Abstract
Exosomes are small membranous vesicles about 30~100 nm in diameter and formed from inward budding of the limiting membrane of multi-vesicular bodies (MVB). Exosomes are secreted by most cell types (including hepatocellular carcinoma cells) into the extracellular environment and can be isolated from various body fluids. Exosomes have broad biological function through delivering contained molecules to the target cells. Although limited studies on hepatocellular carcinoma (HCC) exosomes, increasing observations suggest that exosomes are important in HCC metastatic and prognosis, and exosomes are potential new molecular biomarkers for diagnosis and prognosis of HCC. In this review, we briefly summarize the latest findings on HCC exosomes, and their potential functions for novel diagnostic and therapeutic approaches of HCC.
Keywords: Exosomes, HCC, tumor metastasis, diagnosis, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent tumors worldwide and it has high rate of metastasis and poor prognosis. HCC is the leading cause of cancer-related deaths in many Asian countries and is the third most frequent cause of cancer deaths worldwide [1,2]. There were more than half a million deaths per year. Although surgical resection, transarterial chemo embolization (TACE) and liver transplantation may be effective therapeutic approaches of this disease, many patients are not eligible due to the advanced stage at diagnosis or insufficient liver function in the setting of cirrhosis [3,4]. Therefore, the identification of sensitive and specific biomarkers for detection of HCC or recurrence is urgently needed.
Exosomes are small membranous vesicles about 30~100 nm in diameter and formed from inward budding of the limiting membrane of multi-vesicular bodies (MVB). Exosomes are secreted into the extracellular environment by most cell types and can be isolated from various body fluids such as serum, urine, and malignant ascites [5-7]. Exosomes were firstly found in adult mammal reticulocyte; most prokaryotic and eukaryotic cells release exosomes, including cancer cells such as liver, lung, breast, ovarian, and melanoma [8]. Exosomes have broad biological function [9-11]. Different cellular exosomes contain a different set of molecules (proteins, mRNAs and miRNAs) which may reveal genetic information of their parent cells [12]. Exosomes play physiological roles through delivering these molecules to the target cells and they are important supporter for the transport of various biological molecules [9,10]. Previous studies found that exosomes may be involved in tumor cells communication, migration, tumor angiogenesis [10,13]. Liver cells are exosome-releasing cells as well as targets for exosomes derived from cells of other organs. Although limited studies on HCC exosomes, increasing observations suggest that exosomes are important in HCC metastatic and prognosis, and exosomes are potential new molecular biomarkers for diagnosis and prognosis of HCC, moreover, exosomes are new potential treatment of HCC else. In this review, we briefly summarize the latest findings on HCC exosomes, and their functions and significance for novel diagnostic and therapeutic approaches of HCC.
Composition and functions of exosomes
Morphological studies showed that exosomes belongs to multivesicular body (MVB), exosomes are small membranous vesicles (30-100 nm) and wrapped by bilayer lipid membrane [14]. MVBs fuse with the plasma membrane and release these microvesicles in an exocytic manner into biological fluids such as blood, urine, in vivo [9-11].
Exosomes contain a set of unique miRNAs, mRNAs, and proteins which may reveal genetic information of their parent cells [12]. Most of exosomes have the same evolutionary conserved protein molecules, such as heat shock proteins (HSP), CD63 and transmembrane protein et al., they are the most typical conserved protein in exosomes [15]. Meanwhile, Exosomes can also carry cell signal pathway protein such as Wnt-β-catenin [16], interleukin [17], tumor antigen and immune suppressive protein FasL, Trail and TGF-β. Exosomes derived from different cellular types can participate in diverse biological processes, including the mechanism of nonclassical secretory protein (HSP70, TCTP), regulation of immune response, signal transmission between cells, transport of RNA and protein [15,18].
Exosomes in HCC metastasis
Exosomes are removable and RNAs, protein contained in exosomes extensively involved in regulating tumor invasion and metastasis, for example, exosomes secreted by tumor cells can promote the tumor circulartory me-tastasis through their specific contents (such as TGF-β). It can also promo-te fibroblasts differentiate into myofibroblasts,reduce the production of type I collagen and up-regulate degradation of collagen fibers, and then exosomes promote tumor metastasis [19]. mRNA and miRNA contained in exosomes could regulate tumor development in plenty of pathways since entering the target cells. Glioma exosomes mRNA was translated and expressed after entering the cells, it stimulate cell proliferation and promote the growth of tumor [20]. Exosomes derived from metastatic gastric cancer cells release let-7 family miRNA, these miRNA can suppress antagonist of their target genes and transfer signals caused by tumor metastasis [21]. To date, the studies on the potential involvement of exosomes is limited to the metastasis of HCC. Previous studies have shown that the human hepatoma cell line, Hep3B, releases exosomes containing a specific subset of miRNAs that are taken up by an hepatocellular carcinoma cell line, HepG2, resulting in inhibition of transforming growth factor b activated kinase-1 (TAK1), loss of which has been implicated in hepatocarcinogenesis [22].
It is well-known that the tumor microenvironment can promote the metastatic cascade and that intercellular communication is necessary for this to occur. Exosomes as an important carrier in tumor microenvironment, they promote angiogenesis, invasion, and proliferation in recipient cells to support tumor growth and a prometastatic phenotype. Previous studies have shown that tumor and stromal cells can regulate the invasiveness of cancer cells through exosome-mediated delivery of protein and miRNA [15,23,24]. Various cytokines, growth factors, adhesion molecules and extracellular matrix protein secreted by tumor cells which can mediate intercellular communication in tumor microenvironment, and these molecules provide suitable environment for the survival and growth of cancer cells. Recent researches suggested that exosomes as an important carrier in tumor microenvironment maybe an effective way that these molecules enter into the microenvironment, and then they promote immune suppression, angiogenesis and formation of microenvironment before tumor metastasis. Rana et al. found that tumor exosomes educate selected host tissues toward a prometastatic phenotype [23]. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease, which can activate the tumor cells, endothelial cells and fibroblasts cells. These results suggested that exosomes are involved in tumor metastasis and promote the development of tumor [24].
Moreover, exosomes can also regulate hepatocellular carcinoma metastasis by promoting angiogenesis and epithelial-mesenchymal- transition (EMT) (Figure 1). EMT is a process whereby epithelial cells undergo a shift in plasticity and acquire the ability to disseminate, invade, and cause metastasis [25]. The protein composition of exosomes has been analyzed extensively, inducers of EMT have been found in association with exosomes including TGF-β [26], TNFα, IL-6, TSG101, AKT, ILK1, β-catenin [27,28], hepatoma-derived growth factor, casein kinase II (CK2), annexin A2 [29], and matrix metalloproteinases [30]. In addition, a growing number of miRNAs have been implicated in the regulation of EMT-related pathways in cancer [31], miR15a could be transported from bone marrow-derived mesenchymal cells via exosomes to promote multiple myeloma (MM) cell invasion [32]. In the last decade, experimental evidence has come to light defining exosomes induced plasticity in recipient cells as EMT, however, it’s not clear the role of exosomes in HCC metastasis through EMT pathway, it is the direction of our future research.
Figure 1.
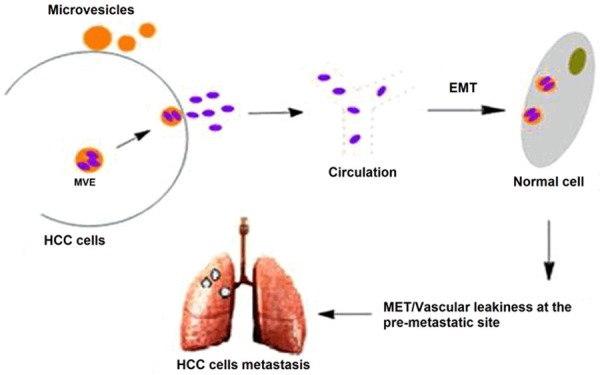
Functional contribution of exosomes during the EMT and MET process in HCC progression and metastasis.
HCC exosomes as diagnostic and prognostic biomarkers
Currently, exosomes from urine and other body fluids (saliva) are considered as noninvasive sources of molecular biomarkers for early detection and prognosis of various diseases, including HCC [33], Parkinson’s disease [34], and other tumors. This approach is based on the observations that exosomes present in blood and urine contain specific proteins, mRNAs and miRNAs derived from multiple organs, including liver, and that the exosomal cargo may be associated with progression of HCC [35,36] (Table 1). Exosomal miR-21 may serve as a potential biomarker for HCC diagnosis, and sensitivity of detection using serum miR-21 is much lower than using exosomal. It suggests that miR-21 in exosomes is one of effective diagnostic markers of HCC [37]. In addition, miRNA in exosomes as a diagnostic marker of HCC has been applied in clinic and achieved some results. miR-718 showed significantly different expression in the serum exosomes of HCC cases with recurrence after liver transplantation compared with those without recurrence, HOXB8 as a potential target gene of miR-718, and its upregulation was associated with poor prognosis. These results indicate that circulating miRNAs in serum exosomes have potential as novel biomarkers for predicting HCC recurrence [38].
Table 1.
Exosome and exosomal cargo as biomarkers or therapeutics of HCC
| Sample type | Exosome or exosomal cargo | Outcome | Reference | |
|---|---|---|---|---|
| HCC patients with LT | Serum | miR-718 | Biomarker for HCC recurrence and prognosis | [52] |
| HCC/Hepattis B patients | Serum | miR-221, miR191, miR-181a and miR-26a | Biomarker for early-stage HCC diagnosis | [53] |
| HCC rats | Serum | Exosome | Biomarker for early-stage HCC diagnosis | [54] |
| HCC patients | Serum | miR-21 | Biomarker for HCC diagnosis | [55] |
| HCC patients | Serum | miR-27b-3p, miR-92a-3p | Targets of HCC therapy | [56] |
| HCC cell lines | Cell culture medium | Exosome | miRNA transfer and contribute to HCC metastases | [57] |
In addition, miR-92a is markedly down-regulated in HCC, and the change is associated with cancer development and progression [39]. miRNA expression profile significantly varies when a blood sample is processed into either serum or plasma. And the relative amount of exosomal miR-92a is significantly decreased in the plasma of HCC patients compared with healthy donors. Although the pathophysiological significance of the decrease of miR-92a in HCC patient’s plasma is still unknown, deregulation of miR-92a expression in cells and plasma is implicated in the development of HCC, and the level of exosomal miR-92a in plasma is considered as a potential diagnostic marker of HCC [40,41].
In a word, although exosomes as diagnostic and prognostic biomarkers of HCC is still in the initial stage, present research results indicate that the method may have a wide application prospect with the development of extraction and analysis technology of exosomes.
Exosomes as novel therapeutics for HCC
Nowadays, despite a wide range of combined therapies are involved in the treatment of HCC, the efficacy of these methods and the prognosis of patients with HCC in late stage is still extremely poor. The 5-year overall survival rate of stage 3 and stage 4 HCC is less than 20% though chemotherapy and TACE could result in transiently regression of tumor. At present new treatment for different cancers (including HCC) based on tumor exosomes research has been making remarkable strides. Aucher A et al. utilized human macrophages transfer microRNAs (miRNAs) to hepato-carcinoma cells (HCCs) in a manner that required intercellular contact and involved gap junctions, they found that transfer of these miRNAs influenced post-transcriptional regulation of proteins in HCCs, including decreased expression of reporter proteins and endogenously expressed stathmin-1 and insulin-like growth factor-1 receptor. Importantly, transfer of miRNAs from macrophages functionally inhibited proliferation of these hepatocarcinoma cells. These data indicated that intercellular transfer of miRNA from immune cells could serve as a new defense against tumor growth [42].
Based on observations that exosomes released by tumor cells (including HCC) under stress condition contain HSPs that can improve tumor immunogenicity and induce NKT cell anti-tumor responses, the potential use of HSP-bearing exosomes for immunotherapy of HCC was proposed [43]. Ma B et al. demonstrated that murine bone marrow stromal cells pulsed with homologous tumor-derived exosomes inhabit proliferation of liver cancer cells, they suggested that exosomes have the potential to be used for therapy of HCC in future [44]. Xiao W et al. showed that the epigenetic drug MS-275 modified exosomes enhance the cytotoxic effect of NK cells significantly through upregulating the expression of MICA, MICB and HSP70. MS-275 may function as potential tumor vaccines against liver cancer [45]. The exosomes elicited CTL-specific cytotoxicity was enhanced by cisplatin, the therapy of cisplatin combined with exosomes had significant synergistic effect against tumor. The mechanism of synergistic effect includes enhancement of CTL activity [46].
Exosomes derived from human adult liver stem cells (HLSC) inhibited the growth and stimulate apoptosis of primary HCC cells in vitro by delivering antitumor miRNAs [47]. Herrera MB et al. found that MVs derived from HLSC induced in vitro proliferation and apoptosis resistance of human and rat hepatocytes. When administered in vivo, MVs accelerated the morphological and functional recovery of liver in a model of 70% hepatectomy in rats, in addition, they found the expression of human AGO2 mRNA and protein in the liver of hepatectomized rats treated with MVs [48]. Vps4A as a key regulator of exosome biogenesis was frequently down-regulated in HCC tissues, the reduction of Vps4A in HCC tissues was associated with tumor progression and metastasis. Vps4A may be a tumor suppressor and utilize exosomes as mediators to regulate the secretion and uptake of miRNAs in hepatoma cells [49]. Moreover, exosomes can also directly induced tumor cell apoptosis. Ristorcelli E et al. found that interactions of exosomal nanoparticles with target cells hampered the functioning of the Notch-1 survival pathway and activated the apoptotic pathway, which determines tumoral cell growth [50,51].
Conclusions
Exosomes play an important role in the development and progression of HCC. To date, studies on liver exosomes are limited and most studies stemmed from cytology experiment. Previous studies suggested that exosomes as a carrier in tumor microenvironment play an important role in the development and progression of HCC, it is also a potential and effective biomarker for the diagnosis and prognosis of HCC. miRNA secreted by HCC exosomes can induce a variety of biological functions, it may be a new direction for HCC targeted therapy through inactivation of oncogenic miRNA. On the other hand, although the progress of exosomes research is exciting, many problems remain to be further elucidated, for example, role of exosomes in the EMT pathway of HCC metastasis and mechanism of exosomes between HCC and the target cells remains to be further studied.
Acknowledgements
This study was supported by Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (No. ZKX12011).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Singal AG, Marrero JA. Interferon therapy and prevention of hepatocellular carcinoma in hepatitis C. Dig Dis Sci. 2012;57:832–834. doi: 10.1007/s10620-012-2069-8. [DOI] [PubMed] [Google Scholar]
- 4.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vella LJ. The Emerging Role of Exosomes in Epithelial-Mesenchymal-Transition in Cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HG, Grizzle W. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, Zhang HG. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–347. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, Zacharias W, Hao H, McMasters KM. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imani Fooladi AA, Mahmoodzadeh Hosseini H. Biological Functions of Exosomes in the Liver in Health and Disease. Hepatitis Monthly. 2014;14:e13514. doi: 10.5812/hepatmon.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa H, Thomas HJ, Schooley K, Born TL. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011;53:74–83. doi: 10.1016/j.cyto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods. 2008;335:98–105. doi: 10.1016/j.jim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 20.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valadi H, Ekstrom K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 22.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Vizio D, Morello M, Dudley AC. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vella LJ. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 27.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R, Deep G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2015;54:554–65. doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjøt L, Ørntoft TF, Larsen MR, Ostenfeld MS. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics. 2014;14:699–712. doi: 10.1002/pmic.201300452. [DOI] [PubMed] [Google Scholar]
- 30.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31:653–662. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Ho DH, Yi S, Seo H, Son I, Seol W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int. 2014;2014:704678. doi: 10.1155/2014/704678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wetmore BA, Brees DJ, Singh R, Watkins PB, Andersen ME, Loy J, Thomas RS. Quantitative analyses and transcriptomic profiling of circulating messenger RNAs as biomarkers of rat liver injury. Hepatology. 2010;51:2127–2139. doi: 10.1002/hep.23574. [DOI] [PubMed] [Google Scholar]
- 36.Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, Valle M, Luka Z, Elortza F, Wagner C, Lu SC, Mato JM, Falcon-Perez M. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics Clin Appl. 2010;4:416–425. doi: 10.1002/prca.200900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, Ochiya T, Maehara Y, Mimori K. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohyashiki JH, Umezu T, Kobayashi C, Hamamura RS, Tanaka M, Kuroda M, Ohyashiki K. Impact on cell to plasma ratio of miR-92a in patients with acute leukemia: in vivo assessment of cell to plasma ratio of miR-92a. BMC Res Notes. 2010;3:347. doi: 10.1186/1756-0500-3-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno S, Ishikawa A, Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398–401. doi: 10.1016/j.addr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. 2013;59:621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma B, Jiang H, Jia J, Di L, Song G, Yu J, Zhu Y, Lu Z, Wang X, Zhou X, Ren J. Murine bone marrow stromal cells pulsed with homologous tumor-derived exosomes inhibit proliferation of liver cancer cells. Clin Transl Oncol. 2012;14:764–773. doi: 10.1007/s12094-012-0860-9. [DOI] [PubMed] [Google Scholar]
- 45.Xiao W, Dong W, Zhang C, Saren G, Geng P, Zhao H, Li Q, Zhu J, Li G, Zhang S, Ye M. Effects of the epigenetic drug MS-275 on the release and function of exosome-related immune molecules in hepatocellular carcinoma cells. Eur J Med Res. 2013;18:61. doi: 10.1186/2047-783X-18-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SH, Shen Y, Li J, Xiang ZW, Fan WK, Chen L. [Experimental studies on anti-mouse hepatocellular carcinoma effects of cisplatin combined with exosomes] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:49–52. [PubMed] [Google Scholar]
- 47.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R, Salizzoni M, Tetta C, Camussi G. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61:1284–94. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:a70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 51.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 52.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, Ochiya T, Maehara Y, Mimori K. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Zhang L, Liu F, Xiang G, Jiang D, Pu X. Identification of endogenous controls for analyzing serum exosomal miRNA in patients with hepatitis B or hepatocellular carcinoma. Dis Markers. 2015;2015:893594. doi: 10.1155/2015/893594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu WH, Ren LN, Wang X, Wang T, Zhang N, Gao Y, Luo H, Navarro-Alvarez N, Tang LJ. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol. 2015;141:1767–78. doi: 10.1007/s00432-015-1943-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61:1284–1294. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]


