR was performed on cardiopulmonary bypass before LTx. Cardiopulmonary bypass was continued during implantation. However, if it was possible to wean from the bypass after implantation of one side was completed, bypass weaning was attempted, and the other side was performed without a bypass. The approach and procedure used for TVR, as well as the severity of tricuspid regurgitation and RV dysfunction, based on intraoperative transesophageal echocardiogram findings are shown in Table 3. A standard DLTx procedure was used, and our current lung protection protocol, which has been reported in detail,2,7 was used for all cases in the present study.
Table 3.
—Combing TVR With DLx: Approach, Procedure, Intraoperative TR Severity, and RV Dysfunction
| Characteristic | TVR (n = 20) | Non-TVR (n = 58) |
| Approach | ||
| Bilateral anteroaxillary thoracotomy | 5 | 26 |
| Median sternotomy | 4 | 2 |
| Clamshell | 11 | 30 |
| Annuloplasty procedure | ||
| Ring annuloplasty | 12 | N/A |
| Without ring (Kay, De Vega) | 8 | |
| Intraoperative TR severity through TEE | ||
| Severe | 20 | 0 |
| Moderate | 0 | 13 |
| Mild or less | 0 | 45 |
| Intraoperative RV function through TEE | ||
| Severe | 13 | 0 |
| Moderate | 7 | 15 |
| Mild or less | 0 | 43 |
N/A = not applicable. See Table 1 legend for expansion of other abbreviations.
Early Outcomes, Mortality, and Long-term Survival
We collected data on postoperative complications, requirement for inotropic support, and duration of mechanical ventilation from our LTx database, which documents all adverse outcomes on the basis of prospective data collection from patient clinical records. Inotropic support was assessed using a score based on the doses of various inotropes administered as described by Rhodes and colleagues.8 Renal dysfunction was defined as severe renal insufficiency requiring either temporary or permanent dialysis treatment. PGD and acute rejection were defined and graded using International Society for Heart and Lung Transplantation definitions.9
It is our routine practice to perform pulmonary function tests at 1, 3, 6, and 12 months and annually thereafter until 5 years after LTx. Additional testing was performed when clinically necessary. We assessed graft quality by comparing pretransplant pulmonary function with pulmonary function 6 months after LTx. Long-term clinical outcomes were assessed by overall survival 3 years after transplantation.
Data Analysis
Statistical analysis was performed using Statview, version 5.0 (SAS Institute Inc; Cary, North Carolina) software. Continuous variables are expressed as the mean ± SD. Comparisons between groups were done using Student t test, whereas categorical variables were analyzed by Fisher exact test. Survival was calculated and assessed with the Kaplan-Meier method and a log-rank test. P < .05 was considered statistically significant.
Results
Operative Data
Intraoperative data are shown in Table 4. The operative time was longer in the TVR group than in the non-TVR group (P < .05). Ischemic time was not significantly different between the TVR and non-TVR groups (362 min vs 352 min). There were no significant differences in cardiopulmonary bypass time between the groups. In the non-TVR group, 49 patients (84%) required a bypass; among these cases, the mean bypass time was 195 min.
Table 4.
—Operative Data
| Characteristic | TVR (n = 20) | Non-TVR (n = 58) | P Value |
| Procedure | |||
| DLx, % | 100 | 100 | .89 |
| Ischemic time, min | 362 ± 118 | 325 ± 121 | .10 |
| Operative time, min | 529 ± 102 | 371 ± 139 | < .05 |
| Without CPB, % | 0 | 16 | < .05 |
| CPB time, min | 202 (62-321) | 195 (70-305) | 15 |
CPB = cardiopulmonary bypass. See Table 1 legend for expansion of other abbreviations.
Postoperative Outcomes
Postoperative outcomes are shown in Table 5. The TVR group required significantly less inotropic support (inotropic score, 1.1 vs 3.2; P < .05), and fewer patients required prolonged postoperative mechanical ventilation (20% vs 38%, P < .05) than in the non-TVR group. However, there were no significant differences in the rates of postoperative bleeding, neurologic complications, or renal insufficiency requiring dialysis and no differences in 30-day mortality (Table 5).
Table 5.
—Early Outcomes, 6-Mo Posttransplant Improvement in PFTs and 6MWT, and Follow-up Echocardiographic Findings
| Characteristic | TVR (n = 20) | Non-TVR (n = 58) | P Value |
| Early outcomes | |||
| Median inotropic score | 1.1 ± 0.9 | 3.2 ± 2.8 | < .05 |
| Mechanical ventilation duration (> 5 d), % | 20 | 38 | < .05 |
| Reoperation for bleeding, % | 5 | 6 | .35 |
| Stroke/neurological complication, % | 0 | 5 | .11 |
| Renal insufficiency on dialysis, % | 15 | 9 | .10 |
| 30-d mortality, % | 5 | 9 | .11 |
| Primary graft dysfunction | |||
| Grade 0-1 | 16 (80) | 35 (61) | < .05 |
| Grade 2-3 | 4 (20) | 23 (39) | |
| Acute rejection episodes (first year), No. | 1.5 | 1.2 | .21 |
| 6-mo posttransplant improvement in PFTs and 6MWT | |||
| Improvement in FVC, % | 31 ± 25 | 25 ± 29 | .35 |
| FEV1, % | 40 ± 32 | 20 ± 28 | < .05 |
| 6MWT, % | 55 ± 47 | 33 ± 39 | < .05 |
| Follow-up echocardiography at 1-mo postoperation | |||
| LVEF, % | 63.3 ± 5.5 | 58.8 ± 9.2 | .12 |
| RV dysfunction (> mild), % | 5 | 41 | < .05 |
| Residual TR (grade 2 or 3), % | 0 | 31 | < .05 |
| Normalization of ventricular septal position, % | 100 | 75 | .37 |
The incidence of PGD was significantly lower in the TVR group than in the non-TVR group (grade 2 or 3, 20% vs 39%; P < .05). Only one recipient in the TVR group was given a diagnosis of grade 3 PGD within 72 h after transplantation. This female patient had been receiving extracorporeal membrane oxygenation preoperatively because of rapid exacerbation with progressive pulmonary fibrosis but then recovered allograft function following DLTx, allowing extracorporeal membrane oxygenation to be weaned.
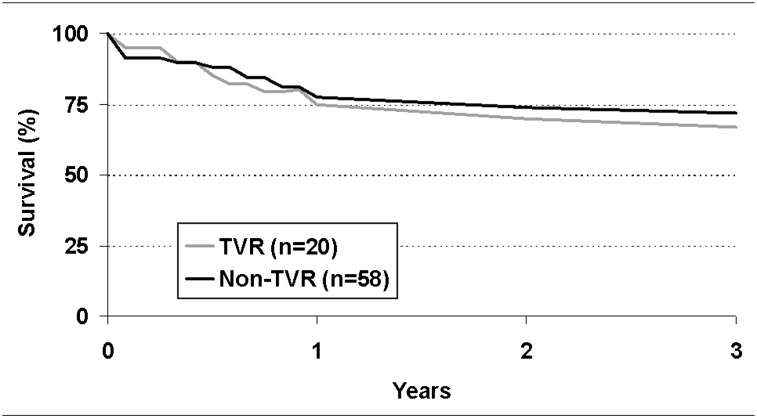
Pulmonary function tests 6 months after DLTx revealed greater FEV1 improvement in the TVR group (40.1% increase in FEV1) than in the non-TVR group (19.9% increase in FEV1, P < .05). The patients in the TVR group also exhibited greater improvement in the 6-min walk test (55% vs 33% in the non-TVR group, P < .05) (Table 5). Survival 3 years after transplantation, used as an assessment of long-term outcome, was not significantly different between the groups (TVR group, 67%; non-TVR group, 71%) (Fig 2).
Figure 2.
Comparison of patient survival after lung transplant between double lung transplantation concomitant with TVR (TVR group) and without TVR (non-TVR group). See Figure 1 legend for expansion of abbreviation.
Cardiac Functional Recovery Following DLTx With TVR
Follow-up transthoracic echocardiography findings were shown in Table 5. Prior to surgery, all patients in the TVR group showed high-grade RV dilatation and hypertrophy, with moderate to severe RV dysfunction. In contrast, 1 month after DLTx, RV size and function returned to normal in all patients in the TVR group except one (5%), in whom a mild to moderate degree of RV dysfunction was detectable. However, in the non-TVR group, 41% of the patients had persistent RV dysfunction after DLTx. LV ejection fraction increased from 54.8% to 63.3% in the TVR group after transplantation and was not significantly different from the non-TVR group. Color Doppler echocardiography demonstrated that severe tricuspid regurgitation, which was present before transplantation, was significantly decreased after transplantation in all patients in the TVR group. However, in the non-TVR group, 31% of the patients still showed residual tricuspid regurgitation after transplantation, whereas 91% (53 out of 58) had mild or moderate tricuspid regurgitation preoperatively (Table 3).
Discussion
Presently, there are only two surgical options available for patients with end-stage severe pulmonary hypertension disease: DLTx and heart-lung transplantation. Appropriate surgical options for patients with severe pulmonary hypertension have been a topic of longstanding debate. Throughout most of the 1990s, we performed single LTx for pulmonary hypertension.10,11 After reviewing outcomes in 1998, however, we changed to a DLTx or heart-lung transplantation procedure for affected patients.2 Nevertheless, we still encountered patients with severe preoperative pulmonary hypertension and RV dysfunction who also struggled with major complications, such as severe PGD and renal insufficiency, most likely because of persistent RV and LV dysfunction following isolated DLTx. The present data demonstrate that our approach of TVR performed concomitant to DLTx may successfully address the issues of RV dysfunction and its association with PGD in very-high-risk recipients with severe pulmonary hypertension, tricuspid regurgitation, and severe RV dysfunction.
The immediate effects of LTx on RV morphology and cardiac function have been discussed since the 1990s.12,13 According to those excellent studies, which were mainly based on echocardiography and MRI findings, LTx results in (1) immediate reduction in RV pressure afterload, (2) reduction in RV volume overload from diminished tricuspid regurgitation, and (3) normalization of ventricular septal position. Together, these three factors may influence biventricular geometry and filling, theoretically leading to biventricular functional recovery. However, other studies demonstrated that there are different varieties of RV functional recovery after LTx for severe pulmonary hypertension, although the underlying mechanisms remain unclear.13,14 In addition, late recovery of impaired LV function due to severe pulmonary hypertension following DLTx has been reported recently.15 Although the literature shows improved RV function after DLTx alone, studies have been unable to discern whether the actual rate of RV functional improvement has an impact on complications, their duration, and their impact on PGD and ultimate lung allograft function.
In a previous study from our institution, ∼30% of the patients had residual tricuspid regurgitation 1 month after DLTx for severe pulmonary hypertension,16 which was consistent with our current findings in the present non-TVR group (grade 2 or 3 tricuspid regurgitation in 31%). Kasimir et al17 reported that pulmonary artery pressure was normalized in all studied cases (n = 17) 3 months after transplantation, which resulted in a nearly complete reverse remodeling of the distorted cardiac geometry with some residual tricuspid insufficiency. On the other hand, rate of mortality in their study was as high as 17.5% at 3 months, and the mean ICU stay (35 days) clearly was prolonged. The patients in the present TVR group spent an average of 5 days in the ICU (data not shown), which is shorter than that of Kasimir et al,17 but the selection of patients for TVR in our series may or may not have included the patients Kasimir and colleagues reported. However, the differences in early outcomes noted between the TVR and non-TVR groups in the present study, or between the present TVR patients and the Kasimir et al17 series, might certainly be explained by the immediate elimination of the volume overload associated with tricuspid regurgitation in combination with the RV pressure and afterload relief provided by the DLTx, resulting in accelerated improvement in RV function, enhanced LV function, and a cascade of associated physiological benefits.
Published studies have suggested that additional cardiac procedures concomitant to LTx do not increase the morbidity or mortality related to transplantation.18,19 Although the TVR group required cardiopulmonary bypass more frequently than the non-TVR group and had higher pulmonary artery pressures, it is important to note that the TVR group had a lower incidence of grade 2 and 3 PGD. Nonetheless, the addition of TVR may have some disadvantages, such as longer operative time, addition of a prosthesis in the circulation, the cost of a ring, and the increased potential for surgical bleeding. Thus, because of these potential disadvantages as well as the present data showing that older patients had renal insufficiency more frequently with one early mortality, we recommend remaining selective when deciding whether to add TVR to LTx, specifically in older patients with comorbidities such as chronic renal insufficiency, and developing guidelines for defining selection criteria for this new aggressive option.
Combining TVR and DLTx attempts to improve and accelerate cardiac remodeling and functional recovery by addressing both RV volume overload (tricuspid regurgitation, immediately) and pressure overload (DLTx gradually). Concomitant correction of a cardiac anatomic abnormality that is presumably the result of longstanding pulmonary hypertension and subsequent RV adaptation has great physiologic appeal in the severely complicated disease of end-stage pulmonary hypertension affecting both lungs and heart.
Tricuspid regurgitation is an adaptive mechanism in a sense, so there might be a plausible point that some residual tricuspid regurgitation, as well as some residual pulmonary hypertension, might protect the transplanted lungs from being “flooded” in the early posttransplant period. Nevertheless, it is noted that despite the selection of a group of DLTx patients with more severe tricuspid regurgitation, RV dysfunction, and PAH, this combination strategy-treated cohort had less PGD. Further advances in imaging technologies, such as intraoperative three-dimensional echocardiography or PET,20,21 may help to clarify the involved mechanisms and to more clearly define the indications for this combined operative technique for severe pulmonary hypertension and RV dysfunction.
The primary limitations of this study are its retrospective nature and the sample size. In addition, although we objectively compared our results in patients with combined TVR and DLTx to patients not having TVR and DLTx for PAH, we acknowledge that the non-TVR group is not by any means a perfect control because these were selected patients without severe tricuspid regurgitation and RV dysfunction. We consider that criticism of the sample size can be tempered with the fact that this report is, to the best of our knowledge the first and largest reported series of combining TVR and DLTx.
In conclusion, our initial experience demonstrates that concomitant TVR and DLTx surgery for patients with severe pulmonary hypertension, tricuspid regurgitation, and severe RV dysfunction can be performed without increasing operative mortality and morbidity. Further, it contributes to minimizing the incidence of PGD with greater pulmonary functional improvement.
Acknowledgments
Author contributions: Dr Shigemura had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Shigemura: contributed to the data collection, data analysis, and the writing of the manuscript.
Dr Sareyyupoglu: contributed to the data collection and analysis and final approval of the manuscript.
Dr Bhama: contributed to the data collection and final approval of the manuscript.
Dr Bonde: contributed to the data collection and final approval of the manuscript.
Dr Thacker: contributed to the data collection and final approval of the manuscript.
Dr Bermudez: contributed to the data collection and final approval of the manuscript.
Dr Gries: contributed to the data collection and final approval of the manuscript.
Dr Crespo: contributed to the data collection and final approval of the manuscript.
Dr Johnson: contributed to the data collection and final approval of the manuscript.
Dr Pilewski: contributed to the data collection and final approval of the manuscript.
Dr Toyoda: contributed to the data analysis and the writing and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- DLTx
double lung transplantation
- IPAH
idiopathic pulmonary arterial hypertension
- LTx
lung transplantation
- LV
left ventricle
- PAH
pulmonary arterial hypertension
- PGD
primary graft dysfunction
- RV
right ventricle
- TVR
tricuspid valve repair
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Christie JD, Edwards LB, Aurora P, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult lung and heart-lung transplantation report—2009. J Heart Lung Transplant. 2009;28(10):1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Toyoda Y, Thacker J, Santos R, et al. Long-term outcome of lung and heart-lung transplantation for idiopathic pulmonary arterial hypertension. Ann Thorac Surg. 2008;86(4):1116–1122. doi: 10.1016/j.athoracsur.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 3.Hinderliter AL, Willis PW, IV, Long WA, et al. PPH Study Group Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol. 2003;91(8):1033–1037. doi: 10.1016/s0002-9149(03)00136-x. [DOI] [PubMed] [Google Scholar]
- 4.Chatila WM, Furukawa S, Gaughan JP, Criner GJ. Respiratory failure after lung transplantation. Chest. 2003;123(1):165–173. doi: 10.1378/chest.123.1.165. [DOI] [PubMed] [Google Scholar]
- 5.Saggar R, Lynch JP, Belperio JA, et al. Pulmonary arterial hypertension and lung transplantation. Semin Respir Crit Care Med. 2010;31(2):147–160. doi: 10.1055/s-0030-1249115. [DOI] [PubMed] [Google Scholar]
- 6.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Shigemura N, Bhama J, Nguyen D, Thacker J, Bermudez C, Toyoda Y. Pitfalls in donor lung procurements: how should the procedure be taught to transplant trainees? J Thorac Cardiovasc Surg. 2009;138(2):486–490. doi: 10.1016/j.jtcvs.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes JF, Blaufox AD, Seiden HS, et al. Cardiac arrest in infants after congenital heart surgery. Circulation. 1999;100(suppl 19):II194–II199. doi: 10.1161/01.cir.100.suppl_2.ii-194. [DOI] [PubMed] [Google Scholar]
- 9.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Bando K, Armitage JM, Paradis IL, et al. Indications for and results of single, bilateral, and heart-lung transplantation for pulmonary hypertension. J Thorac Cardiovasc Surg. 1994;108(6):1056–1065. [PubMed] [Google Scholar]
- 11.Gammie JS, Keenan RJ, Pham SM, et al. Single- versus double-lung transplantation for pulmonary hypertension. J Thorac Cardiovasc Surg. 1998;115(2):397–403. doi: 10.1016/S0022-5223(98)70284-3. [DOI] [PubMed] [Google Scholar]
- 12.Frist WH, Lorenz CH, Walker ES, et al. MRI complements standard assessment of right ventricular function after lung transplantation. Ann Thorac Surg. 1995;60(2):268–271. doi: 10.1016/0003-4975(95)00365-r. [DOI] [PubMed] [Google Scholar]
- 13.Schulman LL, Leibowitz DW, Anandarangam T, et al. Variability of right ventricular functional recovery after lung transplantation. Transplantation. 1996;62(5):622–625. doi: 10.1097/00007890-199609150-00014. [DOI] [PubMed] [Google Scholar]
- 14.Kamler M, Herold U, Piotrowski J, Bartel T, Teschler H, Jakob H. Severe left ventricular failure after double lung transplantation: pathophysiology and management. J Heart Lung Transplant. 2004;23(1):139–142. doi: 10.1016/s1053-2498(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 15.Toyooka S, Kusano KF, Goto K, et al. Right but not left ventricular function recovers early after living-donor lobar lung transplantation in patients with pulmonary arterial hypertension. J Thorac Cardiovasc Surg. 2009;138(1):222–226. doi: 10.1016/j.jtcvs.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Katz WE, Gasior TA, Quinlan JJ, et al. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J Am Coll Cardiol. 1996;27(2):384–391. doi: 10.1016/0735-1097(95)00502-1. [DOI] [PubMed] [Google Scholar]
- 17.Kasimir MT, Seebacher G, Jaksch P, et al. Reverse cardiac remodelling in patients with primary pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg. 2004;26(4):776–781. doi: 10.1016/j.ejcts.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 18.Parekh K, Meyers BF, Patterson GA, et al. Outcome of lung transplantation for patients requiring concomitant cardiac surgery. J Thorac Cardiovasc Surg. 2005;130(3):859–863. doi: 10.1016/j.jtcvs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SB, Allred AM, Cline AM, et al. Cardiac procedures in lung transplant recipients do not increase mortality in selected patients. Ann Thorac Surg. 2006;82(2):460–464. doi: 10.1016/j.athoracsur.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122(6):603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 21.Badano LP, Ginghina C, Easaw J, et al. Right ventricle in pulmonary arterial hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr. 2010;11(1):27–37. doi: 10.1093/ejechocard/jep152. [DOI] [PubMed] [Google Scholar]