Abstract
The association between matrix metalloproteinase 2 (MMP2) gene polymorphisms and cancer risk has been investigated in many published studies; however, the currently available results are inconclusive. Therefore, we performed a meta-analysis to provide conclusive evidence for an association between the MMP2 polymorphism (-735 C/T) and cancer risk. Sixteen case-control studies with 11792 individuals were included in this meta-analysis. The odds ratio (OR) and 95% confidence interval (95% CI) were used to investigate the strength of the association. Overall, the MMP2 polymorphism (-735 C/T) was not associated with cancer risk in any of the models. However, the subgroup analysis revealed that dominant model (C/T+T/T vs. C/C: OR=1.24, 95% CI=1.01-1.53) and codominant 1 model (C/T vs. C/C: OR=1.30, 95% CI=1.05-1.62) were significantly associated with cancer risk in the Caucasian population. In conclusion, our meta-analysis indicated that the MMP2 polymorphism (-735 C/T) might be genetic risk factor for the carcinogenesis in Caucasians. However, more studies with a larger sample size are needed to provide more precise evidence.
Keywords: Matrix metalloproteinase 2, polymorphism, cancer, meta-analysis
Introduction
Cancer is a broad group of diseases characterized by unregulated cell growth and invade healthy cells in human. The cancer may invade or spread to other parts of the body. Cancer invasion and metastasis involve the detachment of tumor cells from the primary tumor, invasion through the basement membrane, intravasation into the circulatory system, extravasation at a distant site, and outgrowth of a secondary tumor [1]. The remodeling of extracellular matrix by matrix metalloproteinase (MMPs) is important for each step of cancer progression [2].
MMPs are a family of Zn2+-dependent endopeptidases. They are essential for tumor growth, angiogenesis, and metastatic processes [3-5]. Among secreted MMPs, MMP2 plays a significant role in cancer invasion and metastasis by degrading extracellular matrix. In addition, it plays an important role in cell proliferation, apoptosis and immune surveillance [2,6-8]. MMP2 is expressed in normal healthy tissues. Expanding studies have shown that the expression of MMP2 is up-regulated in several types of cancers [9-11]. It suggested that MMP2 may play a significant role in the development and progression of cancers.
The MMP2 gene is located on the long arm of chromosome 16 between positions 13 and 21. Several single nucleotide polymorphisms (SNP) in the promoter region of MMP2 gene have recently been identified. Especially, MMP2 polymorphism (-735 C/T) in the promoter region has been characterized functionally [12,13].
Up to now, number of studies have been conducted to evaluate the association between MMP2 promoter polymorphism and risk of different types of cancers in diverse populations including breast cancer, bladder cancer, esophageal cancer, lung cancer, hepatocellular carcinoma, prostate cancer and etc. However, the results from the published studies remain conflicting rather than conclusive. Therefore, we performed a meta-analysis on all eligible case-control studies to clarify the association between MMP2 polymorphism (-735 C/T) and susceptibility to cancer.
Materials and methods
Search strategy
Case and control studies were identified by searching in PubMed, Medline, and Google, up to January 2015 without language restrictions. Relevant studies were identified using the terms: “matrix metalloproteinase2 or MMP2 AND “polymorphism or polymorphisms or variant” AND-735 AND “cancer or carcinoma”. The study was restricted to human. Additional studies were searched by hand search of original or review articles references. If data or data subsets were published in more than one article, only the genetic data from the larger sample size were included.
Inclusion criteria
Studies were included if they met the following criteria: (1) evaluated the association between the MMP2 polymorphism (-735 C/T) and cancer; (2) used a case-control study design; (3) contained sufficient published data for the estimation of an odds ratio (OR) with a 95% confidence interval (CI).
Data extraction
The two investigators independently extracted data and reached consensus on all of the items. If the two investigators generated different results, they would check the data again and had a discussion to come to an agreement. Data extracted from the selected articles including the first author’s name, year of publication, country of origin, ethnicity of study population, number of cases and controls and genotype frequency of MMP2 polymorphism (-735 C/T). The ethnicity was divided into Asian and Caucasian population.
Statistical analysis
The chi-square test was used to calculate the Hardy-Weinberg equilibrium (HWE) in the control group. And meta-analysis was performed using the comprehensive meta-analysis software (Corporation, NJ, USA). The pooled p value, OR and 95% CI were used to investigate association between risk of cancer and MMP2 polymorphism (-735 C/T). The random effects model or the fixed effects model was used. OR with the corresponding 95% CI was calculated for the codominant 1 model (C/C vs. C/T), codominant 2 model (C/C vs. T/T), dominant model (C/C vs. C/T+T/T), recessive model (C/C+C/T vs. T/T), and allele (C vs. T), respectively [14,15]. The P-value less than 0.05 was regarded as statistically significant. A χ 2-test-based Q statistic test was performed to assess heterogeneity of the study. We also determined the effect of heterogeneity using I2 test. The random-effects Mantel-Haenszel method was adopted if the result of the Q test was a P-value less than 0.05 or a I2 statistic was larger than 50%, which indicated the statistically significant heterogeneity between the studies. Otherwise, the fixed-effects Mantel-Haenszel method was adopted. To test for publication bias, the Egger’s regression was applied.
Results
Study characteristics
Our meta-analysis of present study included sixteen studies, as shown in Table 1, comprising 16 articles including 5487 cancer patients and 6305 control subjects were ultimately selected [16-31]. The characteristics of the selected studies in terms of MMP2-735 C/T polymorphism and cancer type are summarized in Table 1. The types of various cancers included were; lung cancer (3), head and neck cancer (2), esophageal cancer (2), hepatocellular carcinoma (1), gastric cancer (1), ovarian cancer (1), colorectal cancer. (1), gallbladder cancer (1), prostate cancer (1), bladder cancer (1), breast cancer (1), and lymphoma (1).
Table 1.
Characteristics of eligible studies included in the meta-analysis
| Study | Cancer type | Country | Ethnicity | Year | Genotyping method | Case/control | Case | Control | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CC | CT | TT | CC | CT | TT | ||||||||
| Rollin et al. [16] | Lung cancer | French | Caucasian | 2007 | PCR-RFLP | 89/90 | 69 | 18 | 2 | 67 | 21 | 2 | 0.82 |
| Zhou et al. [17] | Lung cancer | China | Asian | 2004 | PCR-RFLP | 770/777 | 506 | 230 | 34 | 425 | 313 | 39 | 0.05 |
| Zhou et al. [18] | Head & neck cancer | China | Asian | 2007 | Sequencing | 580/478 | 397 | 159 | 24 | 281 | 167 | 30 | 0.44 |
| Ozgen et al. [19] | Head & neck cancer | Turkey | Caucasian | 2008 | PCR-RFLP | 42/147 | 23 | 19 | 0 | 99 | 51 | 7 | 0.89 |
| Yu et al. [20] | Esophageal cancer | China | Asian | 2004 | PCR-RFLP | 527/777 | 323 | 179 | 25 | 425 | 313 | 39 | 0.05 |
| Sun et al. [21] | Esophageal cancer | China | Asian | 2009 | PCR-RFLP | 335/624 | 222 | 100 | 13 | 408 | 187 | 29 | 0.21 |
| Zhai et al. [22] | Hepatocellular carcinoma | China | Asian | 2007 | Sequencing | 431/478 | 229 | 167 | 35 | 281 | 167 | 30 | 0.44 |
| Sun et al. [15] | Gastric cancer | China | Asian | 2009 | PCR-RFLP | 257/624 | 185 | 63 | 9 | 408 | 187 | 29 | 0.21 |
| Li et al. [23] | Ovarian cancer | China | Asian | 2008 | PCR-RFLP | 246/324 | 164 | 69 | 13 | 181 | 127 | 16 | 0.29 |
| Park et al. [24] | Colorectcal cancer | Korea | Asian | 2010 | PCR-RFLP | 333/318 | 180 | 128 | 25 | 189 | 110 | 19 | 0.58 |
| Sharma et al. [25] | Gallbladder cancer | India | Asian | 2012 | PCR-RFLP | 410/230 | 290 | 112 | 8 | 188 | 40 | 2 | 0.94 |
| Gonzalez et al. [26] | Lung cancer | Spain | Caucasian | 2012 | PCR-RFLP | 879/803 | 596 | 206 | 14 | 465 | 125 | 20 | 0.002 |
| Srivastava et al. [27] | Prostate cancer | India | Asian | 2012 | PCR-RFLP | 190/200 | 132 | 50 | 8 | 135 | 60 | 5 | 0.58 |
| Srivastava et al. [28] | Bladder cancer | India | Asian | 2013 | PCR-RFLP | 200/200 | 122 | 69 | 9 | 135 | 60 | 5 | 0.58 |
| Yari et al. [29] | Breast cancer | Iran | Asian | 2014 | PCR-RFLP | 98/135 | 70 | 28 | 0 | 80 | 52 | 3 | 0.09 |
| Mahmoud et al. [14] | Lymphoma | Egypt | Caucasian | 2014 | PCR-RFLP | 100/100 | 70 | 23 | 7 | 85 | 15 | 0 | 0.42 |
Quantitative synthesis
Table 2 shows the results from the overall meta-analysis. The sixteen studies were analyzed in meta-analysis. And Figure 1 shows OR and 95% CI of individual and pooled data for the MMP2-735 C/T polymorphism and susceptibility of cancer.
Table 2.
Overall analysis between MMP2 polymorphism (-735 C/T) and susceptibility of cancer
| Genetic comparison | Population | OR (95% CI) | P | Heterogeneity | Model | |
|---|---|---|---|---|---|---|
|
| ||||||
| P | I2 | |||||
| C vs. T | All | 0.98 (0.85-1.14) | 0.80 | <0.001 | 76.74 | Random |
| Asian | 0.93 (0.79-1.09) | 0.37 | <0.001 | 77.89 | Random | |
| Caucasian | 1.26 (0.83-1.91) | 0.27 | 0.03 | 65.60 | Random | |
| C/C+C/T vs. T/T | All | 0.95 (0.79-1.14) | 0.58 | 0.29 | 14.40 | Fixed |
| Asian | 0.99 (0.81-1.20) | 0.91 | 0.56 | <0.001 | Fixed | |
| Caucasian | 0.62 (0.34-1.16) | 0.14 | 0.12 | 48.90 | Fixed | |
| C/C vs. C/T+T/T | All | 0.97 (0.81-1.16) | 0.71 | <0.001 | 78.20 | Random |
| Asian | 0.90 (0.74-1.09) | 0.28 | <0.001 | 79.26 | Random | |
| Caucasian | 1.24 (1.01-1.53) | 0.040 | 0.17 | 40.72 | Fixed | |
| C/C vs. C/T | All | 0.99 (0.80-1.15) | 0.63 | <0.001 | 76.41 | Random |
| Asian | 0.89 (0.73-1.07) | 0.22 | <0.001 | 76.84 | Random | |
| Caucasian | 1.30 (1.05-1.62) | 0.015 | 0.42 | <0.001 | Fixed | |
| C/C vs. T/T | All | 0.90 (0.75-1.09) | 0.29 | 0.12 | 30.71 | Fixed |
| Asian | 0.93 (0.76-1.14) | 0.48 | 0.20 | 25.38 | Fixed | |
| Caucasian | 0.66 (0.35-1.23) | 0.19 | 0.12 | 48.76 | Fixed | |
OR, odds ratio; Bold numbers indicant significant association with risk of cancer.
Figure 1.
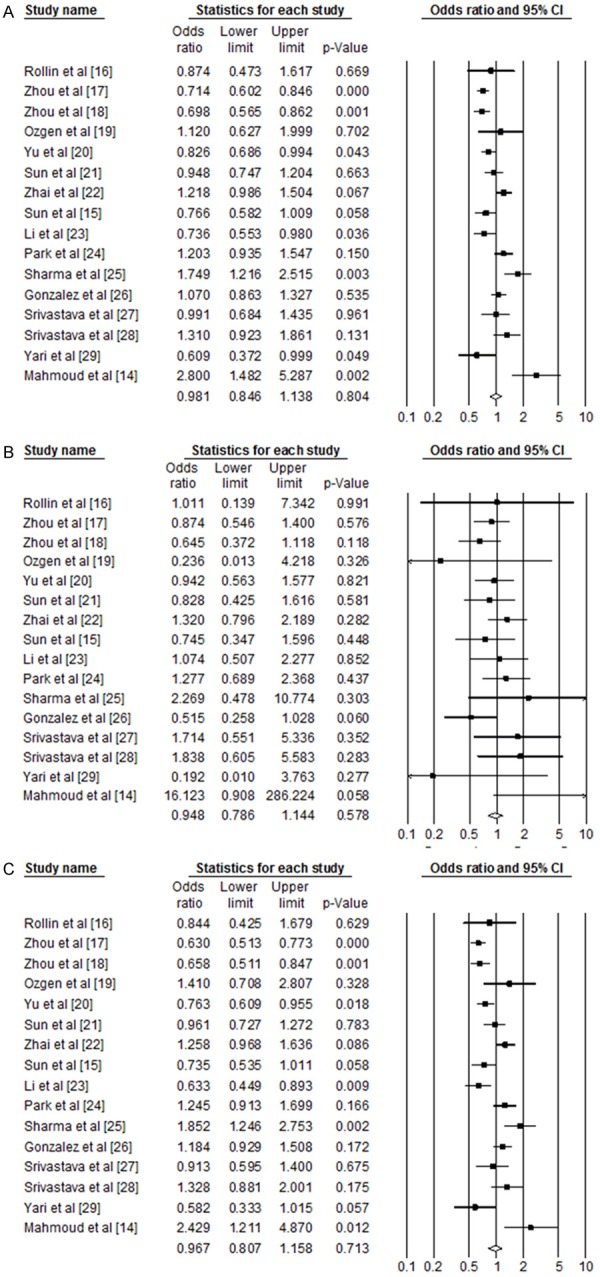
Odds ratio and 95% CI of individual and pooled data for the MMP2 polymorphism (-735 C/T) and susceptibility of cancer. A: C allele vs. T allele; B: C/C genotype + C/T genotype vs. T/T genotype; C: C/C genotype vs. C/T genotype + T/T genotype.
The T allele and genotypes including T allele of the MMP2-735 C/T polymorphism was not associated with susceptibility for cancer when compared with the C allele and genotype including C allele in the overall population (P>0.05, Table 2 and Figure 1A-C).
When stratified for ethnicity, T/T and/or C/T genotypes, and T allele did not show any significant association with cancer in the Asian population (P>0.05). However, there was significant association with risk of cancer in the Caucasian population. When the C/T genotype+T/T genotype of MMP2-735 C/T polymorphism was compared with C/C genotype (dominant model), there was a significant association with risk of cancers in the Caucasian population (fixed model, OR=1.24, 95% CI=1.01-1.53, P=0.040, heterogeneity=40.72, Table 2). And in the co-dominant 1 model, frequency of C/T genotype in the Caucasian population also showed significant association compared C/C genotype (fixed model, OR=1.30, 95% CI=1.05-1.62, P=0.015, heterogeneity <0.001). Although the study by Gonzalez, et al. 2012 was observed that HWE in the control group was not consistent (P=0.002), a publication bias was not observed (Egger’s regression P=0.87 in the codominant 1 model and Egger’s regression P=0.65 in the dominant model).
Discussion
MMP2 is involved in the proteolysis of the extracellular matrix, making it a key enzyme in cancer initiation and progression. MMP2 can regulate the tumor microenvironment and thus, plays a critical role in both tumor invasion and metastasis. Also, numerous studies have shown poor prognosis in tumors overexpressing MMP2 [10,11].
The MMP2-735 C/T polymorphism is located in the promoter region, appears to be closely associated with expression of MMP2, and is involved in the process of tumor progression. The association between MMP2-735 C/T polymorphism and cancer risk has been investigated in a broad range of studies with either a relatively small or larger sample size for the different populations. In previous meta-analysis studies, the MMP2-735 C/T polymorphism was reported to be associated with increased cancer risk [32]. Also, it was reported that MMP2-735 C/T polymorphism was associated with lung cancer risk, and patients with high MMP2 expression levels have poor overall survival compared with those with low MMP2 expression levels [33]. However, because of the difference in the number of participants and genetic background, the evidence provided by each study is not sufficient enough to draw a convincing conclusion. We conducted an up-dated meta-analysis including with 5487 cases and 6305 controls from 16 case-control studies to evaluate the association between MMP2-735 C/T polymorphism and the cancer risk.
In our study, we examined the association of MMP2-735 C/T polymorphism with the risk for cancer by meta-analysis. We found that this polymorphism was related with the increased risk of cancer. Subgroup analysis by ethnicity demonstrated that the MMP2-735 C/T polymorphisms were associated with increased cancer risk among the Caucasian population under the dominant and co-dominant 1 model.
We also have to mention the importance of heterogeneity. The heterogeneity was found in some comparisons in our meta-analysis. To get more full and accurate detail of the precious date, we used random-effects model or fixed model. The results were stable with the sensitivity analysis which did not change the results of the meta-analysis.
This meta-analysis still had some limitations and the results should be evaluated with caution. First, this meta-analysis is a type of secondary and retrospective study, it was limited by the quality of primary studies, and our meta-analysis was so, too. Second, we could not perform the analysis of gene-gene and gene-environment interactions.
In conclusion, this present study investigated the relationship between MMP2-735 C/T polymorphism and the susceptibility to cancer. We found that MMP2-735 C/T polymorphism may contribute to an increase in susceptibility to cancer in the Caucasian population. Further a lager study considering gene-gene and gene-environment interactions is needed to provide more evidence supporting the association of MMP2-735 C/T polymorphism with cancer risk.
Acknowledgements
This study was supported by the R & D program of MKE/KEIT (10040393, development and commercialization of molecular diagnostic technologies for lung cancer through clinical validation).
Disclosure of conflict of interest
None.
References
- 1.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–95. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- 4.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 6.Bodey B, Bodey B Jr, Groger AM, Siegel SE, Kaiser HE. Invasion and metastasis: the expression and significance of matrix metalloproteinases in carcinomas of the lung. In Vivo. 2001;15:175–180. [PubMed] [Google Scholar]
- 7.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 8.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi P. Matrix metalloproteinases and matrix metalloproteinase inhibitors in lung cancer. Semin Oncol. 2002;29:78–86. doi: 10.1053/sonc.2002.31528. [DOI] [PubMed] [Google Scholar]
- 10.Hrabec E, Strek M, Nowak D, Greger J, Suwalski M, Hrabec Z. Activity of type IV collagenases (MMP-2 and MMP-9) in primary pulmonary carcinomas: a quantitative analysis. J Cancer Res Clin Oncol. 2002;128:197–204. doi: 10.1007/s00432-001-0320-3. [DOI] [PubMed] [Google Scholar]
- 11.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 12.Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–7558. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Zhou Y, Miao X, Xiong P, Tan W, Lin D. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res. 2004;64:7622–7628. doi: 10.1158/0008-5472.CAN-04-1521. [DOI] [PubMed] [Google Scholar]
- 14.Seok H, Kim SK, Yoo KH, Lee BC, Kim YO, Chung JH. Association of BID SNPs (rs8190315 and rs2072392) and clinical features of benign prostate hyperplasia in Korean population. J Exerc Rehabil. 2014;10:383–388. doi: 10.12965/jer.140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HK, Lee H, Kim HJ. A polymorphism in DMT1 is associated with lead-related hypertensive status. Mol Cell Toxicol. 2013;9:415–420. [Google Scholar]
- 16.Gouda HM, Khorshied MM, El Sissy MH, Shaheen IA, Mohsen MM. Association between matrix metalloproteinase 2 (MMP2) promoter polymorphisms and the susceptibility to non-Hodgkin’s lymphoma in Egyptians. Ann Hematol. 2014;93:1313–1318. doi: 10.1007/s00277-014-2054-8. [DOI] [PubMed] [Google Scholar]
- 17.Sun DL, Duan YN, Zhang XJ, Zhou RM, Wang N, Cheng ZF, Y L. Association of MMP-2 and TIMP-2 promoter polymorphisms with susceptibility to gastric cardiac adenocarcinoma in a population of carcinoma high incidence region. Chin J Prev Med. 2009;43:342–345. [Google Scholar]
- 18.Rollin J, Regina S, Vourc’h P, Iochmann S, Blechet C, Reverdiau P, Gruel Y. Influence of MMP-2 and MMP-9 promoter polymorphisms on gene expression and clinical outcome of non-small cell lung cancer. Lung Cancer. 2007;56:273–280. doi: 10.1016/j.lungcan.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Yu C, Miao X, Wang Y, Tan W, Sun T, Zhang X, Xiong P, Lin D. Functional haplotypes in the promoter of matrix metalloproteinase-2 and lung cancer susceptibility. Carcinogenesis. 2005;26:1117–1121. doi: 10.1093/carcin/bgi057. [DOI] [PubMed] [Google Scholar]
- 20.Zhou G, Zhai Y, Cui Y, Qiu W, Yang H, Zhang X, Dong X, He Y, Yao K, Zhang H, Peng Y, Yuan X, Zhi L, He F. Functional polymorphisms and haplotypes in the promoter of the MMP2 gene are associated with risk of nasopharyngeal carcinoma. Hum Mutat. 2007;28:1091–1097. doi: 10.1002/humu.20570. [DOI] [PubMed] [Google Scholar]
- 21.Ozgen AG, Karadeniz M, Erdogan M, Berdeli A. Matrix metalloproteinases (MMP)-1, -2, and -9 gene polymorphism in papillary thyroid cancers (PTC) Endocrinologist. 2008;18:137–141. [Google Scholar]
- 22.Yu C, Pan K, Xing D, Liang G, Tan W, Zhang L, Lin D. Correlation between a single nucleotide polymorphism in the matrix metalloproteinase-2 promoter and risk of lung cancer. Cancer Res. 2002;62:6430–6433. [PubMed] [Google Scholar]
- 23.Sun DL, Duan YN, Zhang XJ, Wang N, Zhou RM, Cheng ZF, Li Y. Association of single nucleotide polymorphisms in the promoter region of MMP-2 gene with susceptibility to esophageal squamous cell carcinoma in high prevalence area. Tumor. 2009;29:354–357. [Google Scholar]
- 24.Zhai Y, Qiu W, Dong XJ, Zhang XM, Xie WM, Zhang HX, Yuan XY, Zhou GQ, FC H. Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut. 2007;56:445–7. doi: 10.1136/gut.2006.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XL, Kang S, Zhao XW, Zhang XJ, Zhou RM, Wang N, Jia JH, Zhao J, Li Y. Association of SNPs in the promoter of MMP-2 and TIMP-2 genes with epithelial ovarian cancer. Yi Chuan. 2008;30:455–462. doi: 10.3724/sp.j.1005.2008.00455. [DOI] [PubMed] [Google Scholar]
- 26.Park KS, Kim SJ, Kim KH, Kim JC. Clinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2010;26:391–397. doi: 10.1111/j.1440-1746.2010.06504.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma KL, Misra S, Kumar A, Mittal B. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP-2) genetic variants to gallbladder cancer. Liver Int. 2012;32:1278–1286. doi: 10.1111/j.1478-3231.2012.02822.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Arriaga P, Pascual T, Garcia-Alvarez A, Fernandez-Somoano A, Lopez-Cima MF, Tardon A. Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer. 2012;12:121. doi: 10.1186/1471-2407-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava P, Lone TA, Kapoor R, Mittal RD. Association of promoter polymorphisms in MMP2 and TIMP2 with prostate cancer susceptibility in North India. Arch Med Res. 2012;43:117–124. doi: 10.1016/j.arcmed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava P, Kapoor R, Mittal RD. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in Northern India. Urol Oncol. 2013;31:247–254. doi: 10.1016/j.urolonc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Yari K, Rahimi Z, Moradi MT. The MMP-2 -735 C allele is a risk factor for susceptibility to breast cancer. Asian Pac J Cancer Prev. 2014;15:6199–6203. doi: 10.7314/apjcp.2014.15.15.6199. [DOI] [PubMed] [Google Scholar]
- 32.Peng B, Cao LH, Ma XP, Wang WZ, Wang D, Yu L. Meta-analysis of association between matrix metalloproteinases 2, 7 and 9 promoter polymorphisms and cancer risk. Mutagenesis. 2010;25:371–379. doi: 10.1093/mutage/geq015. [DOI] [PubMed] [Google Scholar]
- 33.Wang JY, Cai Y. Matrix metalloproteinase 2 polymorphisms and expression in lung cancer: a meta-analysis. Tumor Biology. 2012;33:1819–1828. doi: 10.1007/s13277-012-0441-0. [DOI] [PubMed] [Google Scholar]


