Abstract
Purpose:
The purpose of this study was to evaluate the early and midterm results of superficial femoral artery (SFA) stenting with self-expanding nitinol stents and to identify the factors affecting patency.
Materials and Methods:
SFA stenting was performed in 165 limbs of 117 patients from January 2009 to December 2013. Patients were followed-up for the first occurrence of occlusion or stenosis based on computed tomography and duplex scan results and a decrease in ankle brachial index of >15%.
Results:
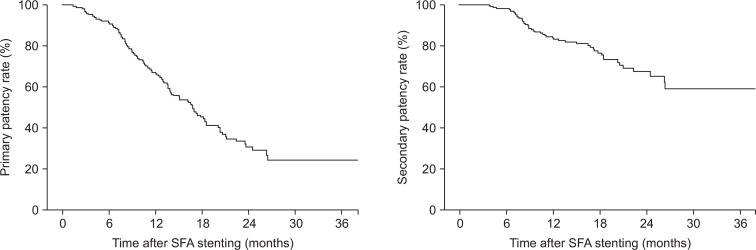
During the follow-up period (mean, 15.3±3.2 months), no early thrombotic reocclusions occurred within 30 days, but in-stent restenosis developed in 78 limbs. The primary patency rates at 6, 12, 18, and 24 months were 78%, 66%, 42%, and 22%, respectively, and the secondary patency rates were 85%, 72%, 58%, and 58%, respectively. TASC II C or D lesions, stent length >8 cm, number of patent tibial arteries and diabetes were significantly associated with reintervention.
Conclusion:
The midterm results of stenting for SFA occlusive disease were disappointing because the primary and secondary patency rates at two years were 22% and 58%, respectively. Reintervention after SFA stenting remains a major problem, particularly in patients with diabetes mellitus or long TASC II C or D lesions.
Keywords: Femoral artery, Peripheral arterial occlusive disease, Stents, Endovascular procedures
INTRODUCTION
Endovascular treatment (EVT) of superficial femoral artery (SFA) disease is common. A paradigm shift has occurred with respect to the role of by ass surgery in the treatment of SFA occlusive disease. Percutaneous transluminal angioplasty (PTA) for the revascularization of the SFA can achieve initial technical success rates of >95% with a low risk of complications [1]. Recent data suggest that stents may also improve the durability of SFA interventions, and might even be able to replace open bypass surgery. However, SFA stenting has many limitations such as stenosis within the stent, fracture, and occlusion. Six-month restenosis rates of approximately 10% were reported for nitinol stents with an average length of 80 mm. After one year of treatment, fractures were observed in approximately 10% of the patients with long lesions (over 10 cm) [1–3].
No reports have been published regarding the midterm results of SFA stenting in Korea. The purpose of this study was to evaluate the early and midterm results of SFA stenting with self-expanding nitinol stents, and to identify factors affecting patency rates.
MATERIALS AND METHODS
We retrospectively reviewed a database of patients who underwent EVT for SFA occlusions from January 2009 to December 2013 in Inha University Hospital (Incheon, Korea). We excluded patients with a history of revascularization intervention such as bypass, angioplasty, or stenting in the SFA, and patients treated for acute limb ischemia. We also excluded patients who only underwent PTA.
The indications for EVT were SFA lesions with TASC II A, B, or C lesions and TASC II D lesions with high levels of operative risk (American Society of Anesthesiologists score >3). Our indications for SFA stenting among SFA EVTs were patients with residual stenosis covering >30% of the lumen after PTA, intimal flap, dissection, ulcerative plaque, and subintimal angioplasty.
All patients were prescribed with antiplatelet or anticoagulation and lipid-lowering medications after the procedure. Stent surveillance was performed using the ankle brachial index (ABI) after stenting and at 3, 6, 12, 18 and 24 months, and computed tomography (CT) angiography or duplex scans were performed at 12 and 24 months after stenting. Reintervention was performed when occlusion or stenosis was observed on CT and duplex scans along with a decrease of >15% in the ABI score.
The reintervention modalities used were EVT, bypass surgery, and conservative treatment. If EVT failed, we performed bypass surgery. However, if patients did not want to undergo bypass surgery or EVT, we performed conservative treatment.
We reviewed preoperative data (sex, indication, comorbidities, ABI, TASC II classification, length of stenosis, number of patent tibial arteries), perioperative data (stent type and number, subintimal angioplasty, concurrent treatment), and postoperative follow-up data (ABI, CT angiography) from hospital records.
Our primary endpoint was to determine the primary and secondary patency rates and the secondary endpoint was to analyze the risk factors related with reintervention.
The chi-square test and Student’s t-test were used to compare characteristics between the patent and reintervention groups. Primary and secondary patency rates were calculated using the Kaplan-Meyer method. To determine risk factors for reintervention after SFA stenting, we also conducted multivariate analysis using the patients’ characteristics, lesion variables, and procedural variables. Multivariate analysis was conducted using a Cox proportional hazard model IBM SPSS Statistice 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
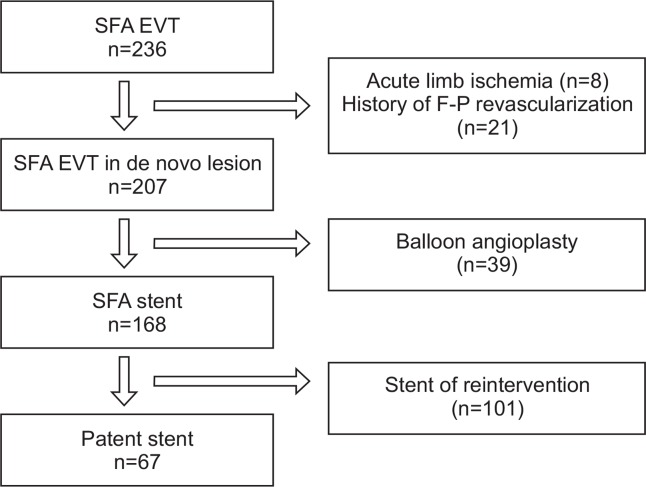
We performed EVT on 236 limbs from January 2009 to December 2013 at a single institution. We excluded 8 limbs due to acute limb ischemia and 21 limbs due to history of femoropopliteal revascularization. We also excluded 39 limbs that were treated by only PTA. Finally, a total of 168 limbs underwent SFA stent insertion (Fig. 1). In these 168 cases, the mean patients’ age was 72.3 years (range, 41–88 years), and 139 limbs were from male patients (82.7%). Mean preoperative ABI was 0.52. The TASC II A or B group lesions were found in 89 limbs (53.0%), and the TASC II C or D group lesions were found in 79 limbs (47.0%) (Table 1).
Fig. 1.
Result of endovascular treatment in superficial femoral artery. SFA, superficial femoralartery; EVT, endovascular treatment.
Table 1.
The characteristics of patients who underwent superficial femoral artery stenting (n=168)
| Variable | Data |
|---|---|
| Demographics | |
| Age (y) | 72.3±9.32 (41–88) |
| Sex, male | 139 (82.7) |
| Indication | |
| Claudication | 121 (72.0) |
| Critical limb ischemia | 47 (28.0) |
| Preoperative ABI | 0.52±0.23 |
| TASC II classification | |
| A or B | 89 (53.0) |
| C or D | 79 (47.0) |
| Comorbidities | |
| Smoking | 99 (58.9) |
| Hypertension | 124 (71.4) |
| Coronary artery disease | 24 (14.2) |
| Diabetes mellitus | 31 (18.4) |
| Cerebrovascular disease | 13 (17.1) |
| Chronic renal failure | 12 (7.1) |
| Hyperlipidemia | 46 (27.6) |
Values are presented as mean±standard deviation (range) or number (%).
Mean lesion length was 1,032 mm (range, 2.5–18.9 mm) and mean reference vessel diameter was 60.2 mm (range, 4.3–7.9 mm). The mean number of tibial arteries per patient was 1.93 (range, 0–3). The mean number of stents used was 1.4 (range, 1–3), and subintimal angioplasty was performed on 78 limbs (46.4%). S.M.A.R.T stents (Cordis, Bridgewater, MJ, USA) were used in 70 limbs (41.7%), Absolute stents (Abbott, Santa Clara, CA, USA) in 42 limbs (25.0 %), Complete stents (Medtronic, Minneapolis, MN, USA) in 17 limbs (10.0%), and Zilver stents (Cook, Limerick, Ireland) in 21 limbs (12.5%). During SFA stenting, simultaneous iliac stenting or PTA was performed in 39 limbs (23.2%), PTA below the knee artery in 21 limbs (12.5%), and endarterectomy in six limbs (3.8%) (Table 2).
Table 2.
The characteristics of lesion and procedure what underwent
| Variable | Data |
|---|---|
| Lesion length (mm) | 103.2±78.0 |
| Reference vessel diameter (mm) | 6.02±0.73 |
| Patent tibial artery (n) | 1.93±0.54 |
| Stent (n) | 1.4±0.72 |
| Subintimal angioplasty | 78 (46.4) |
| Type of nitinol stent | |
| S.M.A.R.T. | 70 (41.7) |
| Complete | 17 (10.1) |
| Absolute | 42 (25.0) |
| Zilver | 21 (12.5) |
| Combined | 18 (10.7) |
| Concurrent treatment | |
| Iliac stent/PTA | 39 (23.2) |
| Below-the-knee PTA | 21 (12.5) |
| Endaterectomy | 6 (3.6) |
Values are presented as mean±standard deviation or number (%). PTA, percutaneous transluminal angioplasty.
S.M.A.R.T stent: Cordis, Bridgewater, MJ, USA; Complete stent: Medtronic, Minneapolis, MN, USA; Absolute stent: Abbott, Santa Clara, CA, USA; Zilver: Cook, Limerick, Ireland.
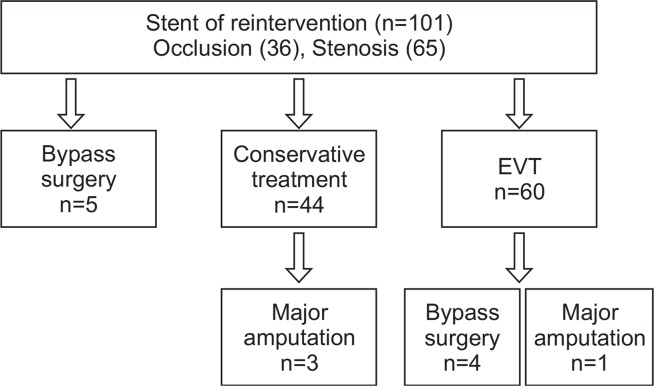
During a mean follow-up period of 15.82 months (range, 1–33 months), ABI was performed in all patients (mean number of ABIs per patient, 3.74). CT angiography was performed more than once in 122 patients and duplex sonography was performed more than once in 17 patients. During follow-up, 101 limbs (60.1%) required reintervention and 67 limbs (39.9%) remained patent without reintervention. Occlusion occurred in 36 limbs and severe stenosis in 65 limbs in the reintervention group. In the reintervention group, bypass surgery was performed on five limbs, EVT on 60 limbs, and conservative treatment on 44 limbs (Fig. 2). The primary patency rates at 6, 12, 18, and 24 months were 78%, 66%, 42%, and 22%, while the secondary patency rates were 85%, 72%, 58%, and 58% at these same time points, respectively (Fig. 3).
Fig. 2.
Result of reintervention after superficial femoral artery stenting. EVT, endovascular treatment.
Fig. 3.
Primary patency rate and secondary patency rate of superficial femoral artery stenting. SFA, superficial femoral artery.
Multivariate analysis revealed that the factors significantly associated with stent reintervention were TASC C or D lesions (hazard ratio [HR], 3.103; 95% confidence interval [CI], 1.676–5.745; P<0.01), stent length >8 cm (HR, 2.904; 95% CI, 1.161–5.197; P=0.048), number of patent tibial arteries (HR, 0.349; 95% CI, 0.125–0.783; P=0.028), and diabetes (HR, 3.420; 95% CI, 1.428–5.774; P=0.035) (Table 3).
Table 3.
Univariate analysis and multivariate analysis of the factor associated to reintervention of superficial femoral artery stenting
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | - | - | 1.019 (0.988–1.050) | 0.239 |
| Sex, male | 1.402 (0.548–3.587) | 0.438 | 2.319 (0.891–6.034) | 0.085 |
| Lesion length >8 cm | 2.624 (1.178–4.389) | 0.042 | 2.904 (1.161–5.197) | 0.048 |
| TASC II C or D | 2.050 (1.044–3.689) | 0.031 | 3.103 (1.676–5.745) | 0.000 |
| Concurrent procedure | 0.894 (0.428–1.945) | 0.614 | 0.938 (0.537–1.865) | 0.752 |
| Critical limb ischemia | 1.161 (0.604–2.073) | 0.378 | 1.029 (0.578–1.830) | 0.924 |
| Patent tibial artery | - | - | 0.349 (0.125–0.783) | 0.028 |
| Hypertension | 1.524 (0.092–1.946) | 0.432 | 0.788 (0.437–1.419) | 0.427 |
| Chronic renal failure | 1.723 (0.282–2.912) | 0.329 | 1.129 (0.387–3.209) | 0.459 |
| Diabetes | 2.503 (1.256–3.888) | 0.039 | 3.420 (1.428–5.774) | 0.035 |
| Smoking | 1.144 (0.618–2.105) | 0.396 | 1.163 (0.620–2.182) | 0.638 |
HR, hazard ratio; CI, confidence interval.
DISCUSSION
Many treatment modalities besides open bypass surgery exist for the treatment of occlusive diseases, such as plain balloons, stents, drug coated balloons, drug eluting stents, stent-grafts, and atherectomy devices. Many of these newly developed devices are supported by a large amount of data. Early results of these new devices have been surprising. The primary patency rate at one year was >80%. However, the patency rates at the two-year mark were disappointing compared with those of open surgery. Although the clinical experience of EVT in SFA occlusive disease is increasing in Korea, outcome of SFA stenting have not been widely reported.
Our study reported that primary patency rates were 78%, 66%, 42%, and 22%, and that secondary patency rates were 85%, 72%, 58%, and 58% at 6, 12, 18, and 24 months after SFA stenting. These results were similar to one-year patency rates of a Western study. Furthermore, the patency rates of new devices were also similar to those in the present study at the one-year mark. Stabile et al. [4] reported that the primary and secondary patency rates of drug-coated balloon (DCB) at 12 months were 92.1%. Tepe et al. [5] reported that the primary patency rate of DCBs was 82.2% at 12 months compared to 52.5% for primary balloon angioplasty. However, the results of this study at the one-year mark were disappointing compared to the results of studies assessing other devices. With new and more flexible stents, Werner et al. [6] reported a 24-month primary patency rate of 72%, and a secondary patency rate of 92%. With drug eluting or coating balloons, Scheinert et al. [7] reported a 24-month primary patency rate of 73%. Furthermore, Ohki et al. [8] reported a 48-month primary patency rate of 75% with drug-eluting stents.
The patency rates of SFA stenting over 24 months were lower than those of open surgical bypass in almost all reports. Our report also demonstrates the limitations of bare metal stents for the treatment of SFA. The new endovascular modalities such as drug-eluting balloons, drug -eluting stents, next generation stents, and atherectomy devices should replace bare metal stents for the treatment of SFA.
We also found that stent length >8 cm, the number of patent tibial arteries, diabetes, and TASC II C/D lesions were risk factors for reintervention. In another study, female sex, critical limb ischemia, and TASC II C/D were identified as risk factors for in-stent occlusion and poor outcomes such as restenosis [9–11]. More attention should be paid to EVT as an alternative to open bypass surgery for the treatment of SFA occlusions with risk factors such as long lesions >8 cm, fewer patent tibial arteries, and diabetes.
The present study has several limitations, including the retrospective design, different indications for intervention and device selection, and loss of patients to follow-up. However, our study is the first to report results of stenting of SFA lesions in Korea.
CONCLUSION
In our study, the midterm results of bare metal stent use in SFA occlusive disease were disappointing. The use of bare metal stents for the treatment of SFA should be avoided in patients with diabetes mellitus and long TASC II C or D lesions with few patent arteries.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Schlager O, Dick P, Sabeti S, Amighi J, Mlekusch W, Minar E, et al. Long-segment SFA stenting–the dark sides: in-stent restenosis, clinical deterioration, and stent fractures. J Endovasc Ther. 2005;12:676–684. doi: 10.1583/05-1672.1. [DOI] [PubMed] [Google Scholar]
- 2.Sabeti S, Mlekusch W, Amighi J, Minar E, Schillinger M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J Endovasc Ther. 2005;12:6–12. doi: 10.1583/04-1359.1. [DOI] [PubMed] [Google Scholar]
- 3.Jens S, Conijn AP, Koelemay MJ, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta-analysis (part 1: above the knee) Eur J Vasc Endovasc Surg. 2014;47:524–535. doi: 10.1016/j.ejvs.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Stabile E, Virga V, Salemme L, Cioppa A, Ambrosini V, Sorropago G, et al. Drug-eluting balloon for treatment of superficial femoral artery in-stent restenosis. J Am Coll Cardiol. 2012;60:1739–1742. doi: 10.1016/j.jacc.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. IN.PACT SFA Trial Investigators Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner M, Paetzold A, Banning-Eichenseer U, Scheinert S, Piorkowski M, Ulrich M, et al. Treatment of complex atherosclerotic femoropopliteal artery disease with a self-expanding interwoven nitinol stent: midterm results from the Leipzig SUPERA 500 registry. EuroIntervention. 2014;10:861–868. doi: 10.4244/EIJV10I7A147. [DOI] [PubMed] [Google Scholar]
- 7.Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10–19. doi: 10.1016/j.jcin.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Ohki T, Yokoi H, Kichikawa K, Kimura T, Snyder SA, Ragheb AO, et al. Two-year analysis of the Japanese cohort from the Zilver PTX randomized controlled trial supports the validity of multinational clinical trials. J Endovasc Ther. 2014;21:644–653. doi: 10.1583/14-4753.1. [DOI] [PubMed] [Google Scholar]
- 9.Pulli R, Dorigo W, Pratesi G, Fargion A, Angiletta D, Pratesi C. Gender-related outcomes in the endovascular treatment of infrainguinal arterial obstructive disease. J Vasc Surg. 2012;55:105–112. doi: 10.1016/j.jvs.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Hunink MG, Donaldson MC, Meyerovitz MF, Polak JF, Whittemore AD, Kandarpa K, et al. Risks and benefits of femoropopliteal percutaneous balloon angioplasty. J Vasc Surg. 1993;17:183–192. doi: 10.1016/0741-5214(93)90022-E. discussion 192–194. [DOI] [PubMed] [Google Scholar]
- 11.Conrad MF, Cambria RP, Stone DH, Brewster DC, Kwolek CJ, Watkins MT, et al. Intermediate results of percutaneous endovascular therapy of femoropopliteal occlusive disease: a contemporary series. J Vasc Surg. 2006;44:762–769. doi: 10.1016/j.jvs.2006.06.025. [DOI] [PubMed] [Google Scholar]