Abstract
Background:
The most serious complications of airway stenting are long term, including infection and granulation tissue formation. However, to our knowledge, no studies have quantified the incidence rate of long-term complications for different stents.
Methods:
To compare the incidence of complications of different airway stents, we conducted a retrospective cohort study of all patients at our institution who had airway stenting for malignant airway obstruction from January 2005 to August 2010. Patients were excluded if more than one type of stent was in place at the same time. Complications recorded were lower respiratory tract infections, stent migration, granulation tissue, mucus plugging requiring intervention, tumor overgrowth, and stent fracture.
Results:
One hundred seventy-two patients with 195 stent procedures were included. Aero stents were associated with an increased risk of infection (hazard ratio [HR] = 1.98; 95% CI, 1.03-3.81; P = .041). Dumon silicone tube stents had an increased risk of migration (HR = 3.52; 95% CI, 1.41-8.82; P = .007). Silicone stents (HR = 3.32; 95% CI, 1.59-6.93; P = .001) and lower respiratory tract infections (HR = 5.69; 95% CI, 2.60-12.42; P < .001) increased the risk of granulation tissue. Lower respiratory tract infections were associated with decreased survival (HR = 1.57; 95% CI, 1.11-2.21; P = .011).
Conclusions:
Significant differences exist among airway stents in terms of infection, migration, and granulation tissue formation. These complications, in turn, are associated with significant morbidity and mortality. Granulation tissue formation develops because of repetitive motion trauma and infection.
Central airway obstruction in patients with cancer develops secondary to intraluminal disease, extrinsic tumor compression, or both.1 For intraluminal obstruction, ablative techniques that destroy tissue are typically used, including laser therapy, electrocautery, and mechanical coring with a rigid bronchoscope, among others. For extrinsic compression, stents are used to strengthen the bronchial wall and provide a barrier against the tumor to keep the airway open. Usually, a multimodality approach is used because findings are often mixed.
Although airway stenting can be a highly effective treatment of central airway obstruction, several complications have been identified.2‐10 Immediate perioperative complications are rare,11 but long-term complications are more common and more serious.1,5 Long-term complications related to stenting include infection, granulation tissue, mucus plugging, stent migration, and stent fracture.1,5 Of these, granulation tissue is the most common serious complication requiring bronchoscopic intervention. Management is further complicated by the difficulty of removing metal stents.12,13
To our knowledge, no randomized controlled studies have been performed to compare the incidence of complications between different airway stents. Most airway stent complication data are from cases series and cohort studies.3‐10 This paucity of data makes clinical decision making difficult and is also problematic from a regulatory and policy perspective. Therefore, the aim of the present study was to compare the incidence of complications of different airway stents and to identify risk factors for each complication type. We hypothesized that different stent types would have different incidence rates of infection and that infection would lead to the development of granulation tissue.14‐16
Materials and Methods
Study Design
We performed a retrospective analysis of all patients who underwent airway stenting for malignant airway obstruction at The University of Texas MD Anderson Cancer Center from January 2005 to August 2010. The protocol DR10-0717 was approved by the Institutional Review Board 4 Committee, and a waiver of informed consent was given. Patients were excluded if more than one type of stent was used. Data were abstracted using a standardized form. All definitions were developed prior to chart abstraction and were entered into a code book to ensure consistency.
We abstracted data on age, sex, smoking status, pulmonary comorbidities, cancer diagnosis, radiation therapy, chemotherapy, airway obstruction type, stenting indication, stent type, stent location, complications, complication treatments, and outcomes, including hospitalizations, respiratory failure, and subsequent airway interventions. All CT images were reviewed by a chest radiologist who was blinded to the clinical data. CT images were evaluated for evidence of stent migration, mucus plugging, granulation, fracture, and tumor overgrowth.
Expandable metal stents were placed using flexible or rigid bronchoscopy, whereas silicone stents were placed with a rigid bronchoscope. Silicone stents were sterilized prior to use. Perioperative and prophylactic antibiotics and steroids were not given.
Definitions
The primary outcome was time to lower respiratory tract infection. Respiratory infections were classified in a manner similar to that described in previously published systematic reviews and cohort studies.2‐10 The presence of a respiratory infection was based on the presence of clinical findings (fever, increased volume and purulence of sputum, and worsening cough), with or without radiographic evidence of pneumonia. The definition also required that the managing physician make a clinical diagnosis of lower respiratory tract infection and that antibiotics be given, with or without additional measures such as bronchoscopy. Respiratory infections were classified as pneumonias if evidence of new consolidation or infiltrates were found on chest radiograph. If no chest radiograph was performed or it did not demonstrate new abnormalities and the patient was treated on the basis of clinical findings, the infection was classified as acute bronchitis.
Secondary outcomes were time to stent migration, granulation tissue formation, mucus plugging requiring bronchoscopic cleaning, tumor overgrowth, and stent fracture. Bronchoscopic confirmation was required for granulation tissue formation, stent migration, stent fracture, and tumor overgrowth.
Statistics
Time to complications was measured from the date of stent insertion to the date of the event; patients who did not experience the relevant end point were censored at last follow-up. Univariate Cox proportional hazards models were fit to determine the association between characteristics and time-to-event outcomes. Time-varying covariates, such as infection, were defined as 0 (absent) at baseline but were changed to 1 (present) after the corresponding time points when they occurred. Variables significant at the 0.1 level in univariate analyses were evaluated in the multivariate extended Cox models. Backward elimination was used, retaining only variables that had a 0.05 level of significance. Kaplan-Meier curves were plotted for significant covariates. P values < .05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc) and S-Plus 7.0 (Insightful Corporation).
Results
Two hundred twelve stent procedures were identified. Eight were excluded because multiple stent types were placed. Three procedures with Montgomery T-tubes and six with Polyflex stents (Boston Medical) were excluded because there were too few of the stents placed to analyze. The study included 172 patients with 195 stent procedures. Ultraflex stents were used in 118 cases (60%), Aero (Merit Endotek) stents were used in 31 (16%), and Novatech Dumon silicone bronchial and Y-stents (Boston Medical) were used in 46 (24%). Patient characteristics and complication rates are summarized in Tables 1 and 2.
Table 1.
—Patient and Clinical Characteristics
| Characteristic | Total Procedures (N = 195) |
| Age (n = 172) | |
| Median (range) | 59 (16-84) |
| Mean (STD) | 59.3 (12.4) |
| Sex (n = 172) | |
| Female | 79 (45.93) |
| Male | 93 (54.07) |
| Lung cancer | |
| Metastatic | 80 (41.03) |
| Primary | 115 (58.97) |
| Smoking status | |
| Nonsmoker | 48 (25.53) |
| Active | 55 (29.26) |
| Quit | 85 (45.21) |
| Stent type | |
| Novatech Dumon | 46 (23.59) |
| Ultraflex | 118 (60.51) |
| Aero | 31 (15.90) |
| Type of Dumon stent (n = 46) | |
| Y-shaped | 28 (60.87) |
| Tube stent | 18 (39.13) |
| Airway pathologic findings | |
| Mixed | 122 (62.56) |
| Endobronchial | 29 (14.87) |
| Extrinsic compression | 32 (16.41) |
| Fistula | 12 (6.15) |
| Stent placed to overcome compression | |
| No | 22 (11.28) |
| Yes | 173 (88.72) |
| Stent placed to provide a barrier effect | |
| No | 39 (20.00) |
| Yes | 156 (80.00) |
| Stent placed to prevent dynamic airway collapse | |
| No | 188 (96.41) |
| Yes | 7 (3.59) |
| Case involved left-sided stents | |
| No | 140 (71.79) |
| Yes | 55 (28.21) |
| Case involved right-sided stents | |
| No | 119 (61.03) |
| Yes | 76 (38.97) |
| Case involved tracheal stents | |
| No | 145 (74.36) |
| Yes | 50 (25.64) |
| Case involved stents at the carina (Y-shaped) | |
| No | 167 (85.64) |
| Yes | 28 (14.36) |
| Prestent chemotherapy | |
| No | 131 (67.18) |
| Yes | 64 (32.82) |
| Poststent chemotherapy (time varying) | |
| No | 131 (67.18) |
| Yes | 64 (32.82) |
| Prestent radiation therapy | |
| No | 136 (69.74) |
| Yes | 59 (30.26) |
| Poststent radiation therapy (time varying) | |
| No | 139 (71.28) |
| Yes | 56 (28.72) |
| Events | |
| Lower respiratory tract infection (time varying) | |
| No | 122 (62.56) |
| Yes | 73 (37.44) |
| Pneumonia | |
| No | 139 (71.28) |
| Yes | 56 (28.72) |
| Granulation | |
| No | 157 (80.51) |
| Yes | 38 (19.49) |
| Mucus impaction | |
| No | 147 (75.38) |
| Yes | 48 (24.62) |
| Hemoptysis | |
| No | 178 (91.28) |
| Yes | 17 (8.72) |
| Stent migration | |
| No | 167 (86.08) |
| Yes | 27 (13.92) |
| Stent strut fracture | |
| No | 191 (97.95) |
| Yes | 4 (2.05) |
| New fistula formation | |
| No | 193 (98.97) |
| Yes | 2 (1.03) |
| Tumor overgrowth | |
| No | 170 (87.18) |
| Yes | 25 (12.82) |
| Death (n = 172 unique patients) | |
| No | 26 (15.12) |
| Yes | 146 (84.88) |
Data are presented as No. (%) unless otherwise noted.
Table 2.
—Crude Incidence Rates of Complications
| Crude Complication Incidence Rate by Stent Type |
||||
| Complication Type | Ultraflex | Aero | Dumon Tube Stent | Dumon Y Stent |
| Infection | 0.00477 | 0.01259 | 0.00393 | 0.00761 |
| Migration | 0.00116 | 0.00278 | 0.00467 | 0 |
| Granulation | 0.00135 | 0.00328 | 0.00368 | 0.00537 |
| Mucus plugging | 0.00188 | 0.00498 | 0.00464 | 0.00373 |
| Stent fracture | 0.00022 | 0.00054 | 0 | 0 |
Rates are expressed in events per stent-day at risk. If patients had their stent removed, they were considered no longer at risk for stent-related complications. Days at risk are, therefore, based on duration of time that the stent was in place.
Infection
Seventy-three patients developed 106 lower respiratory tract infections. The median time to infection was 1 month (range, 0-35 months). Respiratory infections led to significant morbidity and mortality: more than one-half of the patients were hospitalized, and 23% of patients with respiratory infections died within 14 days of their infection (Table 3). On univariate and multivariate analysis (Tables 4, 5), only Aero stents (hazard ratio [HR] = 1.98; 95% CI, 1.03-3.81; P = .041) (Fig 1) had a significant effect on infection risk.
Table 3.
—Clinical Characteristics of Respiratory Infections
| Characteristic | Total Infections (N = 106), No. (%) |
| Infection typea | |
| Acute bronchitis | 34 (32) |
| Pneumonia, not in the stent area | 30 (28) |
| Pneumonia, distal to stent area | 21 (20) |
| Pneumonia, multilobar with area distal to stent involved | 21 (20) |
| Care site | |
| Outpatient | 48 (45) |
| Admitted to hospital floor | 36 (34) |
| Admitted to hospital and required ICU | 22 (21) |
| Ventilator support | |
| No ventilatory support required during hospitalization | 87 (82) |
| Noninvasive positive pressure ventilation | 5 (5) |
| Mechanical ventilation with endotracheal tube | 14 (13) |
| Stent removal required | |
| Yes | 12 (11) |
| No | 94 (89) |
| Death within 14 d of infection | |
| Yes | 24 (23) |
| No | 82 (77) |
Seventy-three patients had 106 lower respiratory tract infections.
Table 4.
—Univariate Cox and Extended Cox Models for Time to Infection
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Age (continuous) | 0.988 | 0.97-1.01 | .20 |
| Sex: male vs female | 0.93 | 0.58-1.48 | .76 |
| Smoking status | |||
| Active vs nonsmoker | 1.60 | 0.82-3.15 | .17 |
| Former smoker, quit vs nonsmoker | 1.50 | 0.81-2.77 | .20 |
| Stent type | |||
| Dumon silicone vs Ultraflex | 1.16 | 0.67-2.02 | .59 |
| Aero vs Ultraflex | 1.98 | 1.03-3.81 | .041 |
| Airway pathology | |||
| Endobronchial vs mixed | 1.02 | 0.51-2.03 | .95 |
| Extrinsic compression vs mixed | 0.66 | 0.34-1.28 | .22 |
| Fistulas vs mixed | 0.95 | 0.38-2.4 | .92 |
| Stent is to overcome compression: yes vs no | 1.18 | 0.56-2.47 | .66 |
| Stent is to provide a barrier effect: yes vs no | 1.47 | 0.8-2.69 | .22 |
| Stent is to prevent dynamic airway collapsibility: yes vs no | 0.52 | 0.13-2.14 | .37 |
| Case involved left-sided stents: yes vs no | 0.81 | 0.46-1.43 | .47 |
| Case involved right-sided stents: yes vs no | 1.41 | 0.89-2.25 | .15 |
| Case involved tracheal stents: yes vs no | 0.84 | 0.48-1.44 | .52 |
| Case involved carinal stents: yes vs no | 1.36 | 0.75-2.49 | .31 |
| Prestent chemotherapy: yes vs no | 0.89 | 0.54-1.49 | .67 |
| Poststent chemotherapy: yes vs no (time varying)a | 0.89 | 0.54-1.47 | .65 |
| Prestent radiation therapy: yes vs no | 0.90 | 0.53-1.54 | .71 |
| Poststent radiation therapy: yes vs no (time varying)a | 1.04 | 0.6-1.81 | .89 |
Extended Cox model.
Table 5.
—Multivariate Model for Time to Infection
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Dumon silicone vs Ultraflex | 1.16 | 0.67-2.02 | .59 |
| Aero vs Ultraflex | 1.98 | 1.03-3.81 | .041 |
Figure 1.
Kaplan-Meier plot of time to first respiratory infection by stent type. Aero stents (dashed line) had a significantly shorter time to infection than did other stents (P = .043).
Stent Migration
Because Y-shaped stents do not typically migrate, we excluded 28 Y stent cases from the migration analysis. Among the remaining 167 procedures, 27 stent migrations occurred. The median time to stent migration was 1.43 months (range, 0-36 months). On univariate and multivariate analysis (Tables 6, 7), only silicone tube stents (HR = 3.52; 95% CI, 1.41-8.82; P = .007) (Fig 2) had a significant effect on migration risk. Overall, 12 stents were electively removed without replacement because of a response to therapy. Of these, two had migration. Among the 155 other patients, 25 had migration (P = 1.0). Silicone stents were more likely to be removed electively than metal stents (P = .02).
Table 6.
—Univariate Cox and Extended Cox Models for Time to Stent Migration
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Age (continuous) | 0.99 | 0.96-1.02 | .56 |
| Sex: male vs female | 0.92 | 0.43-1.96 | .83 |
| Smoking status | |||
| Active vs nonsmoker | 1.08 | 0.30-3.87 | .90 |
| Former smoker, quit vs nonsmoker | 1.83 | 0.64-5.22 | .26 |
| Stent type | |||
| Dumon silicone tube stent vs Ultraflex | 3.52 | 1.44-8.82 | .007 |
| Aero vs Ultraflex | 1.61 | 0.58-4.47 | .37 |
| Airway pathology | |||
| Endobronchial vs mixed | 1.88 | 0.66-5.37 | .24 |
| Extrinsic compression vs mixed | 1.63 | 0.64-4.12 | .30 |
| Fistulas vs mixed | 1.19 | 0.27-5.36 | .82 |
| Stent is to overcome compression: yes vs no | 0.48 | 0.19-1.20 | .11 |
| Stent is to provide a barrier effect: yes vs no | 0.68 | 0.29-1.62 | .39 |
| Stent is to prevent dynamic airway collapsibility: yes vs no | 1.19 | 0.16-8.78 | .87 |
| Case involved left-sided stents: yes vs no | 0.85 | 0.36-2.02 | .71 |
| Case involved right-sided stents: yes vs no | 0.78 | 0.36-1.68 | .53 |
| Case involved tracheal stents: yes vs no | 1.54 | 0.71-3.33 | .28 |
| Case involved carinal stents: yes vs no | 1.36 | 0.75-2.49 | .31 |
| Prestent chemotherapy: yes vs no | 1.21 | 0.55-2.67 | .64 |
| Poststent chemotherapy: yes vs no (time varying) | 0.64 | 0.26-1.58 | .33 |
| Prestent radiation therapy: yes vs no | 1.74 | 0.79-3.81 | .17 |
| Poststent radiation therapy: yes vs no (time varying) | 1.08 | 0.43-2.72 | .88 |
Table 7.
—Multivariate Cox Proportional Hazards Model for Time to Migration by Stent Type
| Stent Type | Hazard Ratio | 95% CI | P Value |
| Dumon silicone vs Ultraflex | 3.52 | 1.41-8.82 | .007 |
| Aero vs Ultraflex | 1.61 | 0.58-4.47 | .37 |
Figure 2.
Kaplan-Meier plot of time to migration by stent type. Silicone tube stents (dashed line) had a higher incidence of stent migration than did non-silicone tube stents (solid line). Y-shaped stents are excluded from this analysis.
Granulation Tissue
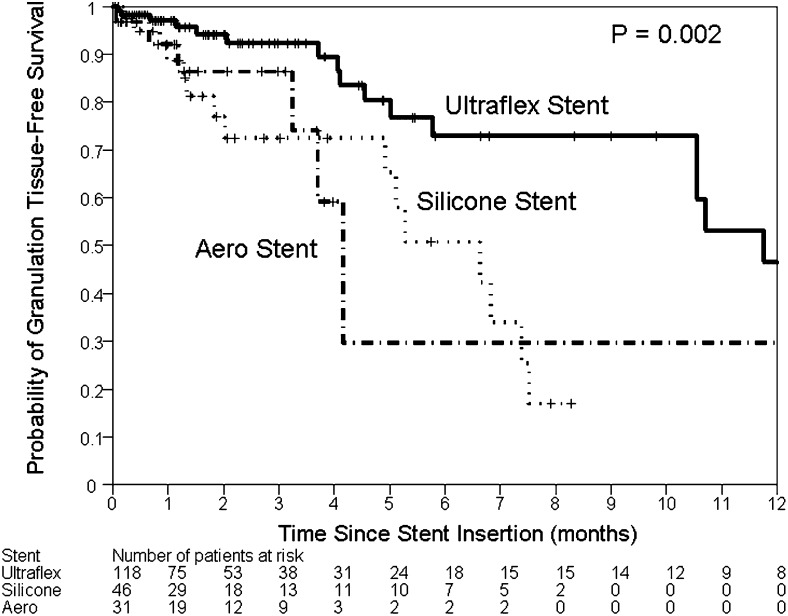
Thirty-eight granulation events occurred among 195 stent procedures. The median time to granulation was 1.4 months (range, 0-36 months). On univariate analysis, lower respiratory tract infections (P < .001) were associated with granulation tissue formation. Compared with Ultraflex stents, both silicone stents (P = .002) and Aero stents (P = .04) were more likely to lead to granulation tissue formation. In the multivariate model, only silicone stents (HR = 3.32; 95% CI, 1.59-6.93; P = .001) and lower respiratory tract infection (HR = 5.69; 95% CI, 2.60-12.42; P < .001) were associated with increased risk for granulation (Fig 3, Tables 8, 9).
Figure 3.
Kaplan-Meier plot of time to granulation tissue formation. Both Aero stents (dashed line) and silicone stents (dotted line) had a higher incidence of granulation tissue formation than did Ultraflex stents (solid line).
Table 8.
—Univariate Cox and Extended Cox Models for Time to Granulation Tissue Formation
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Age (continuous) | 0.99 | 0.96-1.01 | .32 |
| Sex: male vs female | 0.73 | 0.38-1.4 | .34 |
| Smoking status | |||
| Active vs nonsmoker | 0.83 | 0.33-2.09 | .7 |
| Former smoker, quit vs nonsmoker | 1.12 | 0.52-2.38 | .77 |
| Stent type | |||
| Dumon silicone vs Ultraflex | 3.30 | 1.58-6.89 | .002 |
| Aero vs Ultraflex | 2.68 | 1.03-6.96 | .043 |
| Airway pathologic findings | |||
| Endobronchial vs mixed | 1.24 | 0.5-3.04 | .65 |
| Extrinsic compression vs mixed | 0.83 | 0.34-2.05 | .69 |
| Fistulas vs mixed | 0.51 | 0.12-2.18 | .36 |
| Stent to overcome compression: yes vs no | 0.93 | 0.36-2.4 | .88 |
| Stent to provide a barrier effect: yes vs no | 1.06 | 0.48-2.34 | .88 |
| Stent to prevent dynamic airway collapsibility: yes vs no | 2.33 | 0.55-9.87 | .25 |
| Case involved left-sided stents: yes vs no | 0.74 | 0.34-1.62 | .45 |
| Case involved right-sided stents: yes vs no | 0.63 | 0.32-1.25 | .19 |
| Case involved tracheal stents: yes vs no | 1.19 | 0.57-2.46 | .64 |
| Case involved carinal stents: yes vs no | 2.79 | 1.33-5.87 | .007 |
| Prestent chemotherapy: yes vs no | 1.03 | 0.52-2.06 | .93 |
| Poststent chemotherapy: yes vs no (time varying)a | 0.72 | 0.35-1.47 | .37 |
| Prestent radiation therapy: yes vs no | 1.55 | 0.76-3.17 | .23 |
| Poststent radiation therapy: yes vs no (time varying)a | 1.37 | 0.69-2.74 | .37 |
| Infection: yes vs no (time varying)a | 5.69 | 2.65-12.22 | < .001 |
Extended Cox model.
Table 9.
—Multivariate Extended Cox Model of Time to Granulation Tissue Formation
| Characteristic | Hazard Ratio | 95% CI | P Value |
| Dumon silicone vs Ultraflex | 3.32 | 1.59-6.93 | .001 |
| Aero vs Ultraflex | 1.60 | 0.61-4.21 | .34 |
| Infection: yes vs no (time varying) | 5.69 | 2.6-12.42 | < .001 |
Mucus Plugging
There were 48 episodes of mucus impaction requiring intervention among 195 stent procedures. The median time to mucus impaction was 1.3 months (range 0-46 months). On univariate analysis, having a left-sided stent (P = .014) (e-Fig 1A) or a silicone stent (P = .03) (e-Fig 1B) increased the risks of mucus impaction, but older age (P = .027) or having chemotherapy post stent placement (P = .018) were protective (e-Table 1). In the final multivariate model, having a left-sided stent (HR = 3.07; 95% CI, 1.55-6.08; P = .001), age (HR = 0.97; 95% CI, 0.94-0.99; P = .002), having a silicone stent (HR = 2.72; 95% CI, 1.33-5.056; P = .006) vs Ultraflex stents, and having chemotherapy after stent placement (HR = 0.32; 95% CI, 0.14-0.72; P = .006) had significant impact on time to mucus impaction (e-Table 1).
Hemoptysis, Tumor Overgrowth, and Stent Fracture
On univariate analysis, no factors had a significant effect on time to hemoptysis or time to tumor overgrowth. Structural fractures were found in only three Ultraflex and one Aero stent. Thus, we were unable to draw any conclusions regarding fracture risk factors.
Overall Survival
Median follow-up duration was 3 months (range, 0-73 months). One hundred forty-six deaths occurred among 172 patients. On univariate analysis, we found that among individual patients, stents for compression (P = .003) or a barrier effect (P = .05), left-sided stents (P = .03), and lower respiratory tract infections (P = .005) were associated with higher mortality rates; however, silicone stents (P = .036) vs Ultraflex (e-Fig 2A), and tracheal stents (P = .028) (e-Fig 2B) and the presence of extrinsic compression (P = .029) or fistulas (P = .041) vs mixed airway obstruction were associated with lower mortality rates (e-Table 2). Pre-stent radiation therapy (P = .06) (e-Fig 2c) had a marginal effect. In the final multivariate model, silicone stents (HR = 0.56; 95% CI, 0.37-0.84; P = .005), tracheal stents (HR = 0.57; 95% CI, 0.38-0.85; P = .005), pre-stent radiation therapy (HR = 1.44; 95% CI, 1.03-2.00; P = .031), and lower respiratory tract infections (HR = 1.57; 95% CI, 1.11-2.21; P = .011) had significant effects on overall survival (e-Table 2).
Discussion
To our knowledge, this is the first study of airway stents to document and compare the survival and hazard functions of long-term airway complications. Our findings suggest that significant differences exist between airway stent types in terms of infection, migration, and granulation tissue risk. In particular, respiratory infections were more frequent in patients with Aero stents, migration was more common with silicone stents, and granulation tissue was associated with preceding respiratory infections and silicone stents. Stent fracture occurred rarely.
Our findings build on those of other investigators who have studied the incidence proportions of stent complications.2‐10 An incidence proportion is defined as the number of cases with complications divided by the number of cases overall and is an appropriate measure for analyzing immediate perioperative complications. However, the incidence proportion has significant limitations when used to analyze long-term complications because one of the fundamental assumptions is that the groups compared have an equivalent time at risk.17 For example, a higher proportion of stent complications have been observed with benign disease than with malignant disease.6,8‐10,18,19 However, patients with benign disease usually live longer and thus have more time to experience complications, such as infections. Similarly, even if one stent type is less prone to infection than another, given sufficient time, both stents will eventually become infected. Incidence proportions are not useful in these cases because they do not account for time at risk.
A more appropriate measurement of complications is incidence rate because it measures events per person-time at risk. For example, in this study, the incidence proportion of stent infections was 36% to 39% and there was no significant difference between stent types (P = .934) (e-Table 3). However, the incidence rate of Aero stent infections was more than double that of other stents (P = .009). Therefore, when evaluating long-term complication rates, it is desirable to compare hazard functions. Our study adds to the body of evidence previously reported because it is the first to use survival analysis methods to quantify the incidence rates of complications by stent type. To the best of our knowledge, all previous studies used incidence proportions.2‐10
This type of analysis also allows us to gain insight into the mechanisms of disease by evaluating the shape of the hazard function. In the immediate perioperative period, infection rates may be high, but after that initial period, the incidence rate can be expected to decline. Thus, if sterilization is necessary to decrease postprocedural infections, we would expect the infection incidence rate in nonsterilized stents, such as the Ultraflex, to be higher initially and then decrease to levels similar to those of sterilized silicone stents. However, although we found that Aero stents were associated with a higher incidence of infection, we found no difference between Ultraflex and silicone stents. This finding suggests that sterilization does not significantly affect infection. Considering that silicone stent placement requires that the sterilized stent be loaded into a nonsterile deployment device that is passed down a nonsterile rigid bronchoscope, this is not surprising.
Conversely, the finding that the relative risk of infection was persistently higher with Aero stents, rather than being higher initially and then declining, suggests that it is not sterilization that makes the difference but other factors, such as differences in design or manufacturing. It is interesting to note that the Aero stent is unique among airway stents in that it has a hydrophilic coating designed to prevent mucus build-up. Whether this coating affects infection rates warrants further investigation. It is also important to recognize that subtle but important differences in causation, such as absence of sterilization vs design factors, could not be identified by simply using incidence proportions because incidence proportions do not account for time at risk. Future studies should use appropriate analysis methods.
Applying this method to other stent complications, we found higher incidence rates of migration among silicone tube stents than among Aero and Ultraflex stents. Silicone tube stents have been believed to migrate more often,1 but this has been based on anecdotal evidence, and no prior studies have identified or quantified the risk using incidence rates. Because silicone stents are easier to remove than expandable metal stents, quantifying the magnitude of the risks and benefits allows physicians to take these factors into consideration when choosing a stent. The advantages of easier removability are important when considering benign disease, but for patients with advanced cancer the benefits of lower migration rates may outweigh concerns regarding removal.
We also found higher incidence rates of granulation tissue with silicone and Aero stents than with Ultraflex stents. Self-expanding metal stents have been believed to lead to more granulation tissue than silicone stents,20‐22 but again this has been based on anecdotal evidence only.
We believe the observed differences in granulation tissue may be related to repetitive motion trauma and infection. Repetitive stent motion may cause trauma, with mucosal inflammation and granulation tissue formation.16,23,24 Histologic studies of stents have shown a nonspecific inflammatory response without foreign-body giant cells.16,24,25 Presumably, stents with higher migration rates also have a higher degree of motion, putting them at increased risk for developing granulation tissue. This is consistent with our observation that silicone stents have a higher incidence of both migration and granulation.
Bacterial infection may also play a role in granulation tissue formation. The available evidence supporting this is limited, with most of the data coming from studies of laryngotracheal reconstruction, tracheal stenosis, and subglottic stenosis.14‐16,23 In a dog model, trauma alone was insufficient to induce granulation tissue, but trauma and bacterial contamination led to granulation tissue.15 Human studies have shown an association between positive cultures and the presence of granulation tissue,14,16,23 and airway stenting has been found to be associated with frequent bacterial colonization.26 However, whether infections lead to granulation tissue or whether granulation tissue leads to obstruction and subsequent infection is less clear. This situation is termed reverse causation—when the outcome affects the exposure, rather than the reverse. Previous studies have been unable to address this issue because they correlated cultures with the concurrent presence or absence of granulation tissue. By using time-varying covariates, we were able to address the issue of reverse causation. Our results demonstrate that prior infections are a risk factor for subsequent granulation tissue formation.
This is consistent with our observation that Aero stents are associated with a higher incidence of both infection and granulation tissue. In the multivariable model for granulation tissue, after controlling for infection, the Aero stent was not associated with granulation tissue. This finding suggests that the infections associated with the Aero stent drive granulation tissue development, rather than some other structural issue causing trauma. In contrast, silicone stents, which are associated with increased migration, are associated with granulation tissue formation even after adjusting for infection. So both repetitive motion trauma and infection play a role.
This study has some limitations. As with all retrospective studies, residual confounding may be present. Selection bias is also a possibility. Expandable metal stents are easier to place than silicone stents, so it may be that sicker patients with more severe airway obstruction were more likely to have metal stents. Similarly, it may be that physicians placed silicone stents in patients they believed were likely to respond to treatment, since silicone stents are easier to remove. Thus, for the outcome of overall survival, it may be that the associations observed are more a function of patient selection than the stents themselves. However, we did control for many patient and situational variables, such as stent location, type(s), and timing of adjunctive treatments, and type of obstruction. In addition, although such selection bias would likely impact survival, it would not likely affect infection rates and/or granulation tissue formation. In addition, for the outcome of infection, such selection biases would favor silicone stents over metal stents, but our data did not suggest a benefit for silicone stents. This was also a single-center study focusing on malignant airway obstruction. We do not know if the incidence rates of complications are different in patients with benign disease or with other types of stents, so the results should be interpreted cautiously. Additional multicenter studies will be needed to evaluate stent complications in other populations. Finally, follow-up bronchoscopies were only performed when clinically significant symptoms were present. We may have underestimated the occurrence of asymptomatic complications, such as granulation tissue. However, we reviewed all CT images performed subsequent to airway stenting. Because these patients all had malignancy, frequent chest CT imaging was performed for other reasons and did not demonstrate a significant incidence of undetected asymptomatic airway obstruction. Previous studies have shown that CT imaging can detect up to 97% of stent complications; thus, the probability that a large number of complications were missed is small.27‐29
In summary, our findings reveal that not all airway stents are equivalent. Rates of respiratory infections were higher in patients with Aero stents, migration rates were higher with silicone stents, and granulation tissue was more common with both of these than with Ultraflex stents. Stent infection and repetitive motion may be related to the development of granulation tissue. Our findings also highlight the need for better postmarketing surveillance to validate whether existing standards for mechanical testing and airway stent manufacturing are truly correlated with clinical outcomes. Registry data may be useful in this regard, but sufficient detail and follow-up duration must be available to capture long-term outcomes.11,30
Acknowledgments
Drs Ost and Shah contributed equally to this project.
Author contributions: Dr Ost: contributed to study concept, design, data audits, analysis, statistics, writing, and overall study supervision.
Dr Shah: contributed to data collection, interpretation, and analysis and revision of the manuscript.
Dr Lei: contributed to statistical analysis and models, interpretation, and writing of the manuscript.
Dr Godoy: contributed to radiology reading, data collection, and revision of the manuscript.
Dr Jimenez: contributed to data interpretation, revision, and collection and revision of the manuscript.
Dr Eapen: contributed to data interpretation, revision, and collection and revision of the manuscript.
Dr Jani: contributed to data collection and auditing and revision of the manuscript.
Mr Larson: contributed to data collection and interpretation and revision of the manuscript.
Dr Sarkiss: contributed to writing and revision of the manuscript.
Dr Morice: contributed to editing, interpretation, and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: All work was performed at The University of Texas MD Anderson Cancer Center.
Additional information: The e-Figures and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- HR
hazard ratio
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 2.Breitenbücher A, Chhajed PN, Brutsche MH, Mordasini C, Schilter D, Tamm M. Long-term follow-up and survival after Ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration. 2008;75(4):443–449. doi: 10.1159/000119053. [DOI] [PubMed] [Google Scholar]
- 3.Wassermann K. How much incidence is enough? Chest. 2001;120(2):686–687. doi: 10.1378/chest.120.2.686-a. [DOI] [PubMed] [Google Scholar]
- 4.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010;182(5):589–597. doi: 10.1164/rccm.201002-0186CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrafiotis M, Siempos II, Falagas ME. Infections related to airway stenting: a systematic review. Respiration. 2009;78(1):69–74. doi: 10.1159/000213244. [DOI] [PubMed] [Google Scholar]
- 6.Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg. 2007;84(6):1870–1877. doi: 10.1016/j.athoracsur.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Shin JH, Song HY, Shim TS, Yoon CJ, Ko GY. Benign tracheobronchial strictures: long-term results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol. 2007;188(4):1033–1038. doi: 10.2214/AJR.06.0888. [DOI] [PubMed] [Google Scholar]
- 8.Husain SA, Finch D, Ahmed M, Morgan A, Hetzel MR. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg. 2007;83(4):1251–1256. doi: 10.1016/j.athoracsur.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 9.Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg. 2011;6:46. doi: 10.1186/1749-8090-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124(5):1993–1999. doi: 10.1378/chest.124.5.1993. [DOI] [PubMed] [Google Scholar]
- 11.Ernst A, Simoff M, Ost D, Goldman Y, Herth FJ. Prospective risk-adjusted morbidity and mortality outcome analysis after therapeutic bronchoscopic procedures: results of a multi-institutional outcomes database. Chest. 2008;134(3):514–519. doi: 10.1378/chest.08-0580. [DOI] [PubMed] [Google Scholar]
- 12.Alazemi S, Lunn W, Majid A, et al. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest. 2010;138(2):350–356. doi: 10.1378/chest.09-2682. [DOI] [PubMed] [Google Scholar]
- 13.Lunn W, Feller-Kopman D, Wahidi M, Ashiku S, Thurer R, Ernst A. Endoscopic removal of metallic airway stents. Chest. 2005;127(6):2106–2112. doi: 10.1378/chest.127.6.2106. [DOI] [PubMed] [Google Scholar]
- 14.Nouraei SA, Petrou MA, Randhawa PS, Singh A, Howard DJ, Sandhu GS. Bacterial colonization of airway stents: a promoter of granulation tissue formation following laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 2006;132(10):1086–1090. doi: 10.1001/archotol.132.10.1086. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki CT, Horiuchi M, Koss N. Tracheostomy-related subglottic stenosis: bacteriologic pathogenesis. Laryngoscope. 1979;89(6 pt 1):857–865. doi: 10.1288/00005537-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Schmäl F, Fegeler W, Terpe HJ, Hermann W, Stoll W, Becker K. Bacteria and granulation tissue associated with Montgomery T-tubes. Laryngoscope. 2003;113(8):1394–1400. doi: 10.1097/00005537-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ. Epidemiology. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 18.Chung FT, Lin SM, Chou CL, et al. Factors leading to obstructive granulation tissue formation after ultraflex stenting in benign tracheal narrowing. Thorac Cardiovasc Surg. 2010;58(2):102–107. doi: 10.1055/s-0029-1186266. [DOI] [PubMed] [Google Scholar]
- 19.Chung FT, Lin SM, Chen HC, et al. Factors leading to tracheobronchial self-expandable metallic stent fracture. J Thorac Cardiovasc Surg. 2008;136(5):1328–1335. doi: 10.1016/j.jtcvs.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Shlomi D, Peled N, Shitrit D, Bendayan D, Amital A, Kramer MR. Protective effect of immunosuppression on granulation tissue formation in metallic airway stents. Laryngoscope. 2008;118(8):1383–1388. doi: 10.1097/MLG.0b013e318172d686. [DOI] [PubMed] [Google Scholar]
- 21.Burningham AR, Wax MK, Andersen PE, Everts EC, Cohen JI. Metallic tracheal stents: complications associated with long-term use in the upper airway. Ann Otol Rhinol Laryngol. 2002;111(4):285–290. doi: 10.1177/000348940211100401. [DOI] [PubMed] [Google Scholar]
- 22.Chhajed PN, Malouf MA, Tamm M, Spratt P, Glanville AR. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest. 2001;120(6):1894–1899. doi: 10.1378/chest.120.6.1894. [DOI] [PubMed] [Google Scholar]
- 23.Simoni P, Wiatrak BJ. Microbiology of stents in laryngotracheal reconstruction. Laryngoscope. 2004;114(2):364–367. doi: 10.1097/00005537-200402000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Grewe PH, Müller KM, Lindstaedt M, et al. Reaction patterns of the tracheobronchial wall to implanted noncovered metal stents. Chest. 2005;128(2):986–990. doi: 10.1378/chest.128.2.986. [DOI] [PubMed] [Google Scholar]
- 25.Korpela A, Aarnio P, Sariola H, Törmälä P, Harjula A. Comparison of tissue reactions in the tracheal mucosa surrounding a bioabsorbable and silicone airway stents. Ann Thorac Surg. 1998;66(5):1772–1776. doi: 10.1016/s0003-4975(98)00763-2. [DOI] [PubMed] [Google Scholar]
- 26.Noppen M, Piérard D, Meysman M, Claes I, Vincken W. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med. 1999;160(2):672–677. doi: 10.1164/ajrccm.160.2.9812081. [DOI] [PubMed] [Google Scholar]
- 27.Ferretti GR, Kocier M, Calaque O, et al. Follow-up after stent insertion in the tracheobronchial tree: role of helical computed tomography in comparison with fiberoptic bronchoscopy. Eur Radiol. 2003;13(5):1172–1178. doi: 10.1007/s00330-003-1820-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee KS, Lunn W, Feller-Kopman D, Ernst A, Hatabu H, Boiselle PM. Multislice CT evaluation of airway stents. J Thorac Imaging. 2005;20(2):81–88. doi: 10.1097/01.rti.0000149789.28967.03. [DOI] [PubMed] [Google Scholar]
- 29.Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol. 2008;191(5):1576–1580. doi: 10.2214/AJR.07.4031. [DOI] [PubMed] [Google Scholar]
- 30.Ernst A, Simoff M, Ost D, Michaud G, Chandra D, Herth FJ. A multicenter, prospective, advanced diagnostic bronchoscopy outcomes registry. Chest. 2010;138(1):165–170. doi: 10.1378/chest.09-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]