Abstract
BACKGROUND:
Recent emphasis has been placed on methods to predict fluid responsiveness, but the usefulness of using fluid boluses to increase cardiac index in critically ill patients with ineffective circulation or oliguria remains unclear.
METHODS:
This retrospective analysis investigated hemodynamic responses of critically ill patients in the ARDS Network Fluid and Catheter Treatment Trial (FACTT) who were given protocol-based fluid boluses. Fluid responsiveness was defined as ≥ 15% increase in cardiac index after a 15 mL/kg fluid bolus.
RESULTS:
A convenience sample of 127 critically ill patients enrolled in FACTT was analyzed for physiologic responses to 569 protocolized crystalloid or albumin boluses given for shock, low urine output (UOP), or low pulmonary artery occlusion pressure (PAOP). There were significant increases in mean central venous pressure (9.9 ± 4.5 to 11.1 ± 4.8 mm Hg, P < .0001) and mean PAOP (11.6 ± 3.6 to 13.3 ± 4.3 mm Hg, P < .0001) following fluid boluses. However, there were no significant changes in UOP, and there were clinically small changes in heart rate, mean arterial pressure, and cardiac index. Only 23% of fluid boluses led to a ≥ 15% change in cardiac index. There was no significant difference in the frequency of fluid responsiveness between boluses given for shock or oliguria vs boluses given only for low PAOP (24.0% vs 21.8%, P = .59). There were no significant differences in 90-day survival, need for hemodialysis, or return to unassisted breathing between patients defined as fluid responders and fluid nonresponders.
CONCLUSIONS:
In this cohort of critically ill patients with ARDS who were previously resuscitated, the rate of fluid responsiveness was low, and fluid boluses only led to small hemodynamic changes.
Positive fluid balance is associated with worse outcomes in critically ill patients.1‐3 Consequently, some experts have recommended that fluid administration be restricted to patients with commonly accepted indications (eg, oliguria, hypotension) who are “fluid responsive.”4 Between 40% to 70% of patients who are ventilated are deemed fluid responsive5,6 when defined as a 10% to 15% increase in cardiac output following a crystalloid challenge ≥ 500 mL.7 Noninvasive techniques to quantify fluid responsiveness may be difficult to interpret in many critically ill patients8; therefore, further study into the clinical relevance of fluid responsiveness is needed. The relationship between fluid responsiveness and near-term physiologic improvements (eg, heart rate [HR], urine output [UOP], BP) as well as longer-term clinical outcomes (eg, survival) is not well described.9,10
The National Heart, Lung, and Blood Institute ARDSNet Fluid and Catheter Treatment Trial (FACTT) was a factorialized, randomized, protocolized study of fluid management in patients with acute lung injury/ARDS. One-half of patients received a pulmonary artery catheter (PAC) that provided cardiac output measurements.1 Hence, the FACTT dataset offers the opportunity to evaluate short-term physiologic effects of fluids and longer-term outcomes among fluid responsive and non-fluid-responsive patients. As most of these patients received fluids prior to study enrollment, we hypothesized that rates of fluid responsiveness would be < 25% and that additional fluid administration would not lead to durable improvements in hemodynamic parameters. Since the ability to increase cardiac output after a fluid bolus might indicate a more adaptable cardiovascular system, and cardiovascular adaptability has been linked to improved outcomes in other conditions,11‐13 we also hypothesized that clinical outcomes would be better in patients who were fluid responsive.
Materials and Methods
Patient Selection
Deidentified bedside flow sheets from 127 patients with ARDS enrolled in FACTT were collected from the coordinating center and participating sites; this was an unselected sample of available flow sheets. All patients had been previously resuscitated with IV fluids as clinically indicated prior to enrollment. Eligible patients for this study were those randomized to receive a PAC who had received one or more fluid boluses and had complete hemodynamic data available before and after the fluid bolus. Institutional review board approval (LSUHSC #8114) was obtained to conduct these analyses.
Data Collection
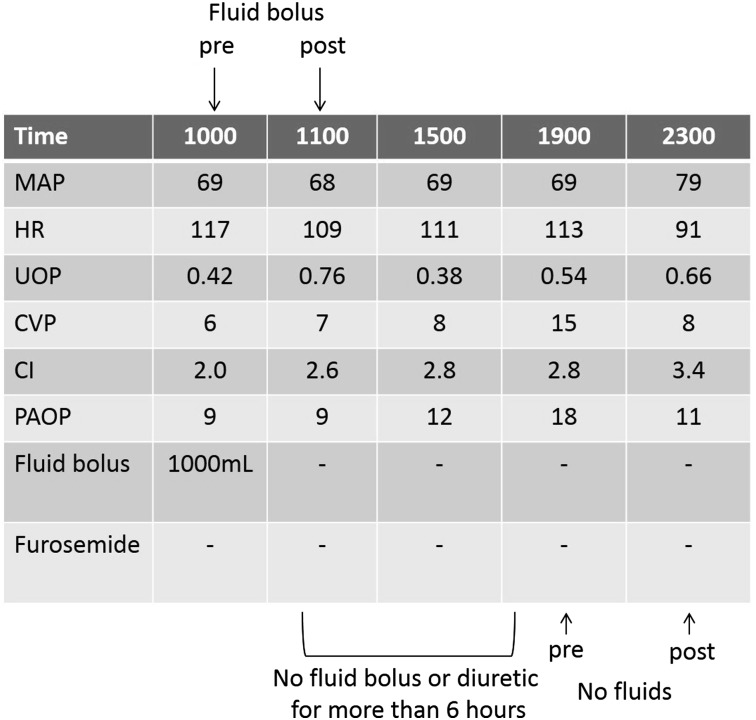
Hemodynamic variables were collected before (“pre”) and 1 to 4 h after (“post”) protocol-directed 15 mL/kg normal saline or 25-g albumin boluses; the fluid bolus could occur at any time during the patient’s enrollment. The FACTT protocol dictated reassessment at 1 h if the indication for fluids was shock, ineffective circulation, or low UOP and 4 h if the indication was only low filling pressure.1 Variables of interest were mean arterial pressure (MAP), HR, UOP, central venous pressure (CVP), cardiac index, and pulmonary artery occlusion pressure (PAOP). To control for unmeasured confounding factors that may have influenced hemodynamic changes, the same variables were also collected from the same patients during periods when fluids had not been administered (Fig 1).
Figure 1 –
Sample bedside flow sheet. To be included in this “no fluids” analysis, variables were collected at a time point at least 6 h after a fluid bolus or diuretic dose; these same variables were then collected 1 to 4 h later. At the top, “pre” (time 1000) represents hemodynamic variables prior to a fluid bolus, and “post” (1100) refers to hemodynamic variables 1 h after the fluid bolus was started. No fluid bolus or diuretic was given for > 6 h from 1100 to 1900; therefore, variables measured at 1900 are “pre” and at 2300 are “post” for the “no fluids” analysis, as described in the Materials and Methods section. CI = cardiac index; CVP = central venous pressure; HR = heart rate; MAP = mean arterial pressure; PAOP = pulmonary artery occlusion pressure; UOP = urine output.
Definitions
We defined fluid responsiveness as an increase in cardiac index of ≥ 15% following a single fluid bolus of 15 mL/kg crystalloid or 25 g albumin. Shock was defined as an MAP < 60 mm Hg or the need for vasopressors to maintain MAP ≥ 60 (except dopamine < 5 μg/kg/min), and oliguria was defined as a UOP < 0.5 mL/kg/h.
Data Analysis
Data are reported as mean ± SD or proportions where appropriate. Pearson correlations were calculated between prebolus hemodynamics and change in cardiac index. Changes in hemodynamic variables after a fluid bolus were compared using paired t tests. To control factors that may influence fluid responsiveness rates, the proportions of boluses leading to a ≥ 15% increase in cardiac index were compared using Fisher exact test for the following comparisons: (1) boluses that caused a CVP increase of ≥ 2 mm Hg vs boluses that did not cause a CVP increase of ≥ 2 mm Hg, as it has been postulated that if a fluid bolus does not increase the CVP there is not expected to be a cardiac index change14; (2) boluses that had repeat measurements 1 or 4 h later, as time from bolus to cardiac index measurement may affect fluid responsiveness rates; and (3) boluses given while a patient was on a vasoactive medication (dopamine, norepinephrine, dobutamine, epinephrine, or phenylephrine) vs boluses given while a patient was not on a vasoactive medication. Fisher exact test was also used to compare proportions of patients having a ≥ 15% increase in cardiac index either after a fluid bolus was given or after no bolus was administered.
Patients were defined as “fluid responders” if they had a ≥ 15% increase in cardiac index after the initial fluid bolus given during the study. Baseline characteristics (age, sex, APACHE [Acute Physiology and Chronic Health Evaluation] III score, Pao2/Fio2 ratio) of fluid responders and fluid nonresponders were compared using unpaired t tests. We analyzed the differences in clinical outcomes (survival to 90 days, need for hemodialysis, and return to unassisted breathing) between responders and nonresponders using Fisher exact test. Return to unassisted breathing was defined as extubated, T-tube breathing, tracheostomy mask breathing, or CPAP ≤ 5 mm Hg without pressure support or intermittent mandatory ventilation support.1
Results
A total of 127 patients (50.2 ± 16.6 years, 56% men) were included, with a mean APACHE III score of 101.4 ± 28.5 (Table 1). Seventy-six patients (60%) were randomized to the FACTT liberal fluid strategy, and 51 (40%) were randomized to the conservative fluid strategy. A total of 569 fluid boluses were administered per protocol (mean, 4.5 ± 3.9 boluses/patient); reasons for fluid administration are shown in Table 2. Twenty-four boluses of albumin were given; the other 545 fluid boluses were crystalloid. The average volume of crystalloid per bolus was 1,025 ± 243 mL. Compared with patients not included in this analysis, the included patients were not different in age, sex, or Pao2/Fio2 ratio but had a higher APACHE III score (Table 3).
TABLE 1 ] .
Baseline Patient Characteristics
| Characteristic | Total | Fluid Responders | Fluid Nonresponders |
| No. of patients | 127 | 34 | 93 |
| Age, y | 50.2 ± 16.6 | 50.5 ± 16.7 | 49.1 ± 15.4 |
| Sex, % male | 56 | 52 | 58 |
| APACHE score | 101.4 ± 28.5 | 102.1 ± 30.0 | 99.5 ± 25.0 |
| Pao2/Fio2 ratio | 130 ± 65 | 118 ± 63 | 134 ± 64 |
| Prerandomization 24 h fluid balance, mL | 4,558 ± 3,335 | 4,914 ± 4,339 | 4,402 ± 2,810 |
| Fluid strategy | |||
| Liberal | 76 (60) | 12 (35)a | 64 (69)a |
| Conservative | 51 (40) | 22 (65)a | 29 (31)a |
Data presented as mean ± SD or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation.
Denotes a significant difference between fluid responders and fluid nonresponders (P = .007).
TABLE 2 ] .
Fluid Bolus Characteristics
| Characteristic | Measure |
| No. of fluid boluses | 569 |
| Fluid boluses per patient, mean ± SD | 4.5 ± 3.9 |
| Time interval between measurements, mean ± SD, h | 2.9 ± 1.3 |
| Indications for bolus, % | |
| Shock | 33 |
| Low UOP/ineffective circulation | 25 |
| Low PAOP | 42 |
| Boluses leading to a ≥ 15% increase in cardiac index, % | 23.0 |
PAOP = pulmonary artery occlusion pressure; UOP = urine output.
TABLE 3 ] .
Comparison of Baseline Characteristics Between Patients Included in this Analysis vs the Remainder of the FACTT Cohort (ie, Those Not Included in This Analysis)
| Characteristic | Included Patients | Remainder of the FACTT Cohort | P Value |
| No. of patients | 127 | 873 | N/A |
| Age, y | 50.2 ± 16.6 | 49.8 ± 16.1 | .88 |
| Sex, % male | 56 | 53 | .67 |
| APACHE III | 101.4 ± 28.5 | 93.5 ± 31.2 | .01 |
| Pao2/Fio2 ratio | 130 ± 65 | 126 ± 59 | .60 |
Data presented as mean ± SD unless otherwise noted. FACTT = Fluid and Catheter Treatment Trial; N/A = not applicable. See Table 1 legend for expansion of other abbreviation.
Predictors of Changes in Cardiac Output
There was no significant association between prebolus MAP, UOP, or CVP and change in cardiac index after a bolus (Table 4). In contrast, there were significant, but extremely weak, negative correlations between prebolus cardiac index and PAOP and change in cardiac index (Table 4). There was also a weak positive correlation between prebolus HR and increase in cardiac index.
TABLE 4 ] .
Correlations Between Prebolus Hemodynamic Variables and Change in Cardiac Index After a Fluid Bolus
| Prebolus Variable | r2 | P Value |
| Mean arterial pressure | 0.0004 | .62 |
| HR | 0.008 | .04 |
| UOP | 0.002 | .26 |
| CVP | 0.004 | .15 |
| Cardiac index | 0.081 | < .0001 |
| PAOP | 0.009 | .03 |
CVP = central venous pressure; HR = heart rate. See Table 2 legend for expansion of other abbreviations.
Changes in Hemodynamics Following Fluid Boluses
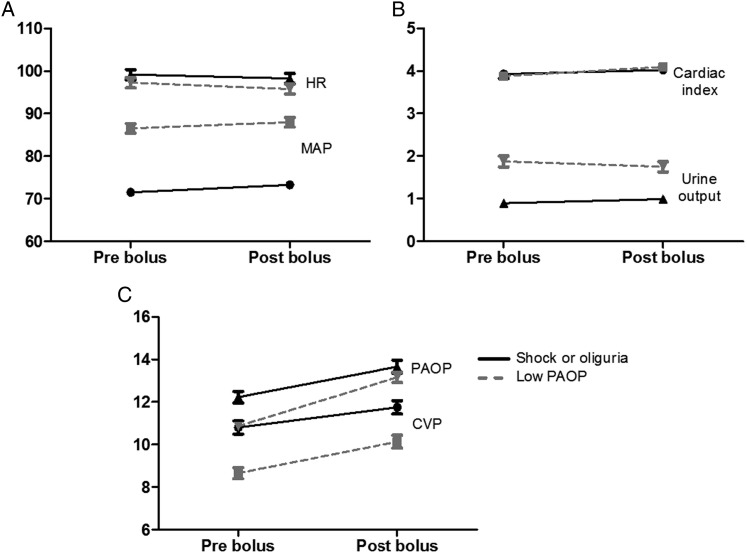
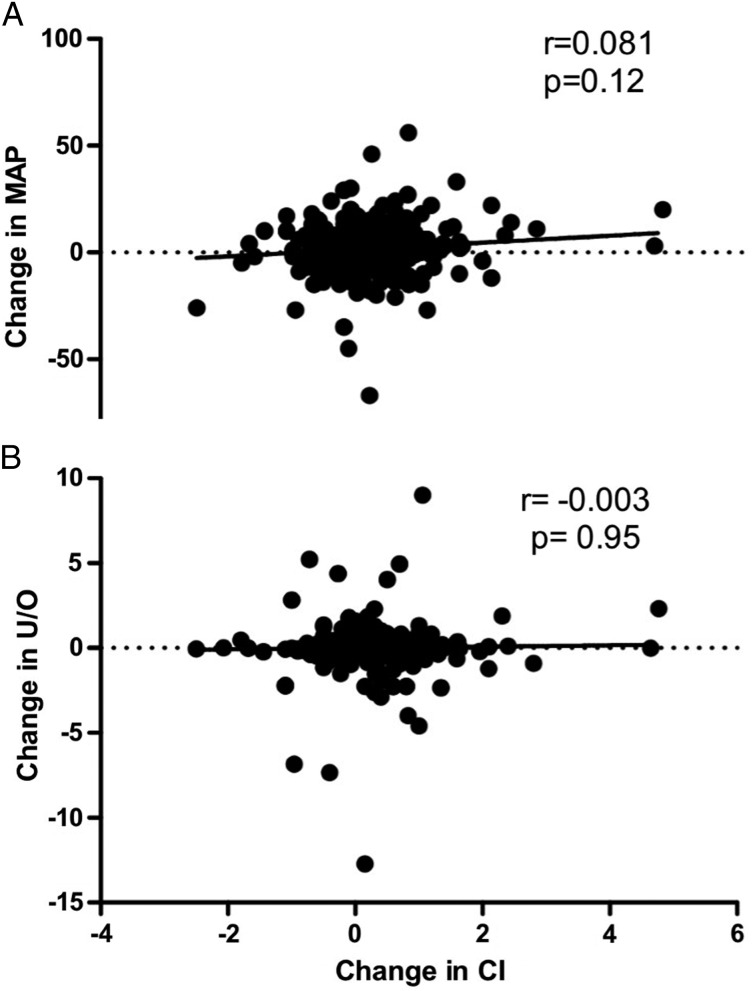
There were significant increases in mean CVP (9.9 ± 4.5 to 11.1 ± 4.8 mm Hg, P < .0001) and mean PAOP (11.6 ± 3.6 to 13.3 ± 4.3 mm Hg, P < .0001) following fluid boluses. However, UOP did not significantly change in the 1 to 4 h after the fluid bolus (1.28 ± 1.46 to 1.28 ± 1.36 mL/kg/h, prebolus vs postbolus, respectively; P = .06) (Fig 2). There were statistically significant but small changes in MAP (78.3 ± 16.4 to 80.4 ± 16.5 mm Hg, P < .0001), HR (96.3 ± 18.9 to 95.2 ± 18.4 beats/min, P < .0001), and cardiac index (3.82 ± 1.28 to 3.99 ± 1.30 L/min/m2, P < .0001) after a fluid bolus. Changes in cardiac index were not correlated with either changes in MAP (r = 0.081) or changes in UOP (r = −0.003) after a fluid bolus (Fig 3). When hemodynamic changes occurring after a fluid bolus were compared with time-matched hemodynamic changes when no fluid bolus was given (232 measurements), there were only small differences in change in MAP, HR, UOP, or cardiac index between fluids and no fluids (Table 5).
Figure 2 –
Change in hemodynamic variables before a fluid bolus (“pre”) and after a fluid bolus (“post”). Changes are stratified by indication, with solid lines and black symbols representing patients with shock or low UOP and dotted lines and gray symbols representing patients with acceptable MAP and UOP but low PAOP. A, MAP (mm Hg) and HR (beats/min). B, Cardiac index (L/min/m2) and UOP (mL/kg/h). C, CVP (mm Hg) and PAOP (mm Hg). See Figure 1 legend for expansion of abbreviations.
Figure 3 –
A, B, Correlations between change in cardiac index and change in MAP (A) and change in U/O (B). U/O = urine output. See Figure 1 legend for expansion of other abbreviation.
TABLE 5 ] .
Comparison of Changes (△) in Hemodynamics 4 h After a Fluid Bolus Was Either Given or Not Given per Protocol
| Hemodynamic | Fluid Bolus Given | No Fluid Bolus Given | P Value |
| △ MAP, mm Hg | 1.9 ± 11.2 | 0.7 ± 12.5 | .17 |
| △ HR, bpm | −1.1 ± 8.9 | 1.4 ± 9.1 | .0004 |
| △ UOP, mL/kg/h | 0.03 ± 1.4 | −0.04 ± 0.6 | .53 |
| △ CVP, mm Hg | 1.1 ± 3.2 | −0.1 ± 3.7 | < .0001 |
| △ Cardiac index, L/min/m2 | 0.16 ± 0.79 | −0.04 ± 0.79 | .001 |
| △ PAOP, mm Hg | 1.7 ± 3.6 | −0.4 ± 3.8 | < .0001 |
Fluid Response Rates
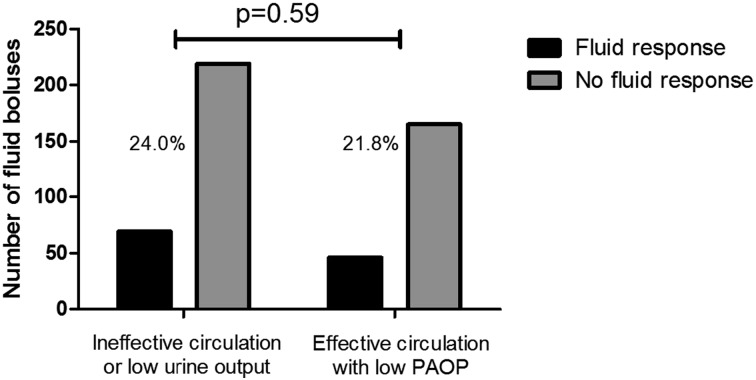
Only 23% of fluid boluses led to a ≥ 15% change in cardiac index. There was no difference in the frequency of fluid responsiveness between boluses given for shock or oliguria vs boluses given only for low PAOP (24.0% vs 21.8%, respectively; P = .59 (Fig 4). The frequency of fluid responsiveness was similar when we analyzed separately those boluses that raised CVP by ≥ 2 mm Hg (n = 211, 25.1%) compared with boluses that did not raise CVP to this degree (n = 358, 22.4%; P = .48). However, fluid boluses given when patients were on vasoactive medications (n = 157) were less likely to demonstrate fluid responsiveness than those given when patients were not on these medications (n = 412; 14.9% vs 26.5%, respectively; P = .003).
Figure 4 –
Frequency of fluid responsiveness stratified by indication for fluid bolus. “Fluid response” is a ≥ 15% increase in cardiac index after a fluid bolus, and “No fluid response” is a < 15% increase in cardiac index after a fluid bolus. See Figure 1 legend for expansion of abbreviation.
Furthermore, there was no difference in the frequency of fluid responsiveness when hemodynamic measurements were made either 1 h postbolus (n = 81) or 4 h postbolus (n = 274; 24.7% vs 20.8%, respectively; P = .45). When comparing hemodynamic changes after a fluid bolus to time-matched changes after no fluid was given, there was no significant difference in the proportion of patients experiencing a ≥ 15% change in cardiac index (23.0% after a fluid bolus vs 17.7% after no fluid bolus, P = .07).
Fluid Responders vs Fluid Nonresponders
In classifying patients based on the response to the first fluid bolus during the study, 34 patients (26.8%) were classified as fluid responders. There was no significant difference between fluid responders and fluid nonresponders in baseline APACHE III score, prerandomization fluid balance, Pao2/Fio2 ratio, age, or sex (Table 1). Patients deemed to be fluid responders based on their first fluid bolus were more likely to have been randomized to the conservative fluid strategy. There were no statistically significant differences in outcomes (90-day survival, need for hemodialysis, or return to unassisted breathing) between fluid responders and fluid nonresponders (Table 6).
TABLE 6 ] .
Clinical Outcomes in Fluid Responders (n = 34) Compared With Fluid Nonresponders (n = 93)
| Outcome Variable | Fluid Responders | Fluid Nonresponders | OR (95% CI) | P Value |
| Alive at 90 d | 19 | 51 | 1.34 (0.60-3.03) | .48 |
| Dead at 90 d | 15 | 30 | … | … |
| No need for hemodialysis | 31 | 76 | 0.57 (0.15-2.10) | .40 |
| Need for hemodialysis | 3 | 13 | … | … |
| Return to unassisted breathing | 26 | 68 | 0.91 (0.36-2.29) | .84 |
| No return to unassisted breathing | 8 | 23 | … | … |
Missing values account for differences in total number of patients.
Discussion
We have demonstrated that fluid boluses were unlikely to cause an increase in cardiac index of ≥ 15% in patients with ARDS enrolled in FACTT, even when given for shock or oliguria. On average there was no change in UOP and only small changes in HR, MAP, and cardiac index after a fluid bolus. There were also no clinically relevant correlations between baseline hemodynamic variables and change in cardiac index after a fluid bolus. These data add to a growing body of evidence suggesting that the routine administration of fluids to patients with shock or oliguria who were previously resuscitated may not improve organ function or clinical outcomes, even if such patients are classified as “fluid responders.”9,10,15 There were no differences in clinical outcomes based on responsiveness to the first fluid bolus, but this study was likely underpowered to detect a significant difference. Patients who were characterized as fluid responders were more frequently randomized to the conservative fluid strategy, although this is likely a chance finding, since fluid strategy should not influence the patients’ response to the first bolus.
Over the last decade, increasing emphasis has been placed on the administration of fluids to only those patients who are fluid responsive and have shock or low UOP. Oliguria and hypotension are among the most common reasons to consider fluid administration in the ICU; however, we have demonstrated that fluid responsiveness as commonly defined is not associated with clinically important improvements in MAP or UOP in a selected cohort of critically ill patients with ARDS. Our observations are consistent with those of a similar study demonstrating modest effects of fluid boluses on MAP and no effect on UOP.16 We extend these observations by demonstrating that among patients who have been previously resuscitated, fluid responsiveness is not a predictor of change in MAP or UOP following a fluid bolus (Fig 3). We also found that an increase in cardiac index by > 15% was less common when the patient was on a vasoactive medication, which is consistent with a prior study in which patients who were fluid responsive had decreased preload dependency while on norepinephrine.17
There are at least two possible explanations for these observations. First, it is likely that the duration of effect of fluid boluses is very short. Experimental data from a rodent model of sepsis demonstrate that plasma volume is unchanged just 20 min after a saline fluid bolus of 32 mL/kg.18 A recent observational study demonstrated that, even if a patient is fluid responsive, the cardiac index returns to baseline by 90 min after the fluid bolus.19 Per protocol, we obtained hemodynamic measurements at discrete snapshots in time between 1 and 4 h after fluid boluses were given and, therefore, may have missed early transient physiologic improvements. If so, any postbolus changes in cardiac index that we observed may simply represent random noise and would, therefore, not be expected to correlate with changes UOP and MAP. We do not consider this to be a shortcoming of this study, because if fluid boluses do not result in sustained improvements in cardiac index for even an hour,19 it is unlikely that they would be of clinical benefit.
Second, the absence of an effect on UOP is not surprising, since increasing global cardiac index with fluid boluses does not improve Doppler-derived renal resistive index, an indirect measure of renal blood flow.15 This observation suggests that in critical illness, renal blood flow and glomerular filtration rate are controlled by the kidney and not by the heart. In fact, normal saline, which was the predominant IV fluid used in FACTT, has been shown to impair renal function. In 12 healthy volunteers, 2 L of normal saline decreased renal artery blood flow velocity and reduced renal cortical tissue perfusion; this effect was not seen with an infusion of balanced crystalloids.20 Moreover, chloride-liberal IV fluid resuscitation has been associated with increased rates of acute kidney injury and need for renal replacement therapy.21
One of many strengths of our study was the use of data obtained during FACTT, a carefully controlled clinical trial. Thus, we studied a relatively homogenous population of critically ill patients who received protocol-based fluid boluses. Specifically, patients who had known heart failure or a clinical suspicion of elevated left atrial pressure were excluded. Additionally, the fluid bolus volume was standardized and weight based, and hemodynamic measurements were obtained rigorously per protocol. It is important to note that our main analysis focused on the physiologic response to a single bolus, rather than outcomes to repeated boluses; therefore, we did not focus on adjusting for the clustering of fluid boluses per patient. Finally, clinical outcomes were defined by protocol and, therefore, were reproducible across the participating centers.
We recognize that there may be limitations to our study. The retrospective design of our study is simply hypothesis generating and does not allow us to conclusively exclude a physiologic benefit to fluid boluses when studied using a more rigorous prospective design. Second, we used a convenience sample of patients who had complete physiologic data. Included patients must have been randomized to receive a PAC and must have received at least one bolus of IV fluids, either for low PAOP or shock/oliguria. Although only 12.7% of the patients randomized in FACTT were included, we do not believe that these inclusion criteria created any systematic bias in our interpretation of the results. Compared with patients not included in this analysis, the included patients were not different in age, sex, or Pao2/Fio2 ratio but did have a higher APACHE III score (Table 3). Third, we did have baseline cardiac index measurements immediately before each bolus, but we did not have repeat cardiac index measurements immediately after each bolus. Instead, repeat cardiac output measurements were obtained 1 h after boluses given for shock, ineffective circulation, or low UOP and 4 h after boluses given for decreased filling pressure. Therefore, we could have underestimated the number of patients who would have been defined as fluid responsive using previous criteria.5 We do not believe that our definition of fluid responsiveness is less valid than prior definitions, because transient (eg, 15 min) increases in cardiac output would be unlikely to have clinical benefit.19 Because of this time lag between fluid bolus and hemodynamic measurements, it is possible that unmeasured confounders (eg, pain, anxiety, and so forth) may have altered parameters independent of the influence of fluids. However, we found no difference in cardiac index change with or without fluid administration. Fourth, this study was underpowered to find a difference in longer-term clinical outcomes such as death. Finally, all of the patients had ARDS and met the inclusion criteria for FACTT, with the majority having sepsis as a cause of ARDS; therefore, generalizing our findings to other critically ill patient populations might be limited.
Conclusions
These data provide evidence that fluid responsiveness is uncommon among critically ill patients with ARDS who were previously resuscitated. Furthermore, fluid boluses in patients who were fluid responsive did not improve either BP or UOP. When considered with other data demonstrating benefits from fluid restriction in patients who were previously resuscitated, there appears to be justification for performing a randomized trial of fluid restriction in normovolemic patients with hypotension or oliguria who were fluid responsive.
Acknowledgments
Author contributions: M. R. L. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to published article, including and especially any adverse effects. M. R. L. contributed to the study concept and design, data acquisition, data analysis and interpretation, and drafting and critical revision of the manuscript; B. A., G. T. B., and T. R. contributed to the data analysis and interpretation and drafting and critical revision of the manuscript; I. S. D., A. P. W., and B. P. d. contributed to the study concept and design, data interpretation, and drafting and critical revision of the manuscript; and all authors provided final approval for manuscript submission.
Conflict of interest: B. P. d. has received pharmaceutical grant monies from Actelion Pharmaceuticals Ltd, Gilead, and Bayer AG. None declared (M. R. L., B. A., G. T. B., T. R., I. S. D., A. P. W.).
Role of sponsors: The sponsors had no role in the design, analyses, or writing of the manuscript.
ABBREVIATIONS
- APACHE
Acute Physiology and Chronic Health Evaluation
- CVP
central venous pressure
- FACTT
Fluid and Catheter Treatment Trial
- HR
heart rate
- MAP
mean arterial pressure
- PAC
pulmonary artery catheter
- PAOP
pulmonary artery occlusion pressure
- UOP
urine output
Footnotes
Part of this article has been presented in abstract form (Lammi MR, Corcoran-Aiello B, Rehman T, et al; the NHLBI ARDS Network Investigators. Am J Resp Crit Care Med. 2013:A3948).
FUNDING/SUPPORT: This study was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health [Award U54 GM104940] and the National Heart, Lung, and Blood Institute [Grants NO1-HR-16150, NO1-HR-16054-64, and NO1-HR-16146-54].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Wiedemann HP, Wheeler AP, Bernard GR, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575. [DOI] [PubMed] [Google Scholar]
- 2.Sakr Y, Vincent J-L, Reinhart K, et al. ; Sepsis Occurence in Acutely Ill Patients Investigators. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128(5):3098-3108. [DOI] [PubMed] [Google Scholar]
- 3.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259-265. [DOI] [PubMed] [Google Scholar]
- 4.Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008;133(1):252-263. [DOI] [PubMed] [Google Scholar]
- 5.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000-2008. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647. [DOI] [PubMed] [Google Scholar]
- 7.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103(2):419-428. [DOI] [PubMed] [Google Scholar]
- 8.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134-138. [DOI] [PubMed] [Google Scholar]
- 9.Natalini G, Rosano A, Militano CR, et al. Prediction of arterial pressure increase after fluid challenge. BMC Anesthesiol. 2012;12(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glassford NJ, Eastwood GM, Bellomo R. Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Crit Care. 2014;18(6):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8(4):311-315. [DOI] [PubMed] [Google Scholar]
- 12.Ramos RP, Arakaki JS, Barbosa P, et al. Heart rate recovery in pulmonary arterial hypertension: relationship with exercise capacity and prognosis. Am Heart J. 2012;163(4):580-588. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama Y, Morita H, Harada H, et al. Systolic blood pressure response to exercise as a predictor of mortality in patients with chronic heart failure. Int Heart J. 2010;51(2):111-115. [DOI] [PubMed] [Google Scholar]
- 14.Magder S. Fluid status and fluid responsiveness. Curr Opin Crit Care. 2010;16(4):289-296. [DOI] [PubMed] [Google Scholar]
- 15.Schnell D, Camous L, Guyomarc’h S, et al. Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med. 2013;41(5):1214-1220. [DOI] [PubMed] [Google Scholar]
- 16.Bihari S, Prakash S, Bersten AD. Post resuscitation fluid boluses in severe sepsis or septic shock: prevalence and efficacy (price study). Shock. 2013;40(1):28-34. [DOI] [PubMed] [Google Scholar]
- 17.Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39(4):689-694. [DOI] [PubMed] [Google Scholar]
- 18.Bark BP, Öberg CM, Grände PO. Plasma volume expansion by 0.9% NaCl during sepsis/systemic inflammatory response syndrome, after hemorrhage, and during a normal state. Shock. 2013;40(1):59-64. [DOI] [PubMed] [Google Scholar]
- 19.Nunes TSO, Ladeira RT, Bafi AT, de Azevedo LCP, Machado FR, Freitas FGR. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care. 2014;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18-24. [DOI] [PubMed] [Google Scholar]
- 21.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566-1572. [DOI] [PubMed] [Google Scholar]