Abstract
This study aims to assess the treatment effects of the molecular adsorbent recirculating system (MARS) in patients with acute and acute-on-chronic liver failure. We searched MEDLINE, EMBASE, and the Cochrane Controlled Trials Registry database between January 1966 and January 2014. We included randomized controlled trials, which compared the treatment effects of MARS with standard medical treatment. Study quality assessed according to Consolidated Standards of Reporting Trials (CONSORT) criteria. The risk ratio was used as the effect-size measure according to a fixed-effects model. The search strategy revealed 72 clinical studies, 10 of which were randomized controlled trials that met the criteria and were included. Four addressed ALF (93 patients) and six addressed AOCLF (453 patients). The mean CONSORT score was 15 (range 10-20). By meta-analysis, MARS significantly improved survival in ALF (risk ratio 0.61; 95% CI 0.38, 0.97; P = 0.04). There was no significant survival benefit in AOCLF (risk ratio 0.88; 95% CI 0.74, 1.06; P = 0.16). MARS significantly improved survival in patients with acute liver failure, however, there is no evidence that it improved survival in patients with acute-on-chronic liver failure. In conclusion, the present meta-analysis indicates that MARS therapy can improve survival in patients with ALF. It is necessary to develop MARS treatment because of the increasing demand for liver transplantation and the risk of liver failure.
Keywords: Hepatic failure, liver-assisted device, liver failure, meta-analysis, molecular adsorbent recirculating system
Introduction
Liver failure describes the development of hepatic encephalopathy, jaundice, and coagulopathy, which lead to multi-organ failure with an exceedingly high mortality rate [1]. Liver failure is divided into two types: acute liver failure (ALF) and acute-on-chronic liver failure (AOCLF). ALF is defined as the onset of coagulopathy and encephalopathy within eight weeks of symptom presentation in an individual with no known underlying liver disease [2]. AOCLF refers to an acute deterioration in liver function in a patient with previously well-compensated chronic liver disease caused by a precipitating event, such as sepsis or upper gastrointestinal bleeding [3].
Liver transplantation for organ failure is currently the most effective method to improve survival [4,5]. However, a large number of patients die before a transplant organ becomes available, due to the shortage of donor grafts [6]. Therefore, liver support systems have attracted more and more focus as an alternative or a bridge to liver transplantation. Liver support is provided by bioartificial (those involving living hepatocytes) and artificial (noncellular) systems. The molecular adsorbent recirculating system (MARS; Gambro Lundia, Lund, Sweden) is an artificial liver support system that provides detoxification via membranes and adsorbents. MARS has been in clinical use since 1996 and is currently one of the most extensively used liver support systems [7,8]. It has been used to reduce the serum levels of bilirubin, bile acids, ammonia, urea, lactate, and creatinine in patients with both ALF and AOCLF [8-10]. However, its survival impact is not well known. Several randomized clinical trials have indicated that albumin dialysis may improve survival in AOCLF [10-12], and other trials have demonstrated that MARS significantly improves survival in ALF [13,14]. We performed a systematic review and meta-analysis to evaluate the effect of MARS treatment for patients with ALF and AOCLF.
Materials and methods
The meta-analyses were performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15].
Literature search and eligibility criteria
Clinical trials citing the MeSH terms and key words “liver failure” or “hepatic failure”, “liver”, “artificial”, “liver-assisted device”, and “MARS” or “Molecular Adsorbent Recirculating System” were identified by searching the MEDLINE and EMBASE databases and the Cochrane database of randomized controlled trials (RCTs) for articles published between January 1966 and January 2014.
We applied the following selection criteria: i) study design: randomized controlled trials; ii) language of publication: English and other languages; iii) study population: patients with ALF or AOCLF; and iv) study groups: intervention group, MARS; control group, standard medical treatment.
We applied the following exclusion criteria: i) study design: non-randomized concurrent control trials; ii) the studies writing in Chinese; iii) the studies involve the patients with chronic liver failure.
Study selection and data extraction
Two independent reviewers (Feng L, Hu X) screened the titles and abstracts of all citations. The full-text articles were retrieved for comprehensive review and rescreened. The following data from full-text articles were extracted: country of origin, year published, period of study, duration of follow-up, population setting (ALF or AOCLF), patient characteristics, and characteristics of MARS. The primary outcome measure was cause of death. Secondary outcome measures included effects on hepatic encephalopathy and bilirubin levels. Safety endpoints were also extracted, including data on hemodynamic instability, prothrombin activity, and other adverse events. Disagreements were resolved through consensus.
Quality assessment
Both reviewers assessed the overall quality of the studies using the Consolidated Standards of Reporting Trials (CONSORT) criteria [16]. The mean CONSORT score was calculated for each trial; a score of 22 was considered to correspond to the “best” quality on a 0-22 scale.
Statistical analysis
RevMan 5.1 (17) was used for statistical analysis in this meta-analysis. The results were calculated as a risk ratio (RR) with a 95% confidence interval (CI) using the fixed effects model, which aims to estimate effectiveness. Statistical significance was considered for 95% CIs that did not include 1. Statistical heterogeneity between studies was evaluated by the Cochran Q test and I2 index. Clinical heterogeneity was assessed by reviewing patient characteristics and study design. Publication bias was investigated by Begg’s and Egger’s tests of funnel plots. A P < 0.05 was considered significant.
A sensitivity analysis was conducted using STATA, version 12.0 (Stata Corp., College Station, TX, USA) to assess the stability of conclusions. Subgroup analyses of patients with AOCLF and ALF were conducted in order to describe differences in the effects of intervention.
Results
Patient characteristics
A total of 72 articles were identified for review (Figure 1). Ten randomized trials [11-14,18-23] met the criteria for inclusion, which included 546 patients; 269 (49.2%) had received MARS treatment and 277 (50.8%) had received standard medical treatment (Table 1). Of these, six trials included 453 patients with AOCLF. The etiologies of hepatic cirrhosis in these patients included hepatitis virus infections, drugs, Budd-Chiari syndrome, toxicity, Wilson’s disease, biliary cirrhosis, and alcohol, which was the most important factor. Another four trials included 93 patients with ALF. The etiology of ALF was related to paracetamol/acetaminophen (n = 46), cardiogenic shock (n = 44), hepatitis B infection (n = 2), and disulfiram (n = 1). All trials used MARS plus standard medical treatment or single MARS treatment and standard medical treatment for the control.
Figure 1.
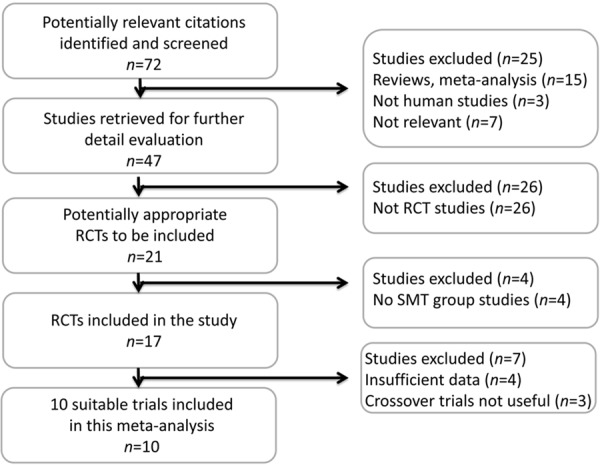
Trial flow diagram. The meta-analyses were performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and (Tunon, Alvarez et al. 2009) Meta-Analyses (PRISMA) statement. RCT, randomized controlled trial; SMT, standard medical therapy.
Table 1.
Characteristics of randomized controlled trials included in the meta-analysis
| Reference | Primary outcome | Follow-up | Etiology of liver failure | MARS: SMT (n) | RP (mo) | Treatment | Centers (n) | Treatment regimen | CONSORT score | Allocation | Adverse events (n) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Active | Control | |||||||||||
| Mitzner et al. 2000 [12] | 30 d survival or turn to OLT | 30 d | ALD, chronic HBV, Budd-Chiari/chronic HBV, PBC, secondary biliary cirrhosis | 8:5 | 24 | MARS + HDF + SMT | HDF + SMT | 2 | 6-8 h/treatment, bilirubin level not increased | 16 | Sealed envelope | Severe hepatic encephalopathy (1), mild thrombocytopenia |
| Heemann et al. 2002 [11] | Serum bilirubin | 30 d | Alcoholic hepatitis, HCV, HBV | 12:12 | NR | MARS | SMT | 1 | 8 h/treatment for 3 consecutive d | 17 | Sealed envelope | Anemia (9), hemorrhage (2), hypotension (2), coagulopathy (3), dyspnea (1), paresthesia |
| El Banayosy et al. 2002 [13] | Survival | NR | Cardiogenic shock | 8:9 | 12 | MARS | SMT | 1 | 8 h/d for 3 d, bilirubin < 6 mg/dL | 12 | NR | Thrombocytopenia |
| Schmidt et al. 2003 [19] | Hemodynamics/oxygen consumption | NR | Acetaminophen, HBV, disulfiram | 8:5 | NR | MARS + SMT | SMT + TM | 1 | 6 h per treatment | 10 | NR | Arterial hypotension |
| El Banayosy et al. 2004 [14] | Survival | NR | Cardiogenic shock | 14:13 | 16 | MARS | SMT | 1 | 8 h/d for 3 d, bilirubin < 6 mg/dL | 11 | NR | Thrombocytopenia, hepatorenal syndrome |
| Sen et al. 2004 [18] | Clinical and biochemical | NR | Alcoholic liver disease | 9:9 | NR | MARS + SMT | SMT | 1 | 8 h/d for 7 d | 15 | Sealed envelope | Variceal bleeding, multiorgan failure |
| Hassanein et al. 2007 [23] | HE | 180 d | Cirrhosis and HE grade 3 or 4 | 39:31 | 39 | MARS + SMT | SMT | > 1 | 6 h/d for 5 d | 20 | Blinded envelope | Hepatic encephalopathy, GI bleeding, hypotension |
| Hessel et al. 2010 [22] | Survival | 3 y | Alcoholic liver disease, infections/intoxications, autoimmune hepatitis | 67:82 | 51 | MARS + SMT | SMT | 1 | until death | 14 | NR | NR |
| Saliba et al. 2013 [21] | Survival | 1 y | Paracetamol, non-paracetamol | 53:49 | 40 | MARS + SMT | SMT | 16 | 6 h/d for 3 d | 20 | Central automated system | Neurologic, psychiatric, and renal disorders |
| Banares et al. 2013 [20] | Survival | 90 d | Alcohol, HCV + alcohol | 90:89 | 71 | MARS + SMT | SMT | 19 | 6 h/d for 21 days | 19 | Randomization system | Bacterial infection, variceal bleeding, pneumonia |
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; GI, gastrointestinal; HBV, hepatitis B virus; HCV, hepatitis C virus; HDF, hemodiafiltration; HE, hepatic encephalopathy; MARS, molecular adsorbent recirculating system; NR, not reported; OLT, orthotopic liver transplantation; PBC, Primary biliary cirrhosis; RP, recruitment period; SMT, standard medical treatment; TM, temperature-matched.
Study quality
The overall average CONSORT score was 15. Seven of the trials used an intention-to-treat analysis. Randomization and sequence generation procedures were adequately reported. Details of allocation concealment were reported in five of the 10 included studies (Table 1).
Effect of MARS on mortality in ALF and AOCLF
We performed separate meta-analyses for the six trials including patients with AOCLF and the four trials including patients with ALF. MARS treatment significantly reduced mortality in ALF compared with standard medical treatment (RR = 0.61, Z = 2.07; P = 0.04) (Figure 2). There was no significant heterogeneity in effect size (χ2 = 2.25). In AOCLF, there was no beneficial effect on survival with MARS versus standard medical therapy (RR = 0.88, Z = 1.33; P = 0.18), with no significant heterogeneity.
Figure 2.
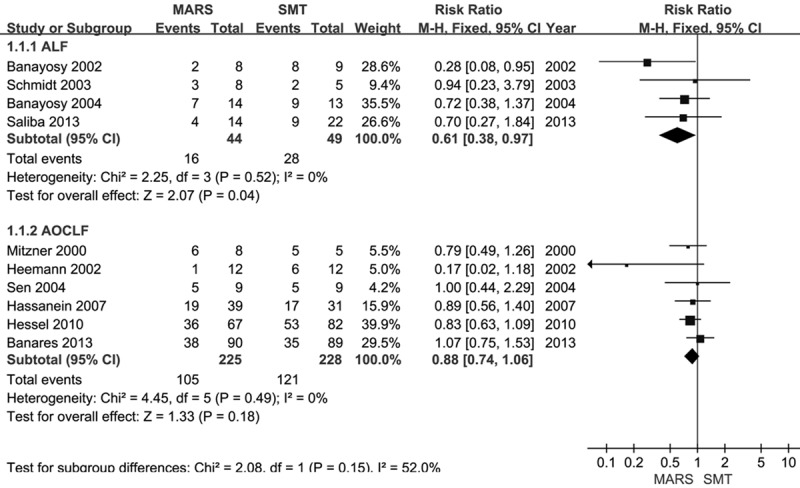
Forest plots showing risk ratios for studies comparing MARS with SMT in ALF and AOCLF. The fixed effects model method was used. ALF, acute liver failure; AOCLF, acute-on-chronic liver failure; CI, confidence interval; MARS, molecular adsorbent recirculating system; SMT, standard medical therapy.
Sensitivity analysis
A sensitivity analysis was performed to assess the stability of the conclusions, and no individual study significantly affected the values of the clinical events (Figure 3).
Figure 3.
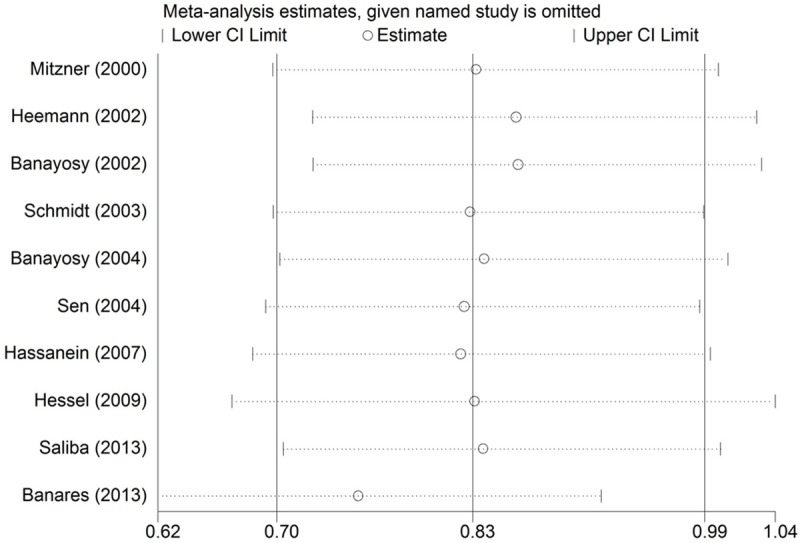
Sensitivity analysis of the summary relative risk of mortality for MARS. The two ends of the dotted lines represent the 95% confidence intervals (CI).
Publication bias
The funnel plot for publication bias is shown in Figure 4. The P values for Begg’s and Egger’s tests were 0.21 and 0.16, respectively (continuity-corrected), demonstrating no significant publication bias in this meta-analysis.
Figure 4.
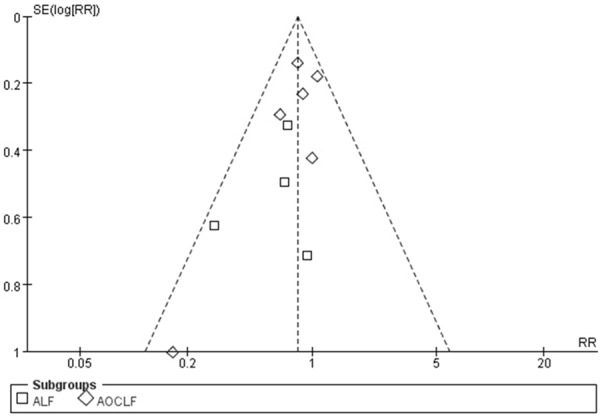
Funnel plot of the randomized controlled trials. ALF, acute liver failure; AOCLF, acute-on-chronic liver failure; RR, relative risk; SE, standard error.
Adverse events
The adverse events reported are shown in Table 2. El Banayosy et al. [13,14] reported that 12 patients receiving 91 MARS treatment sessions experienced 17 adverse events. The complications included bleeding, thrombocytopenia, coagulopathy, hypotension, fever, hepatorenal syndrome, and anemia. None of these resulted in death. In most of the trials, MARS treatments were well tolerated [11,12,19,20].
Table 2.
Outcomes of randomized controlled trials included in the meta-analysis according to significant benefit
| Reference | Overall survival | Subgroup survival | HE | Bilirubin | Prothrombin activity | Hemodynamics |
|---|---|---|---|---|---|---|
| Mitzner et al. [12] | No | NA | NA | Reduced serum bilirubin | Yes (increased) | NA |
| Heemann et al. [11] | Yes | NA | Yes | Reduced serum bilirubin | No | Yes (increased MAP with MARS) |
| El Banayosy et al. [13] | No | No | NA | Reduced serum bilirubin | No | NA |
| Schmidt et al. [19] | No | NA | NA | No | No | Yes (increased MAP with MARS) |
| El Banayosy et al. [14] | No | No | NA | Reduced serum bilirubin | No | NA |
| Sen et al. [18] | No | NA | Yes | No | No | Yes (not changed) |
| Hassanein et al. [23] | No | NA | Yes | No | NA | NA |
| Hessel et al. [22] | Yes | No | NA | No | NA | NA |
| Saliba et al. [21] | No | No | Yes | Reduced serum bilirubin | NA | NA |
| Banares et al. [20] | Yes | Yes (HE ≥ II, MELD > 20, HRS) | Yes | Increased serum bilirubin | No | No |
Abbreviations: HE, hepatic encephalopathy; HRS, hepatorenal syndrome; MAP, mean arterial pressure; MELD, Model for end-stage liver disease; NA, not assessed; No, no significant benefit; Yes, significant benefit demonstrated.
Discussion
Previous systematic reviews and meta-analyses indicated that MARS has no significant survival advantage in liver failure, though the findings were limited by study quality and the number of included patients [24,25]. This meta-analysis compared the effect of MARS therapy with standard medical treatment for liver failure reported in ten randomized trials comprising 546 patients. The test for subgroup differences indicated that the ALF and AOCLF groups were heterogeneous. Given the differences in disease etiology, management, and outcome, these two groups were therefore analyzed separately, in line with previous reports [24,26]. The data suggest that MARS treatment can significantly improve survival in ALF subgroups, while having no effect on survival in AOCLF subgroups.
ALF is a dramatic clinical syndrome characterized by sudden and massive hepatic necrosis that results in jaundice, coagulopathy, and hepatic encephalopathy in the absence of pre-existing liver disease [27]. In 2002, El Banayosy and colleagues [13] concluded that MARS significantly improved survival in 17 hypoxic liver failure patients. However, in 2013, Saliba et al. [21] concluded that MARS provided no definitive efficacy or safety advantages to 102 patients with ALF, likely because many of the patients had previously received transplants. Because of the number of patients with ALF, there should be more randomized trials to test this conclusion, as small RCTs can produce unreliable results. For AOCLF, there was no beneficial effect on survival with MARS versus standard medical therapy, consistent with results of three of the randomized trials [22,23], including the largest randomized trial on MARS to date (involving 179 patients) conducted by Banares and colleagues [20], as well as previous meta-analyses [24,26].
Bile acids and bilirubin are toxic to hepatocytes in vitro and in animal studies [28,29]. The use of MARS appeared to reduce serum bilirubin and hepatic encephalopathy in several studies [11-14,20,21], which may be attributed to MARS’ albumin dialysis component [30]. The clinical significance of this has been confirmed in previous meta-analyses and randomized trials.
The results of the present study are in agreement with a systematic review and meta-analysis conducted by Stutchfield et al. [31] that analyzed four types of liver support systems (two artificial and two bioartificial) in eight trials (five of which involved MARS) involving 332 patients with liver failure. Although their study demonstrated that liver support systems did not affect mortality, subgroup analyses similarly showed significantly reduced mortality in patients with ALF, but not with AOCLF. This is in contrast to the Cochrane review evaluating the use of extracorporeal liver support [32] and the meta-analysis by Kjaergard and colleagues [33]. Additional randomized trials are needed to test this conclusion. Vaid et al. [26] found that MARS treatment had no significant survival advantage in liver failure. However, the results of their meta-analysis should be viewed with caution because of the low Jadad score and the combination of ALF and AOCLF data.
The present meta-analysis indicates that MARS therapy can improve survival in patients with ALF. The available evidence does not suggest a significant survival benefit in those with AOCLF. It is necessary to develop MARS treatment because of the increasing demand for liver transplantation and the risk of liver failure.
The major limitation of this study was the small number of patients with ALF, which may lead to incorrect conclusions. In addition, the meta-analyses may be affected by bias. However, the studies included in this analysis were homogenous, and the sensitivity analysis revealed stability of the conclusions.
Disclosure of conflict of interest
None.
References
- 1.Li Y, Zhang Z, Shi J, Jin L, Wang L, Xu D, Wang FS. Risk factors for naturally-occurring early-onset hepatocellular carcinoma in patients with HBV-associated liver cirrhosis in China. Int J Clin Exp Med. 2015;8:1205–1212. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 4.Chan AC, Fan ST, Lo CM, Liu CL, Chan SC, Ng KK, Yong BH, Chiu A, Lam BK. Liver transplantation for acute-on-chronic liver failure. Hepatol Int. 2009;3:571–581. doi: 10.1007/s12072-009-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Grady JG. Acute liver failure. Postgrad Med J. 2005;81:148–154. doi: 10.1136/pgmj.2004.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pless G. Artificial and bioartificial liver support. Organogenesis. 2007;3:20–24. doi: 10.4161/org.3.1.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stange J, Mitzner SR, Klammt S, Freytag J, Peszynski P, Loock J, Hickstein H, Korten G, Schmidt R, Hentschel J, Schulz M, Lohr M, Liebe S, Schareck W, Hopt UT. Liver support by extracorporeal blood purification: a clinical observation. Liver Transpl. 2000;6:603–613. doi: 10.1053/jlts.2000.7576. [DOI] [PubMed] [Google Scholar]
- 8.Tan HK. Molecular adsorbent recirculating system (MARS) Ann Acad Med Singapore. 2004;33:329–335. [PubMed] [Google Scholar]
- 9.Inderbitzin D, Muggli B, Ringger A, Beldi G, Gass M, Gloor B, Uehlinger D, Regli B, Reichen J, Candinas D. Molecular absorbent recirculating system for the treatment of acute liver failure in surgical patients. J Gastrointest Surg. 2005;9:1155–1161. doi: 10.1016/j.gassur.2005.07.026. discussion 1161-1152. [DOI] [PubMed] [Google Scholar]
- 10.Jalan R, Sen S, Steiner C, Kapoor D, Alisa A, Williams R. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003;38:24–31. doi: 10.1016/s0168-8278(02)00334-3. [DOI] [PubMed] [Google Scholar]
- 11.Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M, Klammt S, Loehr M, Liebe S, Mitzner S, Schmidt R, Stange J. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002;36:949–958. doi: 10.1053/jhep.2002.36130. [DOI] [PubMed] [Google Scholar]
- 12.Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J, Hickstein H, Loock J, Lohr JM, Liebe S, Emmrich J, Korten G, Schmidt R. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–286. doi: 10.1002/lt.500060326. [DOI] [PubMed] [Google Scholar]
- 13.El Banayosy A, Kizner L, Schueler V, Bergmeier S, Cobaugh D, Koerfer R. The role of MARS in patients suffering from hypoxic liver failure secondary to cardiogenic shock. ITBM RBM. 2002;23(Suppl 1):61–66. [Google Scholar]
- 14.El Banayosy A, Kizner L, Schueler V, Bergmeier S, Cobaugh D, Koerfer R. First use of the Molecular Adsorbent Recirculating System technique on patients with hypoxic liver failure after cardiogenic shock. ASAIO J. 2004;50:332–7. doi: 10.1097/01.mat.0000131251.88146.cd. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 17.Review Manager (RevMan) [computer program] Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008. [Google Scholar]
- 18.Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, Jalan R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109–1119. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl. 2003;9:290–297. doi: 10.1053/jlts.2003.50051. [DOI] [PubMed] [Google Scholar]
- 20.Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–62. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 21.Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, Perrigault PF, Belnard M, Ichai P, Samuel D. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522–531. doi: 10.7326/0003-4819-159-8-201310150-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hessel FP, Bramlage P, Wasem J, Mitzner SR. Cost-effectiveness of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Eur J Gastroenterol Hepatol. 2010;22:213–220. doi: 10.1097/MEG.0b013e3283314e48. [DOI] [PubMed] [Google Scholar]
- 23.Hassanein TI, Tofteng F, Brown RS Jr, McGuire B, Lynch P, Mehta R, Larsen FS, Gornbein J, Stange J, Blei AT. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46:1853–1862. doi: 10.1002/hep.21930. [DOI] [PubMed] [Google Scholar]
- 24.Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10:1099–1106. doi: 10.1002/lt.20139. [DOI] [PubMed] [Google Scholar]
- 25.Atienza Merino G. [Evaluation of extracorporeal liver support systems in the treatment of liver failure. A systematic review] . Gastroenterol Hepatol. 2010;33:352–62. doi: 10.1016/j.gastrohep.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Vaid A, Chweich H, Balk EM, Jaber BL. Molecular adsorbent recirculating system as artificial support therapy for liver failure: a meta-analysis. ASAIO J. 2012;58:51–59. doi: 10.1097/MAT.0b013e31823fd077. [DOI] [PubMed] [Google Scholar]
- 27.Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36–45. doi: 10.1055/s-0032-1301733. [DOI] [PubMed] [Google Scholar]
- 28.Bomzon A, Holt S, Moore K. Bile acids, oxidative stress, and renal function in biliary obstruction. Semin Nephrol. 1997;17:549–562. [PubMed] [Google Scholar]
- 29.Chieco P, Romagnoli E, Aicardi G, Suozzi A, Forti GC, Roda A. Apoptosis induced in rat hepatocytes by in vivo exposure to taurochenodeoxycholate. Histochem J. 1997;29:875–883. doi: 10.1023/a:1026446008712. [DOI] [PubMed] [Google Scholar]
- 30.Drexler K, Baustian C, Richter G, Ludwig J, Ramlow W, Mitzner S. Albumin dialysis molecular adsorbents recirculating system: impact of dialysate albumin concentration on detoxification efficacy. Ther Apher Dial. 2009;13:393–398. doi: 10.1111/j.1744-9987.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 31.Stutchfield BM, Simpson K, Wigmore SJ. Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg. 2011;98:623–631. doi: 10.1002/bjs.7418. [DOI] [PubMed] [Google Scholar]
- 32.Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev. 2004:CD003628. doi: 10.1002/14651858.CD003628.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217–222. doi: 10.1001/jama.289.2.217. [DOI] [PubMed] [Google Scholar]


