Abstract
Background:
CO2 excretion is impaired in pulmonary arterial hypertension (PAH) due to underlying vascular obstruction and increased dead space. Our aim was to determine whether resting end tidal CO2 (Etco2) could differentiate patients with PAH from those with pulmonary venous hypertension (PVH) or patients without pulmonary hypertension (PH) and whether successful treatment of PAH resulted in higher Etco2 values.
Methods:
We performed Etco2 measurements for five breaths at rest and after a 6-min walk test (6MWT) in patients seen at our pulmonary vascular center. Mean Etco2 values were correlated with 6-min walk distance and right-sided heart catheterization data.
Results:
We enrolled 84 patients with PAH, 17 with PVH without left ventricular systolic dysfunction, and seven with no PH and no severe alterations in pulmonary function testing. Etco2 was significantly lower in patients with PAH than in those with no PH and PVH (P < .0001 PAH vs both groups). Etco2 correlated with the pulmonary artery diastolic pressure-to-pulmonary artery occlusion pressure gradient (r = −0.50, P = .0002) and pulmonary vascular resistance (r = −0.44, P = .002). Etco2 after 6MWT correlated with walk distance (r = 0.34, P = .003). In patients with prostaglandin therapy escalation, Etco2 increased in those who had clinical improvement, whereas in patients who did not improve clinically, Etco2 failed to rise (P = .04).
Conclusions:
Etco2 is a promising tool to differentiate patients with PAH from those with PVH or no PH, correlates with diagnostic and prognostic hemodynamic indicators, and may increase with successful treatment of PAH.
Distinguishing pulmonary arterial hypertension (PAH) from other forms of pulmonary hypertension (PH), such as pulmonary venous hypertension (PVH), can be difficult at the bedside, even with use of echocardiography or other noninvasive techniques. Although recent reports have suggested a potential role for analysis of “notch” pattern in right ventricular outflow tract Doppler echocardiographic flow velocity, right-sided heart catheterization (RHC) with provocative procedures usually is required for accurate distinction of PAH from PVH associated with nonsystolic heart failure.1‐4 This distinction is crucial because therapies for these two conditions and prognoses are different. Moreover, determining response to therapy in PAH is challenging, with many well-described limitations of standard noninvasive 6-min walk test (6MWT)3,5 and logistic challenges and expense with frequent RHC. Efficient, noninvasive testing to distinguish PAH from PVH and to determine response to therapy in PAH is needed.
The central pathologic finding in PAH is the presence of pulmonary arteriolar obstruction; although arterial changes may be present in PVH, obstruction is not described.6 Because pulmonary arterioles are variably obstructed in PAH, reduction or absence of perfusion in subtended alveoli results in increased dead space ventilation in which the partial pressure of CO2 is the same as atmospheric air at nearly 0 mm Hg. This is in contrast to the normally perfused Paco2 of 40 mm Hg. During exhalation, air from both dead space and normal alveoli mix in the large airways. End tidal CO2 (Etco2), representing mixed alveolar CO2 tension, falls in proportion to dead space ventilation and, thus, may be useful in the evaluation of PH.
Measured as part of exercise testing protocols using state-of-the-art exercise testing equipment, reduction in Etco2 has been shown at rest and during exercise in patients with PAH compared with normal control subjects by Yasunobu et al.7 Ventilatory efficiency differences in PAH compared with advanced left ventricular systolic failure have been described.8‐11 These investigations used formal exercise testing equipment not readily available at the bedside. Previously, we demonstrated that a simple, rapid technique of uncoached resting ventilation at the bedside yielded Etco2 measurements that are reproducible and that accurately differentiated patients with pulmonary embolism from those without.12 This technique involves breathing through a simple plastic tube connected to a handheld capnograph.
We hypothesized that Etco2 discriminates patients with PH with pulmonary arterial obstruction from those with diastolic dysfunction and passive PH and sought to determine the utility of this technique in the evaluation and treatment of PH. We undertook a study of patients with well-defined PH to determine the predictive value of Etco2 in the differential diagnosis of PH and the change of Etco2 after therapeutic change or escalation.
Materials and Methods
Study Design
This prospective, single-center study was designed to investigate the potential role of Etco2 in the diagnosis of PH. The Vanderbilt University Medical Center Institutional Review Board (Nashville, Tennessee) approved the study (approval number 070270), and all subjects gave informed consent.
Setting and Population
All new or returning patients aged ≥ 18 years who were evaluated in the Vanderbilt University Center for Pulmonary Vascular Disease from August 2009 through March 2010 were eligible for enrollment. PH was defined as mean pulmonary artery pressure (mPAP) of ≥ 25 mm Hg. PAH required a pulmonary artery occlusion pressure (PAOP) of ≤ 15 mm Hg.3 Additionally, we required a PAOP-pulmonary artery diastolic pressure (PAd) difference of > 11 mm for a diagnosis of PAH.13‐16 Patients with PVH had no other cause of PH identified after evaluation and had a PAOP > 15 mm Hg at rest, increased PAOP > 7 mm Hg after infusion of 1 L normal saline as previously described,16 or left atrial enlargement on echocardiography and mild to modest elevation in right ventricular systolic pressure with no other etiology of PH found after standard evaluation and no identified risk factors for PAH. RHCs were performed as previously described.16 Exclusion criteria were ≥ 5 L/min nasal cannula oxygen, portopulmonary hypertension (due to cirrhosis-associated hyperventilation), serum bicarbonate level of > 34 mmol/L, pregnancy, known neuromuscular disease, moderate or severe mitral stenosis, mitral or aortic regurgitation, left ventricular ejection fraction < 55% by echocardiography, known hypercarbic respiratory failure, untreated hypothyroidism or hyperthyroidism, hereditary hemorrhagic telangiectasia, uncertain diagnosis because of incomplete testing, diagnosis of World Health Organization group 3 or 4 PH, or mixed PH phenotype after thorough evaluation according to published guidelines.3
Measurements
Etco2 was measured by a trained tester blinded to diagnosis using the Nellcor NPB 75 handheld capnograph (Mallinckrodt/Nellcor; St. Louis, Missouri).17 Device calibration and oral modification were performed as previously described.12 Etco2 measurements were recorded as previously described for five breaths after resting for 5 min minimum and immediately upon completion of 6MWT, if performed.12 Demographic data; results of blood tests, 6MWT, and pulmonary function testing; and RHC data were extracted from the medical record. The 6MWTs were performed according to American Thoracic Society criteria,18 although patients were not required to perform 6MWT for study enrollment.
Healthy Control Subjects: 6MWT
The 6MWT was performed in 13 healthy control subjects (mean age ± SD, 30 ± 7 years; seven men). Etco2 was recorded as described in the “Measurements” section.
Determination of the Effects of PAH Treatment on Etco2
The effects of PAH treatment on Etco2 was measured in two groups of patients: (1) patients initiating treatment with IV or subcutaneous prostaglandin followed by clinically prescribed dose uptitration and (2) patients currently receiving IV or subcutaneous prostaglandin in whom a dose increase of > 2 ng/kg/min of epoprostenol or treprostinil was prescribed for treatment of worsening symptoms. Etco2 was measured at rest prior to and within 3 months of the therapeutic change. Poor clinical response was defined by death related to PAH or failure to improve one functional class or increase 6MWT distance by > 10%.3,19
Statistical Analysis
Based on prior publications,16 we assumed a 60% positive rate for PAH in patients seen in our clinic for pulmonary vascular disease. Given this rate and SD of 3 mm Hg in Etco2 measurements in normal volunteers, a sample size calculation determined that 102 patients would be required to detect a difference in Etco2 of 2 mm Hg between groups with 90% power at α = .05. Continuous variables are presented as mean ± SD, unless otherwise noted, and analyzed using an unpaired t test or Wilcoxon rank sum testing. Patients were enrolled only once except for those initiating or increasing prostaglandin therapy. The effects of PAH therapy were analyzed using paired t test. Categorical variables are reported as percentages and were analyzed using Fisher exact test. Receiver operating characteristic curves with areas under the curve were generated for determining sensitivity and specificity of different Etco2 cutoff levels in discrimination of patients with PAH from those without PAH. All P values are two tailed, and P ≤ .05 was considered significant. Data analyses were performed using SPSS for Windows, version 19.0 (SPSS Inc; Chicago, Illinois) and GraphPad Prism, version 5.0c (La Jolla, California).
Results
Study Patients
A total of 280 patients were evaluated for PH over the enrollment period; 66 were new patients, and 214 were return patients. Consenting to enrollment were 168 patients, of whom 56 were excluded because of portopulmonary hypertension (n = 5), advanced parenchymal lung disease (n = 5), nongroup 1 or 2 PH (n = 30), miscellaneous causes (bicarbonate > 34, n = 5; no final diagnosis, n = 3; incomplete data, n = 7; uncontrolled hyperthyroidism, n = 1). Therefore, 112 patients were included in the final analysis.
Diagnoses included PAH (n = 84), PVH (n = 17), and no PH (n = 11). Of the 84 patients with PAH, 17 were functional class 1, 37 were functional class 2, 29 were functional class 3, and one was functional class 4. Causes for PAH were idiopathic (n = 33), connective tissue disease (n = 23), heritable PAH (n = 7), congenital heart disease (n = 15), and miscellaneous (n = 6). Seven of the 11 patients with no PH had only mild or moderate pulmonary function testing abnormalities and normal chest imaging; these patients comprised the control cohort (Table 1). The remaining four patients with severely abnormal parenchyma were not included in the control group. Control patients tended to be obese and frequently had diabetes mellitus, systemic hypertension, and hyperlipidemia. However, pulmonary function testing did not show severe impairment, and in the five patients with available RHC data, mPAP and PAOP were normal. Two patients without RHC had a normal physical examination and normal echocardiography.
Table 1.
—Features of Patients Without Pulmonary Hypertension or Severe Pulmonary Function Test Abnormalities
| Value | |
| No. patients | 7 |
| Age, y | 52.1 ± 12.0 |
| Female sex, No. (%) | 6 (86) |
| BMI, kg/m2 | 33.6 ± 9.6 |
| Comorbid conditions, No. | |
| Diabetes mellitus | 4 |
| Systemic hypertension | 5 |
| Hyperlipidemia | 3 |
| Pulmonary function tests | |
| Dlco, % | 75.7 ± 23.9 |
| FEV1, % | 74.2 ± 12.4 |
| FVC, % | 71.2 ± 13.7 |
| Plasma bicarbonate, mmol/L | 27.3 ± 2.7 |
| 6-min walk distance, m | 452.3 ± 114.9 |
| Hemodynamic data (n = 5) | |
| RAP, mm Hg | 4.2 ± 3.6 |
| mPAP, mm Hg | 17.6 ± 5.2 |
| PAOP or LAP, mm Hg | 9.4 ± 2.4 |
| Cardiac index, L/m per m2 | 2.87 ± 0.9 |
| PVR, Wood units | 1.4 ± 1.1 |
| PAd-PAOP, mm Hg | 0.4 ± 1.7 |
Data are presented as mean ± SD, unless otherwise indicated. Dlco = diffusing capacity of the lung for carbon monoxide; LAP = left atrial pressure; mPAP = mean pulmonary artery pressure; PAd = diastolic pulmonary artery pressure; PAOP = pulmonary artery occlusion pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Etco2 in PAH and PVH
In order to determine whether Etco2 was different between patients with PAH and those with PVH, 84 patients with PAH were compared with 17 with PVH (Table 2). The patients with PVH were older but had a similar percentage of women. Plasma bicarbonate level was higher in patients with PVH, but values were within the normal range. In patients with available RHC data, PAOP confirmed PVH or PAH, with a low gradient from the PAd to PAOP in the PVH group. The mPAP and pulmonary vascular resistance (PVR) were significantly higher in patients with PAH than in those with PVH.
Table 2.
—Demographic Data in PAH Compared With PVH
| PAH (n = 84) | PVH (n = 17) | P Value | |
| Age, y | 50.5 ± 14.1 | 63.9 ± 12.2 | .0004 |
| Female sex, No. (%) | 62 (73.8) | 12 (70.6) | .30 |
| Plasma bicarbonate, mmol/L | 25.7 ± 3.2 | 28.1 ± 2.4 | .007 |
| Hemodynamic dataa | |||
| RAP, mm Hg | 7.5 ± 5.9 | 11.9 ± 5.8 | .01 |
| mPAP, mm Hg | 49.6 ± 15.8 | 38.6 ± 11.6 | .02 |
| PAOP or LAP, mm Hg | 9.0 ± 4.6 | 19.9 ± 6.9 | < .0001 |
| CI, L/m per m2 | 2.6 ± 0.9 | 3.3 ± 0.7 | .01 |
| PVR, Wood units | 9.2 ± 5.0 | 3.4 ± 1.7 | .0001 |
| PAd-PAOP, mm Hg | 23.4 ± 11.2 | 4.4 ± 6.0 | < .0001 |
Data are presented as mean ± SD, unless otherwise indicated. The t test was used for continuous variables, χ2 test for nominal variables, and Mann-Whitney U test for ordinal variables. PAH = pulmonary arterial hypertension; PVH = pulmonary venous hypertension. See Table 1 for expansion of other abbreviations.
PAH, n = 84; PVH, n = 14.
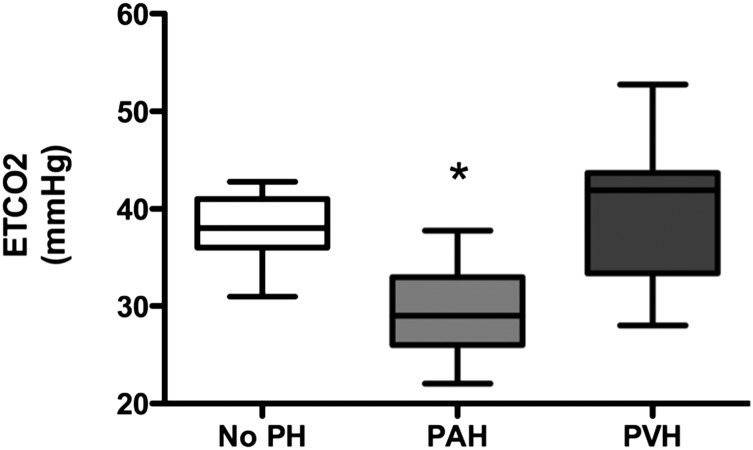
Etco2 was compared among the PAH, PVH, and no-PH groups (Fig 1). Median Etco2 was significantly lower in patients with PAH (29.0 mm Hg; 95% CI, 28.3-30.4 mm Hg) compared with both no PH (38.0 mm Hg; 95% CI, 34.4-41.6 mm Hg) and PVH (41.9 mm Hg; 95% CI, 36.6-43.4 mm Hg; P < .0001 PAH vs both groups). We did not find any difference in Etco2 between idiopathic or heritable PAH and connective tissue disease-associated PAH (data not shown). Etco2 in patients with no PH was not different from our previously published normal mean Etco2 in healthy control subjects.12
Figure 1.
Etco2 in PAH, PVH, and no PH. Etco2 measurements are shown in PH subcategories and patients with no PH. Data are presented as median with 95% CI. Etco2 = end tidal CO2; PAH = pulmonary artery hypertension; PH = pulmonary hypertension; PVH = pulmonary venous hypertension. *P < .0001 vs no PH and PVH, one-way analysis of variance.
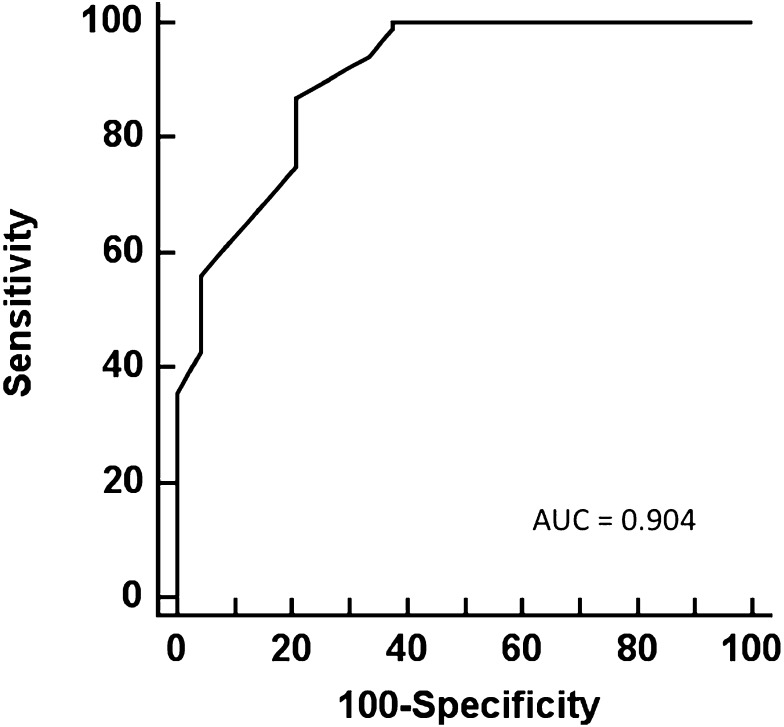
A receiver operating characteristic curve was generated to evaluate Etco2 as a discriminator of PAH vs no PH and PVH (Fig 2). Etco2 showed good performance characteristics with an r2 of 0.904. Sensitivities, specificities, and positive and negative predictive values of Etco2 in detection of PAH are shown in Table 3.
Figure 2.
Receiver operating characteristic curve for sensitivity and specificity of Etco2 in the diagnosis of PAH. AUC = area under the curve. See Figure 1 legend for expansion of other abbreviations.
Table 3.
—Sensitivity and Specificity of a Given Measurement of Etco2
| Mean Etco2, Positive if ≤ x mm Hg | Sensitivity, % | Specificity, % | Positive Predictive Value, % | Negative Predictive Value, % |
| 28 | 42.9 | 95.8 | 97.3 | 32.4 |
| 30 | 60.7 | 91.7 | 96.2 | 40.0 |
| 32 | 75.0 | 79.2 | 92.6 | 47.5 |
| 34 | 86.9 | 79.2 | 93.5 | 63.3 |
| 36 | 91.7 | 70.8 | 91.6 | 70.8 |
| 38 | 98.8 | 62.5 | 90.2 | 93.5 |
Etco2 = end tidal CO2.
Correlation of Etco2 With Invasive Hemodynamics
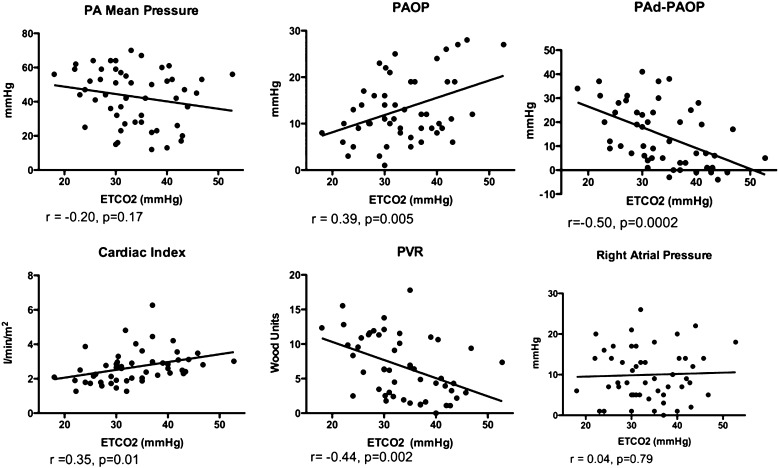
We sought to determine whether Etco2 correlated with invasive hemodynamic measurements differentiating PAH from PVH. Fifty-one patients had RHC within 3 months of Etco2 measurement (Fig 3). Etco2 correlated strongly with PAd-PAOP (P = .0002) and with cardiac index, PAOP, and PVR, important components in the evaluation of PAH vs PVH. There was no significant correlation with mPAP or right atrial pressure. In the same cohort of patients with RHC data, there was no correlation between 6MWT distance and any RHC parameters (data not shown).
Figure 3.
Correlation of invasive hemodynamics with Etco2. Scatterplots showing correlation of right-sided heart catheterization data with Etco2 measurements in patients with PAH, PVH, or no PH. Pearson test was used to determine correlation. PA = pulmonary artery; PAd = diastolic pulmonary artery pressure; PAOP = pulmonary artery occlusion pressure; PVR = pulmonary vascular resistance. See Figure 1 legend for expansion of other abbreviation.
Change in Etco2 vs Change in 6MWT Distance
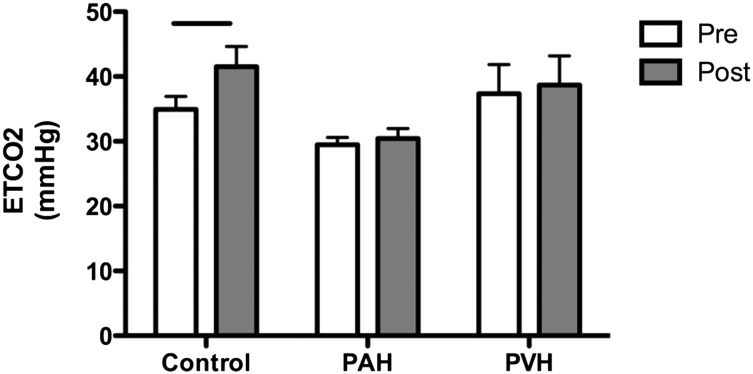
We compared the effect of 6MWT on Etco2 in healthy volunteers, patients with PAH, and patients with PVH in whom both resting and exercise data were available (13, 73, and 10, respectively) (Fig 4). In healthy control subjects, Etco2 increased after 6MWT as expected.7,20 However, mean Etco2 increased in neither PAH nor PVH after exercise. Although Etco2 did not decrease in any healthy volunteers with exercise (e-Figure 1), several patients with PAH and PVH had decreased Etco2 with exercise. Patients with PAH who were in functional class 3 trended toward decreased Etco2 after 6MWT compared with those in functional class 2 (P = .05) (e-Figure 2). In PAH, resting Etco2 did not correlate with 6MWT distance (e-Figure 3); however, after 6MWT, Etco2 directly correlated with 6MWT distance (r = 0.34, P = .003, n = 73).
Figure 4.
Effect of 6-min walk test on Etco2 in healthy control subjects, subjects with PAH, and subjects with PVH. Bars denote P = .0004; other comparisons were not significant. Data are presented as mean with 95% CI. Pre = before 6-min walk test; Post = after 6-min walk test. See Figure 1 legend for expansion of other abbreviations.
Change in Etco2 With Prostaglandin Therapy
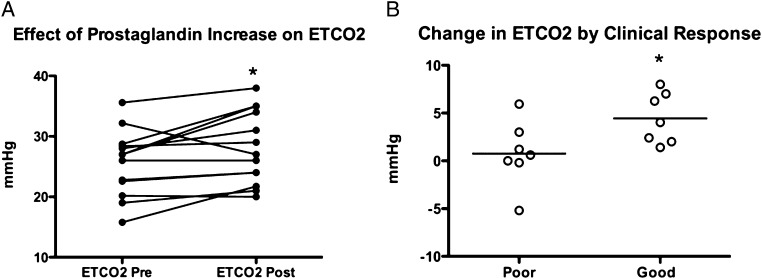
Fourteen patients (three men) with PAH (age, 51 ± 13 years; median follow-up, 11 months) either began prostaglandin therapy (n = 7) (all epoprostenol) or had an increase in dose of prostaglandin to improve symptoms (n = 7) (epoprostenol, six; treprostinil SQ, one). The effects of this change in therapy are shown in Figure 5; Etco2 increased after therapy escalation with prostaglandin. Seven patients were considered to have a poor response (three patients died, one was referred for transplant, and two neither increased 6MWT distance by > 10% nor improved one functional class). Change in Etco2 after treatment differentiated responders from nonresponders (P = .04).
Figure 5.
Effect of prostaglandin therapy on Etco2. A, Change in Etco2 in the group of patients treated with new or escalating doses of IV or subcutaneous prostaglandins (P = .03 paired t test). B, Change in Etco2 as a function of clinical response (P = .04). See Figure 1 legend for expansion of abbreviation.
Discussion
In the present study, we show that safe, simple, inexpensive measurement of Etco2 at the bedside may discriminate patients with PAH from those with PVH or no PH. Etco2 increases with clinical improvement following treatment of PAH, suggesting improved perfusion in potentially obstructed vessels. These findings suggest that Etco2 may aid in the diagnosis and treatment of pulmonary vascular disease.
With rising numbers of patients being evaluated for PH and the substantial cost, time, and potential risk in evaluation of pulmonary vascular disease,21 there is interest in development of new, noninvasive diagnostic techniques to identify patients at low risk for PAH. Currently, final confirmation of a PAH diagnosis requires RHC, in part to rule out PVH. Although clinical and echocardiographic features may make PVH more likely,4,16 often these indicators are not adequately compelling to dissuade clinicians from pursuing RHC in patients with elevated right ventricular systolic pressure on echocardiography or evidence of cor pulmonale. Conversely, clinicians may treat presumptive PAH with expensive and potentially harmful medications based on clinical and echocardiographic findings. A noninvasive bedside test with good negative predictive value for PAH is a much-needed diagnostic tool. Although cardiopulmonary exercise testing reveals differences in exhaled CO2 and ventilatory efficiency between patients with PAH and those with PVH,7,11 this test is not available at the bedside, requires expertise not found at some institutions, and has limitations in nonambulatory patients.
In the present study, resting bedside Etco2 showed potential for a high negative predictive value ruling out PAH. In this cohort, the positive predictive value for an Etco2 of ≤ 38 mm Hg was 90.2%, and the negative predictive value for an Etco2 > 38 mm Hg was 93.5%. If an Etco2 cutoff value of ≤ 38 mm Hg were chosen in this cohort, we would have spared 12 of 17 diagnostic RHCs for PVH and three of seven for no PH and would have missed three of 84 patients with PAH. Etco2 measurements had strong correlation with hemodynamic measurements for both the diagnosis of PAH (PAOP, PVR, PAd-PAOP gradient) and correlated well with cardiac index, an important measure in long-term follow-up in patients with PAH, but they did not correlate with right atrial pressure, a marker of right ventricular failure. These findings fit with the mechanism of depression of Etco2 in PAH and pulmonary arterial obstruction that would not be affected by right ventricular failure.
Other investigators have shown a low Etco2 in patients with PAH and further decrease from baseline with cardiopulmonary exercise testing in PAH.7,11,20 We extended these findings in the present study by using a handheld capnograph and further correlated Etco2 with hemodynamic markers of PAH compared with PVH. We examined the effect of 6MWT on Etco2 in PAH and found that change in Etco2 was highly variable after exercise, which likely reflects the variety of functional classes enrolled in the study because there was a trend toward decreased Etco2 after exercise in functional class 3 patients but not in functional classes 1 or 2 patients. Given the variability in change in Etco2 after 6MWT, we chose to focus on resting values for assessment of therapeutic response.
We showed that Etco2 increased with clinically successful prostaglandin treatment of PAH. The 6MWT distance did not correlate with any invasive hemodynamic variable; therefore, an improvement in Etco2 may be a more useful surrogate marker of hemodynamics in the follow-up of patients with PAH.
These findings are from a single center, and further study of Etco2 in the diagnosis of pulmonary vascular phenotype in a multicenter study would be needed to confirm the utility of this test. We did not collect arterial blood gases in this study to measure volume of dead space to tidal volume ratio; however, we did measure plasma bicarbonate levels, which revealed a numerically small, but statistically significant difference between patients with PVH and those with PAH. This may reflect diuretic use or renal compensation for differences in ventilation between the two populations. Arterial partial pressure of CO2 might identify a small number of low Etco2 values that were false-positives for PAH through alveolar hyperventilation.
In summary, we have shown that (1) resting Etco2 is lower in patients with PAH than in those with PVH and no PH, (2) Etco2 correlates well with hemodynamic markers of etiology of pulmonary vascular disease, and (3) Etco2 increases with clinically successful treatment of PAH. Further studies are needed to confirm these findings and discern their utility in clinical decision making.
Acknowledgments
Author contributions: Dr Hemnes had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Hemnes: contributed to the study design, data collection, data analysis, and manuscript preparation.
Dr Pugh: contributed to the data collection and analysis and manuscript preparation.
Mr A. L. Newman: contributed to the data collection and manuscript drafting and revision.
Dr Robbins: contributed to the study design, data collection, data analysis, and manuscript preparation.
Dr Tolle: contributed to the data analysis and manuscript preparation.
Dr Austin: contributed to the data analysis and manuscript preparation.
Dr J. H. Newman: contributed to the study design, data analysis, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hemnes has served on speakers bureaus for Actelion Pharmaceuticals Ltd and Gilead, has received research support from Actelion, and served on advisory boards for Actelion, Gilead, and United Therapeutics Corporation. Drs Hemnes and J. H. Newman and Mr A. L. Newman have a patent pending for the oral modification of the Etco2 measurement described herein. Dr Robbins has received grants to participate in industry-sponsored studies from Actelion, United Therapeutics, Gilead, and BioMarin Pharmaceutical Inc. He also has served as an advisory board member for Actelion; United Therapeutics; Gilead; Pfizer Inc; Ikaria, Inc; and BioMarin. Drs Pugh, Tolle, and Austin have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Additional information: The e-Figures can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/5/1267/suppl/DC1.
Abbreviations
- 6MWT
6-min walk test
- Etco2
end tidal CO2
- mPAP
mean pulmonary artery pressure
- PAd
diastolic pulmonary artery pressure
- PAH
pulmonary arterial hypertension
- PAOP
pulmonary artery occlusion pressure
- PH
pulmonary hypertension
- PVH
pulmonary venous hypertension
- PVR
pulmonary vascular resistance
- RHC
right-sided heart catheterization
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int J Clin Pract Suppl. 2009;63(suppl s162):4–19. doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(2):268–276. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 5.Peacock A, Keogh A, Humbert M. Endpoints in pulmonary arterial hypertension: the role of clinical worsening. Curr Opin Pulm Med. 2010;16(suppl 1):S1–S9. doi: 10.1097/01.mcp.0000370205.22885.98. [DOI] [PubMed] [Google Scholar]
- 6.Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(12 suppl 1):25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Yasunobu Y, Oudiz RJ, Sun XG, et al. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127(5):1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 8.Methvin AB, Owens AT, Emmi AG, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011;139(3):617–625. doi: 10.1378/chest.10-0318. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto A, Itoh H, Eto Y, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol. 2000;36(1):242–249. doi: 10.1016/s0735-1097(00)00702-6. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe Y, Hosaka Y, Ito M, Ito E, Susuki K. Significance of end-tidal P(CO(2)) response to exercise and its relation to functional capacity in patients with chronic heart failure. Chest. 2001;119(3):811–817. doi: 10.1378/chest.119.3.811. [DOI] [PubMed] [Google Scholar]
- 11.Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007;132(3):977–983. doi: 10.1378/chest.07-0619. [DOI] [PubMed] [Google Scholar]
- 12.Hemnes AR, Newman AL, Rosenbaum B, et al. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Respir J. 2010;35(4):735–741. doi: 10.1183/09031936.00084709. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RF, Beckman SB, Tyburski JG, Scholten DJ. Pulmonary artery diastolic and wedge pressure relationships in critically ill and injured patients. Arch Surg. 1988;123(8):933–936. doi: 10.1001/archsurg.1988.01400320019002. [DOI] [PubMed] [Google Scholar]
- 14.Her C, Cerabona T, Baek SH, Shin SW. Increased pulmonary venous resistance in morbidly obese patients without daytime hypoxia: clinical utility of the pulmonary artery catheter. Anesthesiology. 2010;113(3):552–559. doi: 10.1097/ALN.0b013e3181e4f706. [DOI] [PubMed] [Google Scholar]
- 15.Lappas D, Lell WA, Gabel JC, Civetta JM, Lowenstein E. Indirect measurement of left-atrial pressure in surgical patients–pulmonary-capillary wedge and pulmonary-artery diastolic pressures compared with left-atrial pressure. Anesthesiology. 1973;38(4):394–397. doi: 10.1097/00000542-197304000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136(1):31–36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Operators Manual. NPB 75: Portable Bedside Capnograph/Pulse Oximeter. Pleasanton, CA: Nellcor Puritan Bennet; 1998. [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Provencher S, Sitbon O, Humbert M, Cabrol S, Jaïs X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27(5):589–595. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- 20.Oudiz RJ, Barst RJ, Hansen JE, et al. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol. 2006;97(1):123–126. doi: 10.1016/j.amjcard.2005.07.129. [DOI] [PubMed] [Google Scholar]
- 21.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance–United States, 1980-2002. MMWR Surveill Summ. 2005;54(5):1–28. [PubMed] [Google Scholar]