Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide. Interferon-alpha (IFN-α) has recently been recognized to harbor therapeutic potential in prevention and treatment of HCC. IFN-stimulated gene 15 (ISG15) is an ubiquitin-like molecule that is strongly upregulated by type I interferons as a primary response to diverse microbial and cellular stress stimuli. Several studies have shown that the overexpression of ISG15 is correlated with multiply tumor types. However, the role of ISG15 in hepatitis B virus (HBV)-related HCC remains undetermined. ISG15 expression was found to be obviously higher in HBV-related HCC tissues than that in non-tumor tissues. ISG15 is a novel prognostic marker for predicting 5-year overall survival of HBV-related HCC patients. Overexpression of ISG15 was associated with clinicopathological characteristics and poor patient outcomes. ISG15 may serve as a novel prognostic marker for HBV-related HCC. Therefore, ISG15 may represent a novel HCC marker with prognostic significance and may be helpful in selecting patients for and predicting response to the treatment of HBV-related HCC.
Keywords: Hepatocellular carcinoma, ISG15, interferon-alpha, prognostic biomarker, HBV, HLCZ01
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related deaths worldwide. In 2008, an estimated 748300 new liver cancer cases and 695900 cancer-related deaths occurred worldwide. HCC in men is the fifth most frequently diagnosed cancer worldwide but the second most frequent cause of cancer death. In women, it is the seventh most commonly diagnosed cancer and the sixth leading cause of cancer death, and globally, rates are more than twice as high in males as in females [1]. Approximately 50% of these incident cases and deaths occurred in China, among which over 90% of the incident patients were chronically infected with the hepatitis B virus (HBV) [2]. Hepatic resection is still a major curative treatment for patients with HCC, and only about 10 to 20% of patients with HCC are currently eligible for surgical intervention [3]. However, the long-term prognosis of HCC patients following hepatic resection is poor because of the high rate of recurrence [4]. Previous studies have reported a 5-year cumulative recurrence rate of 70% to 80% for HCC [5]. Several adjuvant treatments have been used primarily in an attempt to decrease recurrence after curative surgical resection for HCC [6]. IFN-α belongs to type I interferon family of cytokines originally identified for their antiviral properties, and further studies revealed the anti-tumor activity of IFN-α against various tumors via direct inhibitory effects on tumor cells, anti-angiogenesis, enhanced immunogenicity of tumors and immunomodulatory effects [7,8].
To survive from viral infection, cells can produce and secrete IFNs, pro-inflammatory cytokines which can block viral infection and replication, cellular proliferation, and inhibit important immunomodulatory activities. One important mechanism by which IFNs mediate their antiviral effects is through the transcriptional regulation of relevant genes, such as IFN-stimulated genes (ISGs) [9,10]. ISG15 is an IFN inducible ubiquitin-like protein and its expression is highly induced upon viral or bacterial infection [11]. Type I-IFNs induce several hundred ISGs, including ISG15, through the Janus kinase/signal transducer and activator of transcription (Jak/STAT) signaling pathway. IFN alpha and beta are the strongest inducers of the ISG15 gene [12]. The ISG15 protein is comprised of tandem ubiquitin homology domains with distinct intracellular and extracellular activities [13]. Within cells, ISG15 is covalently conjugated to cellular proteins in an enzymatic pathway similar to that used by ubiquitin. In contrast to ubiquitin, post-translational modification by ISG15 does not result in the proteasome-dependent degradation of ISG15 conjugates. Multiple target proteins of ISG15 have recently been identified; however, the biological consequences of modification by ISG15 remain to be determined [14].
Many studies have identified specific alterations of ISG15 pathway in human tumors, such as bladder cancer [15], prostate cancer [16], breast cancer [17], pancreatic cancer [18] and squamous cell cancer [19]. Like other innate immune/stress response mediators, appropriately regulated ISG15 expression is associated with a tumor suppressor function, whereas the perturbation of ISG15 regulation is correlated with enhanced tumor progression and leads to aberrant cell signaling and malignant transformation [20].
The present study was designed to further explore the overexpression of ISG15 was associated with clinicopathological characteristics and poor patient outcomes. ISG15 may serve as a novel prognostic marker for HBV-related HCC. Since HBV is thought to be a main causative agent and most liver cirrhosis and HCC are developed from patients with chronic HBV infection. We established previously a hepatoma cell line, HLCZ01, the first cell line, supporting the entire lifecycle of both HBV and HCV. HBV surface antigen (HBsAg)-positive particles can be observed in the supernatant and the lumen of the endoplasmic reticulum of the cells via electron microscopy [21]. Therefore, in this study, we used HLCZ01 to investigate the ISG15 expression and related mechanism were detected by qRT-PCR and immunoblotting using clinicopathological data, cell line and xenograft model.
So far, ISG15 has been associated to a great number of malignancies due to its expression correlating to cancer invasion and metastasis. However, little is known about the clinical role of ISG15 in HBV-related HCC. Recently, Li et al [22] reported that ISG15 was highly expressed in HBV-related HCC through combined functional genome survey, but the role and clinical implications of ISG15 in HCC were not to be investigated. To our knowledge, this is the first study assessing the effect of ISG15 expression on the survival of patients with HBV-related HCC and used HLCZ01 to investigate the ISG15 expression and related mechanism were detected by qRT-PCR and immunoblotting using HLCZ01 cell lines supporting the entire lifecycle of both HBV and HCV and its xenograft model.
In this study, we demonstrate that elevated ISG15 expression is observed in the HCC tissues, cells and its xenograft model. The high expression of ISG15 is correlated with poor clinicopathological features of HCC. Moreover, high ISG15 expression confers a worse 5-year survival of HCC patients. ISG15 knockdown reduces HBV-related HCC cell migration and invasion. Our results demonstrate that overexpression of ISG15 was associated with clinicopathological characteristics and poor patient outcomes. ISG15 may serve as a novel prognostic marker for HBV-related HCC. Therefore, ISG15 may represent a novel HCC marker with prognostic significance and may be helpful in selecting patients for and predicting response to the treatment of HBV-related HCC. ISG15 acts as a potent prognostic marker and contributes to tumor invasion and metastasis in HBV-related HCC.
Materials and methods
Ethics statement
All patients signed consent forms to acknowledge participation in this study approved by the review board of the Hunan Provincial Tumor Hospital, Changsha, China. All procedures followed were in accordance with the ethical commission of Hunan Provincial Tumor Hospital, Changsha, China.
Patient specimens
From January 2001 to December 2008, patients with HBV-related HCC diagnosed pathologically at Hunan province tumor hospital were recruited. Retrospective analysis was conducted based on medical records of patients. These tissues were collected at and the human tissues were obtained and studied in strict adherence to the protocol approved by hospital. A total of 129 snap-frozen normal or paraffin-embedded HCC tissues and surrounding non-tumor hepatic tissues from HBV-related HCC patients who had first undergone radical resection at Hunan Provincial Tumor Hospital in Changsha. Normal human liver tissues were obtained from distal normal liver tissue of liver hemangioma patients.
In this study, we investigated 199 patients, who were diagnosed with primary HCC. The inclusion and exclusion criteria: ① distinctive pathologic diagnosis; ② without pre-operative anticancer treatment and distant metastases; ③ surgery liver resection; ④ with a complete clinico-pathologic and follow-up data. In the period extending from 2001 to 2008, we investigated these patients who had undergone conventional surgery. Before surgery, these patients were not subjected to radiotherapy or chemotherapy. After completion of surgical procedure, they were followed up for 5 years, and their complete clinical data were collected. All primary HCC samples were taken from these 199 patients. These primary samples were fixed in 10% formalin and subsequently embedded in paraffin. Thereafter, they were sectioned consecutively at 4 mm stained by hematoxylin and eosin. According to the criteria of the World Health Organization (WHO) classification system, the histological types were independently assigned by two double-blinded pathologists.
HBV infection of HLCZ01 cells
A normal hepatocyte line (L02) was purchased from the cell bank of Chinese Academy of Sciences. Human HCC cell lines HLCZ01 and normal hepatocyte line (L02) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum at 37°C in 5% CO2. The supernatant of HepG2.2.15 cells derived from human hepatoma cell line HepG2 transfected with the full genome of HBV was collected and filtered. HLCZ01 cells were inoculated overnight with the filtered supernatant at a multiplicity of infection (MOI) of 20 genome equivalents (Geq) per cell. The cells were washed three times with PBS, were maintained in DMEM/F12 medium, and were harvested at the indicated times. HLCZ01 cells were inoculated with the different strains of sera from hepatitis B patients diluted at 1:20 and were cultured for the indicated time periods. The HLCZ01 cells of HBV infection cells were isolated and cultured as described previously [21]. All of cell lines maintained under recommended culture conditions. Cells were incubated in a 37°C humidified incubator containing 5% CO2. RNA was extracted from exponentially growing cells for the purpose of detecting the expression of ISG15.
Quantitative reverse transcription-PCR
Total cellular RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instruction. Real-time RT-PCR for ISG15 was performed using the ABI PRISM 7700 instrument (Perkin-Elmer Applied Biosystems, Foster City, CA, USA) with gene-specific primers and the SYBR Green I protocol. A total of 1.0 μg RNA was reverse-transcribed to complementary DNA (cDNA) in a total volume of 20 μl, and 1 μL of this mixture was used as a template for Quantitative reverse transcription polymerase chain reaction (qRT-PCR). The primers used for amplification are listed in Table 1. Relative expression ratios normalized to that of GAPDH (Santa Cruz, California, USA) were calculated. The relative expression of ISG15 mRNA for each sample was calculated as follows: ΔCt = Ct (TPX2)-Ct (GAPDH), ΔΔCt (sample) = ΔCt (sample)-ΔCt (calibrator), and the fold changes in mRNAs were calculated through relative quantification (2-ΔΔCt). To evaluate the effect of ISG15 mRNA on survival, the weighed expression of ISG15 mRNA were divided into high and low level using the median expression as the cutoff point for the whole group.
Table 1.
List of primers used for real-time PCR
| Gene | Primer sequences |
|---|---|
| GAPDH | f: 5’-TTTGTCAAGCTCATTTCCTG-3’ |
| r: 5’-TGGTCCAGGGTTTCTTACTC-3’ | |
| ISG15 | f: 5’-GCGCAGATCACCCAGAAGAT-3’ |
| r: 5’-GCGCAGATCACCCAGAAGAT-3’ |
Immunohistochemistry
Immunohistochemical staining was done on formalin-fixed and paraffin-embedded tissue using 5-μm section from tissue microarray blocks. Mounted tissue section were baked at 60°C for 30 min, deparaffinized in xylene and rehydrated through graded alcohols. Antigen was retrieved by heating in 1 μM sodium citrate (pH 6.0) in a pressure cooker for 2 min. According to the manufacturer’s instruction, anti-ISG15 ( Santa Cruz Biotechnology, Santa Cruz, CA, dilution 1:1000) and was incubated on the section overnight at 4°C after non-specific staining was blocked, followed by incubation with a horseradish peroxidase conjugated secondary antibody. Staining was visualized using DAB chromogen substrate and counterstained with haematoxylin. The tissue sections were viewed at 400× magnification. Three fields per section were analyzed and ISG15 positive cells were calculated using Image-Pro Plus software.
Western blot analysis
Cells were washed twice with PBS and lysed in buffer containing 20 mM Tris-HCl (pH 7.4), 137 mM NaCl, 10% (wt/vol) glycerin, 1% Triton X-100, 2 mM EDTA, and a protein inhibitor cocktail (Roche) for 30 min on ice. The homogenate was centrifuged at 14,000 rpm for 20 min at 4°C and the supernatants were transferred to fresh tubes. Protein samples were separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The membranes were blocked in 5% nonfat dry milk containing 0.1% Tween-20 for 1.5 h at 37°C, and then probed with primary antibodies at 4°C overnight. After washing, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody followed by ECL detection (Amersham Pharmacia Biotech, USA). The membranes were scanned with a LAS-4000 luminescent image analyzer (Fujifilm, Japan). Subsequently, the membranes were washed in 20 mM glycine (pH 2.3) and subjected to an anti-tubulin antibody (Sigma) to verify for equal protein loading. The bound antibodies were detected by ECL reagent (Pierce, Rockford, IL) according to the manufacturer’s instruction.
Statistical analysis
All results were reported as mean ± SD or median (range). The normality of data distribution was tested by the Kolmogorov-Smirnov test. Pearson’s chi-square test, independent-samples t test, nonparametric Mann-Whitney U or Wilcoxon signed ranks test, logistic regression, and nonparametric Spearman’s rank correlation were used when appropriate. Data analysis was performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). A univariate test was used to examine the influence of each clinical variable on survival. Student t-test was applied to determine statistical significance. P values of < 0.05 were considered statistically significant.
Results
Upregulation of ISG15 in HBV-related HCC cell lines
We started the study by investigating the level of ISG15 mRNA expression in non-HCC and HCC HLCZ01, Huh7 and HepG2 cell lines by RT-PCR and Western blot. Real-time PCR analysis revealed a low ISG15 mRNA expression among the non-HCC epithelial cell lines L02, whereas ISG15 mRNA transcript levels varied more and in summary were clearly elevated in the cancerous cell lines Huh7, HLCZ01 and HBV-HLCZ01, of which HBV-HLCZ01 displayed an exceptionally high level of ISG15 mRNA (Figure 1A). Furthermore, western blot technique revealed that ISG15 was overexpressed in most HCC cell lines. In contrast, ISG15 expression was low or undetectable in the normal hepatocyte line (Figure 1B).
Figure 1.
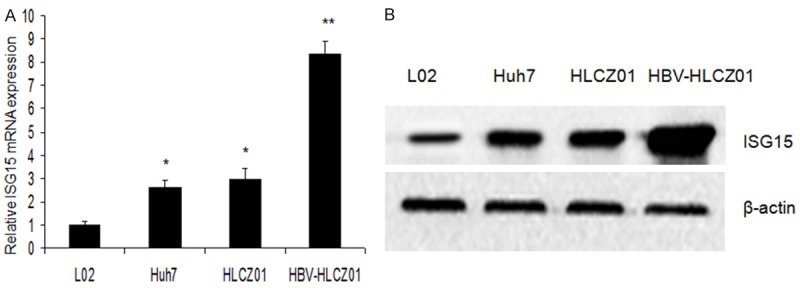
ISG15 expression in normal hepatocyte line and HCC cell lines. A: qRT-PCR was performed to determine ISG15 mRNA expression in L02, Huh7, HLZC01 and HBV-HLCZ01, GAPDH was used as a control; B: Western blot was performed to determine ISG15 protein expression in L02, Huh7, HLZC01 and HBV-HLCZ01.
ISG15 is overexpressed in HCC tissues
ISG15 expression was determined by IHC in the 190 surgical specimens of HCC. An overexpression of ISG15 was exhibited in 166 (87.38%) surgical specimens. In 199 pairs of HCC and normal tumor-adjacent tissues, qRT-PCR and immunoblotting showed that ISG15 expression in HCC was obviously higher than that in adjacent noncancerous liver tissues (P < 0.01, respectively, Figure 2A-C). We also examined the expression of ISG15 protein in 190 cases of HCC and matched tumor-adjacent liver tissues using immunohistochemical staining, and found that the expression score of ISG15 protein was significantly higher in the HCC tissues than that in the noncancerous tissues, especially in HBV related HCC (4.86±0.29 vs. 1.35±0.15; P < 0.01, Figure 2D). These results indicated ISG15 to be more highly expressed in HCC tissues than in adjacent no tumorous liver tissues.
Figure 2.
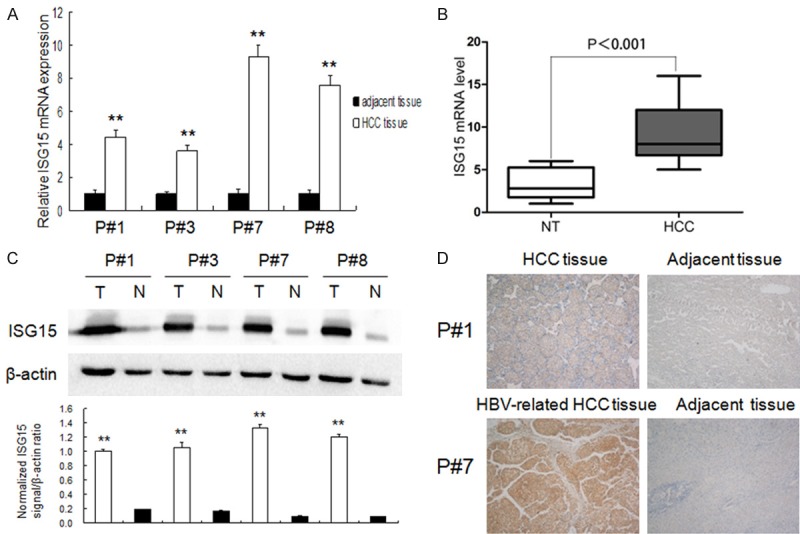
ISG15 expression in HCC tissues. A, B: ISG15 mRNA expression HCC and corresponding adjacent no tumorous liver tissues using qRT-PCR (n = 199, P < 0.01); C: ISG15 protein expression in HCC tissues (T) and corresponding adjacent no tumorous liver tissues (N) using immunoblotting (upper). The protein level of ISG15 was quantified by densitometry in comparison with β-actin (bottom); D: The difference in Protein expression levels of ISG15 in HCC tissues was compared with the corresponding adjacent tissues by immunohistochemistry in patient specimens.
Correlation between ISG15 expression and clinicopathological parameters
To further characterize the clinical role of ISG15 in HBV related-HCC, we tried to precisely identify the correlations of the ISG15 expression with clinicopathological parameters, including patient gender, age, HBsAg, AFP level, tumor size, tumor number, vascular invasion, cirrhosis, capsule formation, grade, time to recurrence and TNM stage. The median expression score of ISG15 protein and mRNA was used as the cutoff point to divide into low-expressing and high expressing groups. In our study, the expression of ISG15 was significantly correlated with the hepatitis status, tumor differentiation, tumor number, and TNM stage (P < 0.05). However, no evident correlation was found between the expression of ISG15 and patient gender, age, hepatitis status, liver cirrhosis, AFP level, tumor size, microvascular invasion, recurrence and capsule formation (P > 0.05). The results are listed in Table 2.
Table 2.
Correlation between ISG15 expression and clinicopathological parameters
| Variables | n | ISG15 mRNA | P | ISG15 protein | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High | Low | High | Low | ||||
| Men | 170 | 109 | 61 | 0.603 | 106 | 64 | 0.502 |
| Women | 20 | 14 | 6 | 14 | 6 | ||
| Age (y)* | |||||||
| > 50 | 133 | 90 | 43 | 0.747 | 89 | 44 | 0.647 |
| ≤ 50 | 57 | 33 | 14 | 31 | 16 | ||
| Hepatitis status | |||||||
| Active | 130 | 85 | 45 | 0.783 | 85 | 45 | 0.349 |
| Non-active | 60 | 38 | 22 | 35 | 25 | ||
| Liver cirrhosis | |||||||
| No | 154 | 89 | 55 | 0.135 | 88 | 56 | 0.301 |
| Yes | 36 | 34 | 12 | 32 | 14 | ||
| Tumor capsule | |||||||
| Presence | 106 | 74 | 32 | 0.101 | 72 | 34 | 0.126 |
| Absence | 84 | 49 | 35 | 48 | 36 | ||
| Tumor size | |||||||
| ≤ 5 cm | 86 | 52 | 34 | 0.262 | 52 | 34 | 0.484 |
| > 5 cm | 104 | 71 | 33 | 68 | 36 | ||
| Tumor nodules | |||||||
| 1 | 106 | 62 | 44 | 0.043 | 59 | 47 | 0.016 |
| ≥ 2 | 84 | 61 | 23 | 61 | 23 | ||
| Recurrence | |||||||
| Yes | 75 | 52 | 23 | 0.284 | 51 | 24 | 0.264 |
| No | 115 | 71 | 44 | 69 | 46 | ||
| Microvascular invasion | |||||||
| No | 57 | 32 | 25 | 0.104 | 32 | 25 | 0.189 |
| Yes | 133 | 91 | 42 | 88 | 45 | ||
| Tumor differentiation | |||||||
| I-II | 102 | 60 | 42 | 0.066 | 59 | 43 | 0.102 |
| III-IV | 88 | 63 | 25 | 61 | 27 | ||
| Serum AFP | |||||||
| > 400 ng/mL | 130 | 92 | 38 | 0.010 | 90 | 40 | 0.011 |
| ≤ 400 ng/mL | 60 | 31 | 29 | 30 | 30 | ||
| TNM stage | |||||||
| I | 38 | 20 | 18 | 0.001 | 19 | 17 | 0.020 |
| II | 76 | 41 | 35 | 43 | 33 | ||
| III | 76 | 61 | 15 | 56 | 20 | ||
year-old.
Correlation between ISG15 expression and patients’ survival
While differentiating patients with high and low ISG15 protein levels, we correlated the prognostic effect of ISG15 with the overall survival of HBV-related HCC patients. By Kaplan-Meier curve assessment, it was found that high ISG15 protein level was a significant prognostic factor in deciphering the poor overall survival in HCC patients. Patients with high ISG15 protein level had a significantly lower 5-year survival rate than those with low ISG15 protein level (Figure 3, P < 0.05). While comparing the Kaplan-Meier survival curves for low IHC (protein) expressions and high IHC (protein) expressions of ISG15, we found a significant separation (P = 0.001, log-rank test) in the 190 HCC patients.
Figure 3.
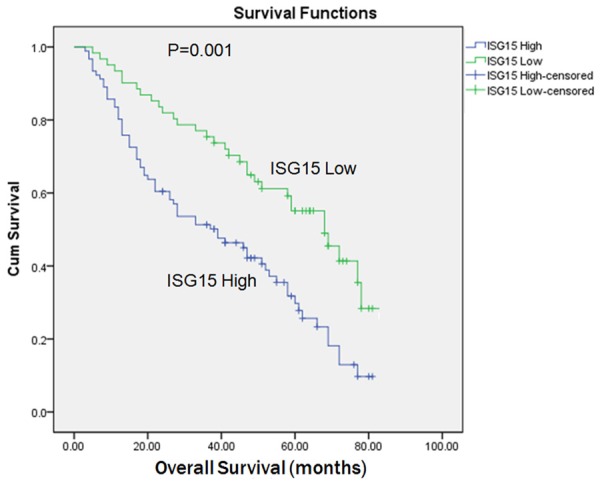
Kaplan-Meier survival analysis of primary HCC patients with different level of ISG15. The survival rate for patients in the ISG15-high group (n = 106) was significantly lower than that for patients in the ISG15-low group (n = 57) (log-rank, P = 0.001).
Univariate and multivariate analyses of prognostic variables in HCC patients
To identify the variables with potential prognostic significance, univariate analysis of each variable was performed in relation to the survival time of patients with HCC. The difference in predicting the prognosis was assessed by examining the ratio hazard and p value for each variable. Then, the relative importance of each variable was determined by multivariate Cox proportional hazards model analysis. The stepwise inclusion of variables in the model was performed through univariate analysis, which proved that the significant prognostic factors were ISG15 expression, gender, tumor size, tumor differentiation, tumor number, TNM stage, hepatitis status, liver cirrhosis, serum AFP level, microvascular invasion, recurrence and capsule formation in the over survival (OS) and time to recurrence of HCC patients. Multivariate analysis results showed that the OS of HCC patients could be predicted on the basis of significant prognostic factors, such as ISG15 expression, tumor differentiation, tumor number, TNM stage and hepatitis status (Table 3). Multivariate analysis results showed that the time to recurrence of HCC patients could be predicted on the basis of significant prognostic factors, such as ISG15 expression, the tumor differentiation, tumor number, TNM stage and and hepatitis status (Table 4).
Table 3.
Univariable and multivariate analyses of Clinicopathological Prognostic Factors for Overall Survival
| Independent factors | Univariable | Multivariable* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| ISG15 | 1.548 | 1.402-2.716 | 0.003 | 1.110 | 1.004-3.766 | 0.002 |
| Number of tumor nodules | 1.345 | 1.061-4.339 | 0.006 | 1.987 | 1.922-8.793 | 0.005 |
| Hepatitis status | 1.381 | 1.104-1.784 | 0.002 | 3.112 | 2.801-10.908 | 0.008 |
| TNM stage | 1.068 | 1.006-2.033 | 0.005 | 1.577 | 1.295-2.128 | 0.003 |
| Differentiation | 1.059 | 1.014-2.078 | 0.007 | 1.629 | 1.199-2.027 | 0.003 |
Abbreviations: HR, Hazard radio; CI, Confidence interval; OS, overall survival.
Statistically significant (P < 0.05).
Table 4.
Univariable and multivariate analyses of Clinicopathological Prognostic Factors for time to recurrence
| Independent factors | Univariable | Multivariable* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| ISG15 | 1.376 | 1.308-2.628 | 0.004 | 1.120 | 1.104-3.959 | 0.005 |
| Number of tumor nodules | 1.486 | 1.098-4.012 | 0.009 | 1.936 | 1.774-6.902 | 0.007 |
| Hepatitis status | 1.542 | 1.269-1.697 | 0.005 | 2.924 | 2.887-9.721 | 0.005 |
| TNM stage | 1.153 | 1.008-2.132 | 0.010 | 1.692 | 1.391-2.105 | 0.006 |
| Differentiation | 1.428 | 1.124-2.098 | 0.008 | 1.474 | 1.325-2.223 | 0.004 |
Abbreviations: HR, Hazard radio; CI, Confidence interval; OS, overall survival.
Statistically significant (P < 0.05).
Discussion
There have been major breakthroughs in the diagnosis and chemotherapy measures used in the treatment of cancer. However, HCC continues to be one of the most deadly human carcinomas, and the prognosis of HCC remains dismal [23]. In clinical practice, prognostic molecular biomarkers are valuable tools in predicting the progression of disease in patients. And these patients we selected are at early or intermediate stage of HCC patients in our study. Clinicians use these prognostic biomarkers in planning strategies that control the proliferation of tumor. ISG15 is conjugated to intracellular proteins by a mechanism called ISGylation, which is similar to ubiquitin (ubiquitination). In this study, we determined the ISG15 expression in HCC patients. We also explored the clinical prognostic significance of ISG15 expression. For this purpose, we analyzed the entire long-term follow-up data of a large cohort of HCC patients along with their corresponding clinical samples. We have found that the ISG15 protein and ISG15 mRAN are expressed much more strongly in major HCCs than in normal liver and cirrhotic liver tissues. We also found the overexpression of ISG15 protein and ISG15 mRAN at the transcriptional and translational levels of HCC cell lines. Our results also indicate that ISG15 probably plays a role in hepatocarcinogenesis. However, we could not completely elucidate the precise molecular mechanism of ISG15 in HCC tumorigenesis. The findings of ISG15 overexpression in our study are in good agreement with most observations reported in previous cancer studies. In summary, our study confirms a close association of ISG15 expression with the genesis and development of tumors.
Recent studies have established that type I interferon modulates expression of large number of cellular genes. While the proteins encoded by some of these genes have a direct antiviral activity, the functions of the majority of the others have not yet been determined. One of the first identified IFN stimulated gene, encodes ubiquitin like protein ISG15 that is also expressed in response to different stress stimuli. Although it was shown that ISG15 functions as protein modifier, it has been only recently that the targets of ISG15 conjugation were identified. Recent studies have also revealed mechanism of ISG15 conjugation and its interaction with the ubiquitin conjugation pathway, it is focused on the possible role of ISG15 in the antiviral response, regulation of cell growth and carcinogenesis [24]. Several research studies have reported that ISG15 plays a pivotal role in cancer progression. Laljee et al [25] reported the overexpression of ISG15 in up to 80% of oral squamous cell carcinoma tissues collected from Indian patients. Hence ISG15 may be explored for the possibility of use as a high confidence diagnostic biomarker in oral cancers. Bektas et al [26] systematically analyzed ISG15 expression in invasive breast carcinomas (n = 910) and normal breast tissues (n = 135). Using semiquantitative real-time PCR, cDNA dot-blot hybridization and immunohistochemistry. The study shown that ISG15 was overexpressed in breast carcinoma cells compared with normal breast tissue, both at the RNA and protein level, and the recurrence-free, event-free and overall survival analyses showed a significant correlation between ISG15 overexpression and unfavourable prognosis. Therefore, ISG15 may represent a novel breast tumour marker with prognostic significance and may be helpful in selecting patients for and predicting response to the treatment of human breast cancer. In contrast, Darb Esfahani et al [27] reported that the ISG15 plays an ambiguous role in the progression and response to chemotherapy of solid cancers, and its protein expression was significantly increased in relapsed carcinomas as compared to primary tumors (P = 0.027). Patients with ISG15-positive carcinomas had a significantly longer overall survival in univariate and multivariate analysis.
Our contingency table analysis, however, showed that ISG15 expression correlate with AFP, which is the important clinicopathological parameters analyzed in this study. On the other hand, our Kaplan-Meier survival analysis revealed that high level ISG15 expression was significantly linked with a poor prognosis of HCC patients after surgical resection. When the expression of ISG15 was higher, the survival time for HCC patients was found to be shorter. The univariate analysis of Cox proportional-hazard model proved that significant prognostic factors, such as ISG15 expression, tumor size, recurrence, metastasis, and serum AFP were associated with an increased risk of death from HCC. In contrast, multivariate analysis proved that ISG15 expression, the tumor differentiation, tumor number, recurrence, TNM stage and hepatitis status were the six significant and independent prognostic factors that could be associated with overall survival of HCC patients. Barring ISG15 expression, the other factors are well-acknowledged indicators of HCC. As ISG15 expression is now considered as a novel and independent predictor of decreased OS survival of patients with prostate cancer, it may also be reckoned as a new and independent predictor of prognosis for HCC patients.
Surprisingly, in human oesophageal squamous cancer cell lines, ISG15 was found to be upregulated [28]. ISG15 mRNA expression was also found to be upregulated in human non-small cell lung cancer cell lines [29]. Our findings suggested that ISG15 is involved in the proliferation and migration of HCC cells HepG2 and HLCZ01. These data cast light on novel mechanisms of HCC progression. ISG15 mRNA and protein levels in HCC cells are higher than non-HCC cell and HCC adjacent tissues. Furthermore, using anti-ISG15 antibody clearly indicated ISG15 protein over-expression in HCC cells and tumor tissues from patients. The ISG15 is an ubiquitin-like protein transcriptionally regulated by IFN-α which shows antivirus and antitumor activities. However, the exact role of ISG15 is unknown. Wan et al [30] reported that IFN-α significantly induced ISG15 expression but failed to induce HepG2 cell apoptosis, whereas transient overexpression of ISG15 dramatically increased HepG2 cell apoptosis. ISG15 overexpression increased overall protein ubiquitination, which was not observed in cells with IFN-α-induced ISG15 expression, suggesting that IFN-α treatment not only induced the expression of ISG15 but also inhibited ISG15-mediated ubiquitination. His results suggest that ISG15 overexpression could be developed into a powerful gene-therapeutic tool for treating IFN-α-resistant HCC. It is possible that ISG15 by itself can function as a signaling pathway related to HCC cell growth and/or survival. Therefore, ISG15 might be a target molecule in the therapy of drug resistant HCC.
In summary, The ISG15 overexpression in HBV-related relative to normal HCC tissue at the RNA and protein level indicating that ISG15 may represent a candidate oncogene in human HCC. Interestingly, ISG15 could function as a novel HCC marker with prognostic or predictive significance. Recently, microarray experiments determined strong deregulation of ISG15 expression in response to diverse cancer chemotherapeutic agents [31]. These findings indicate that ISG15 could represent a predictive biomarker in the treatment of these cancers as well. However, the putative function of ISG15 in liver carcinogenesis and in chemotherapeutic response has to be further analyzed in prospective studies. As a next step we will investigate whether an ISG15-based diagnostic assay is able to predict response to specific chemotherapy regimens in the treatment of HCC.
Acknowledgements
Our study was supported by the Changsha City Third Science and Technology Project (K1403380-31, to Chaohui Zuo).
Disclosure of conflict of interest
None.
Abbreviations
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IFN-α
interferon-alpha
- ISG15
interferon -stimulated gene 15
- OS
overall survival
References
- 1.Jemal A, Brany F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistitics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. In J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 4.Qu LS, Jin F, Huang XW, Shen XZ. Interferon-αtherapy after Curative resection prevents early recurrence and improves survival in patients with hepatitics B virus-related hepatocellular carcinoma. J Surg Oncol. 2010;102:796–801. doi: 10.1002/jso.21741. [DOI] [PubMed] [Google Scholar]
- 5.Fina RS. Current and Future Treatment Strategies for Patients with Advanced Hepatocellular Carcinoma: Role of mTOR Inhibition. Liver Cancer. 2012;1:247–256. doi: 10.1159/000343839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma review. Liver Cancer. 2012;1:144–158. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, Kanzler S, Schulze-Bergkamen H, Galle PR. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independenity of p53. Cancer Res. 2009;69:855–862. doi: 10.1158/0008-5472.CAN-08-2831. [DOI] [PubMed] [Google Scholar]
- 8.Lamm D, Brausi M, O’Donnell MA, Witjes JA. Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer. Urol Oncol. 2014;32:35, e21–30. doi: 10.1016/j.urolonc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Laurence A, Pesu M, Silvennoinen O, O’Shea J. JAK Kinases in Health and Disease: An Update. Open Rheumatol J. 2012;6:232–244. doi: 10.2174/1874312901206010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanai H, Negishi H, Taniguchi T. The IRF family of transcription factors: Inception, impact and implications in oncogenesis. Oncoimmunology. 2012;1:1376–1386. doi: 10.4161/onci.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadier AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridharan H, Zhao C, Krug RM. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J Biol Chem. 2010;285:7852–7856. doi: 10.1074/jbc.C109.095703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Zhang M, Xiao ZZ, Sun L. Cynoglossus semilaevis ISG15: a secreted cytokine-like protein that stimulates antiviral immune response in a LRGG motif-dependent manner. PLoS One. 2012;7:e44884. doi: 10.1371/journal.pone.0044884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakopoulos NV, Luo JK, Papov V, Zou W, Lenschow DJ, Jacobs BS, Borden EC, Li J, Virgin HW, Zhang DE. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem Biophys Res Commun. 2005;336:496–506. doi: 10.1016/j.bbrc.2005.08.132. [DOI] [PubMed] [Google Scholar]
- 15.Andersen JB, Aaboe M, Borden EC, Goloubeva OG, Hassel BA, Orntoft TF. Stage-associated overexpression of the ubiquitin-like protein, ISG15, in bladder cancer. Br J Cancer. 2006;94:1456–1471. doi: 10.1038/sj.bjc.6603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood LM, Pan ZK, Seavey MM, Muthukumaran G, Paterson Y. The ubiquitin-like protein, ISG15, is a novel tumor-associated antigen for cancer immunotherapy. Cancer Immunol Immunother. 2012;61:689–700. doi: 10.1007/s00262-011-1129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadjivasiliou A. ISG15 implicated in cytoskeleton disruption and promotion of breast cancer. Expert Rev Proteomics. 2012;9:7. [PubMed] [Google Scholar]
- 18.Ina S, Hirono S, Noda T, Yamaue H. Identifying molecular markers for chemosensitivity to gemcitabine in pancreatic cancer: increased expression of interferon-stimulated gene 15 kd is associated with intrinsic chemoresistance. Pancreas. 2010;39:473–485. doi: 10.1097/MPA.0b013e3181c0decc. [DOI] [PubMed] [Google Scholar]
- 19.Vincent-Chong VK, Ismail SM, Rahman ZA, Sharifah NA, Anwar A, Pradeep PJ, Ramanathan A, Karen-Ng LP, Kallarakkal TG, Mustafa WM, Abraham MT, Tay KK, Zain RB. Genome-wide analysis of oral squamous cell carcinomas revealed over expression of ISG15, Nestin and WNT11. Oral Dis. 2012;18:469–476. doi: 10.1111/j.1601-0825.2011.01894.x. [DOI] [PubMed] [Google Scholar]
- 20.Cajee UF, Hull R, Ntwasa M. Modification by ubiquitin-like proteins: significance in apoptosis and autophagy pathways. Int J Mol Sci. 2012;13:11804–11831. doi: 10.3390/ijms130911804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Zuo C, Wang X, Meng X, Xue B, Liu N, Yu R, Qin Y, Gao Y, Wang Q, Hu J, Wang L, Zhou Z, Liu B, Tan D, Guan Y, Zhu H. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proc Natl Acad Sci U S A. 2014;111:E1264–1273. doi: 10.1073/pnas.1320071111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, Liu H, Zhu H. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014;5:8429–8441. doi: 10.18632/oncotarget.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maheshwari A, Kantsevoy S, Jagannath S, Thuluvath PJ. Endoscopic ultrasound and fine-needle aspiration for the diagnosis of hepatocellular carcinoma. Clin Liver Dis. 2010;14:325–332. doi: 10.1016/j.cld.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Pitha-Rowe IF, Pitha PM. Viral defense, carcinogenesis and ISG15: novel roles for an old ISG. Cytokine Growth Factor Rev. 2007;18:409–417. doi: 10.1016/j.cytogfr.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laljee RP, Muddaiah S, Salagundi B, Cariappa PM, Indra AS, Sanjay V, Ramanathan A. Interferon stimulated gene-ISG15 is a potential diagnostic biomarker in oral squamous cell carcinomas. Asian Pac J Cancer Prev. 2013;14:1147–1150. doi: 10.7314/apjcp.2013.14.2.1147. [DOI] [PubMed] [Google Scholar]
- 26.Bektas N, Noetzel E, Veeck J, Press MF, Kristiansen G, Naami A, Hartmann A, Dimmler A, Beckmann MW, Knüchel R, Fasching PA, Dahl E. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008;10:R58. doi: 10.1186/bcr2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darb-Esfahani S, Sinn BV, Rudl M, Sehouli J, Braicu I, Dietel M, Denkert C. Interferon-stimulated gene, 15 kDa (ISG15) in ovarian high-grade serous carcinoma: prognostic impact and link to NF-κB pathway. Int J Gynecol Pathol. 2014;33:16–22. doi: 10.1097/PGP.0b013e31827b25a2. [DOI] [PubMed] [Google Scholar]
- 28.Chairatvit K, Wongnoppavich A, Choonate S. Up-regulation of interferon-stimulated gene15 and its conjugates by tumor necrosis factor-α via type I interferon-dependent and -independent pathways. Mol Cell Biochem. 2012;368:195–201. doi: 10.1007/s11010-012-1360-5. [DOI] [PubMed] [Google Scholar]
- 29.Tessema M, Yingling CM, Thomas CL, Klinge DM, Bernauer AM, Liu Y, Dacic S, Siegfried JM, Dahlberg SE, Schiller JH, Belinsky SA. SULF2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-I inhibitors via induction of ISG15. Oncogene. 2012;31:4107–4116. doi: 10.1038/onc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan XX, Chen HC, Khan MA, Xu AH, Yang FL, Zhang YY, Zhang DZ. ISG15 inhibits IFN-α-resistant liver cancer cell growth. Biomed Res Int. 2013;2013:570909. doi: 10.1155/2013/570909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sgorbissa A, Brancolini C. IFNs, ISGylation and cancer: Cui prodest? Cytokine Growth Factor Rev. 2012;23:307–314. doi: 10.1016/j.cytogfr.2012.07.003. [DOI] [PubMed] [Google Scholar]


