Abstract
Our study aimed to explore the differences in short and long-term outcomes about the transthoracic (TH) and abdominal-transhiatal (TH) approaches for treating esophagogastric junction (AEG). A systematic review of PubMed, EMbase, Cochrane Library, China National Knowledge Infrastructure and CBMdisc was performed. All original articles comparing TH with TA were included in the study. Meta-analysis was conducted using odd ratios (OR) and weighted mean differences (WMDs).Thirteen studies including 2489 patients with adenocarcinoma of the esophagogastric junction, with 1050 patients underwent TA and 1437 patients underwent TH were pooled for this study. There were no significant difference between two approaches concerning duration of operation, blood loss, anastomotic leakage and positive of proximal incisal margin. Lymph node excised also showed no significant differences between two procedures in RCTs while in TA group of Non-RCTs, the number of lymph node dissection is higher. TH approach was associated with a longer length of hospital stay and had higher incidence of respiratory and cardiovascular complications and early postoperative mortality. Overall analysis of 1, 3, 5-year survival showed no significant difference between two approaches. Based on the study, TA approach had a positive impact than TH for AEG with respect to respiratory and cardiovascular complications, hospital stay and early mortality rates. There were no significant differences between the two approaches for long-term survival. Therefore, two surgical approaches are acceptable, and the elders with poor cardiopulmonary function, we recommended TA approach for treating it.
Keywords: Gastric cancer, adenocarcinoma of the esophagogastric junction, Siewert type II/III, surgical resection, prognosis, meta-analysis
Introduction
Adenocarcinoma of the esophagogastric junction (AEG) was first described by Siewert in 1987. In contrast to the remarkably decreasing incidence of distal gastric cancer, it is evidently increasing in recently years, especially in the western countries [1-5]. AGE is a malignant tumor with early hematogenous and lymphatic dissemination. Though the use of chemotherapy, the 5-year survival rate was reported to be below 30% for AEG [6,7]. Controversies exist in the literatures about the etiology and classification of AGE according to the borderline location, which is between the stomach and the esophagus. Consequently, the effectiveness of different surgical approaches have also been controversial, resulting in the different long-term survival rates that were reported in recent literatures [4,8-12].
At present, en-bloc resection is still considered as the best way to treat the AEG type II/III. Due to the location of tumors’ centers is different, the Siewert classification of AEG provide a variety of surgical approaches. Over the past few decades, two major surgical approaches have emerged to improve survival rates-the the transthoracic approach (TH) and the abdominal-transhiatal (TA). TH provides superior visualization of the operative field and allows complete dissection of the tumor and the mediastinal lymph nodes [13-15], sometimes in combination with a thoracoabdominal resection. It has often been associated to higher complications and mortality rates [16-18]. Some scholars have attempted to reduce the mortality rates by limiting the extent of the resection. It might be achieved by abdominal-transhiatal resection (TA), thus avoiding a formal thoracotomy and limiting the extent of surgical trauma. Although three reviews have not revealed the superiority of TA to TH resection for AEG, Sasako M demonstrated the TA approach show better trend for survival of type II and III AEG than TH approach while some randomized controlled trials revealed opposite conclusion for type I and II AEG.
Although previous studies have sought to determine the most effective method, no conclusive evidence has been provided. This study aimed to compare the treatment effects and prognosis of the two operative approaches with respect to early postoperative mortality rate, postoperative complications and long-term survival rates. The pooled data of patients with Siewert type II/III adenocarcinoma of the esophagogastric junction were also evaluated in a systematic review of literature and examined by meta-analyses.
Materials and methods
This meta-analysis was conducted following the Cochrane Handbook (updated March 2011) and the Quality of Reporting Meta-analysis statement (QUOROM).
Methods used for literature search
We searched the electronic databases of PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, WeiPu, CBMdisc and WanFang systematically, using medical subject headings [((“Gastroesophageal Junction” [Mesh] AND “Carcinoma” [Mesh]) OR (“StomachNeoplasms” [Mesh] AND “Carcinoma” [Mesh]) OR (“Cardia” [Mesh] AND “Carcinoma” [Mesh]) OR (“Carcinoma” [Mesh] AND “Esophageal Neoplasms” [Mesh])) OR ((((((((gastroesophageal junction) OR gastroesophageal junction) OR gastroesophageal junction) OR distal esophageal) OR lower third esophageal) OR cardia) OR subcardial) OR siewert)] AND (((((transhiatal [Title/Abstract]) OR transabdominal [Title/Abstract]) OR transthoracic [Title/Abstract]) OR thoracoabdominal [Title/Abstract]) OR abdominothoracic [Title/Abstract]). Only those studies published in English and Chinese were included. The search was limited to the time between 1998 and 2015. To identify further relevant studies, the reference lists of articles identified were manually searched to find other related articles.
Inclusion criteria
Types of study
Randomized controlled trials (RCTs), clinical controlled trials, cohort studies, case-control studies and case series were all considered for inclusion.
Types of participants
1) Patients with pathologically diagnosed primary adenocarcinoma of cardia or subcardia according to Siewert classification (type II and III). 2) Patients who received TA or TH approach. 3) The studies which have intention to performing curative operation were included.
Types of interventions
Surgical interventions assessed the difference in outcomes between TH resection and TA resection as management for Siewert type II/III adenocarcinoma of the esophagogastric junction.
Types of outcome measures
The outcomes of the studies were reviewed with attention to early postoperative complications and mortality rates and long-term survival. The following outcome variables analyzed were included: (1) lymph nodes excised; (2) duration of operation; (3) volume of blood loss; (4) positivity of proximal incisal margin; (5) postoperative complications; (6) in-hospital mortality rate; (7) hospital stay and (8) 1, 3, 5-year survival rates.
Exclusion criteria
a) Patients who had other type of carcinomas rather than adenocarcinoma, and who had undergone other surgical approaches rather than TH or TA were excluded. b) Patients with important complication (such as benign diseases) or with distant metastases were both excluded. c) Unpublished RCTs and abstracts of RCTs presented at national and international meetings were also excluded to prevent the duplication of data. d) Studies not published in English and Chinese were excluded. e) Uncertain trials or ones with inequality of characteristics on baselines between groups were excluded. f) The studies with many cases lost during the follow-up period were excluded.
Date collection and analysis
Selection of studies
Three reviewers (J.Z, T.Z, K.W) screened the quality of the articles independently. To start with, they read through the title and abstract to eliminate unrelated studies, whereas, the related papers were retrieved for further identification. We managed disagreements through discussion and/or a deciding arbiter (MVD). All search results were put in Review Manager 5.2.
Data extraction and management
Three review authors (J.C, J.Y, Z.Z) designed a data extraction sheet for trial reports, which was a pilot study using the sample studies and revised by the other authors. The review authors (J.C, J.Y, Z.Z) then extracted data from the reports independently. We extracted data from each report separately and then combined it in the event of multiple reports for the same study. The discrepancies were addressed with discussion until consensus was achieved. We used the Newcastle-Ottawa Scale (NOS) described by Wells et al, to assess the quality of all the non-randomized controlled studies [19]. The quality of each study was assessed in three parameters: Selection, comparability and exposure. The full score was considered 10 stars. If studies evaluated with more than 6 stars, we were inclined to consider it as high quality trials. The Cochrane Library Handbook was used to assess the quality of randomized controlled studies (RCTs), which contained seven items: randomization, allocation concealment, baseline features, eligibility criteria, blinding, lose to follow-up and selection bias. Studies valued with six or seven “yes” were recognized as high quality, while four or five “yes” as moderate quality and not more than three “yes” as low quality.
All related data including the primary author, the year of publication, origin country, the type and period of study, the number of patients who performed operation by two approaches (TA and TH) and their characteristics were extracted by the reviewers independently.
Statistical analysis
Review Manager Version 5.2 supplied by the Cochrane Collaboration was employed for the analysis. Meta-analyses were conducted using odds ratios (ORs) for dichotomous data, which corresponds to the odds of adverse events appearing in the treatment group (TA), compared to the control group (TH). The OR >1 indicates the probability of a result to most likely occur in the treatment group, P<0.05 and 95% CI does was considered statistically significance. The Mantel-Haenszel method was used to combine the ORs for the results of interest. If the heterogeneity of results had significance, we would use a random effects model. On the contrary, a fixed effect was used. For continuous variables, we used the random effects weighted mean difference (WMD) as summary statistic. P<0.05 was considered statistically significant. Statistical heterogeneity in each meta-analysis was assessed using theτ2 (tau-squared), χ2 and I2 statistics [20]. For the computations, CI estimates and standard deviation are required. However, some published clinical trials only reported the size, median and range of the trial, rather than reporting the mean and standard deviation. For these available statistics, estimates of the mean and standard deviation were obtained using formulas proposed by Hozo et al [21]. For survival analysis, if trials reported survival curves only, the 1, 3, 5-year survival rates were extracted from the figures [22]. If the data which extracted could not be used for meta-analysis, we would like to present the conclusion in a descriptive and qualitative method [23]. Heterogeneity was considered as significant if either the P value of χ2 analysis was <0.10 or I2>50%.
Subgroup analyses included randomized controlled trials. Sensitivity analyses included studies which considered as high quality trials. Funnel plots were performed to determine the presence of publication bias in the meta-analysis.
Results
Selected studies: There were 2564 studies selected in the preliminary research, including 967 from Pubmed, 628 from EMbase, 542 from Cochrane Library, 201 from CNKI, 119 from Wan Fang, 71 from WeiPu and 36 from CBMdisc. After cross-browsing the title and abstracts by the reviewers, 233 potential studies were included and after reading the full-text, only 12 articles were selected. Meanwhile, by further searching the potential trials, only 1 study were in accordance with the inclusion criteria. Finally, there were 13 trials included in the meta-analysis [24-26,27-36] the flow chart containing selection information has been summarized in Figure 1. As a whole, selected studies included 3 randomized controlled trials, 1 prospective study and 9 retrospective studies. There were two studies which reported on the same patients with AEG, so we integrated with the pooled data from the two studies [28,34]. Among a total of 2489 patients, 1050 (42.2%) patients underwent TA and 1437 (57.7%) patients who underwent TH were compared. The detailed information of included studies and patient demographics are presented in (Tables 1 and 2).
Figure 1.
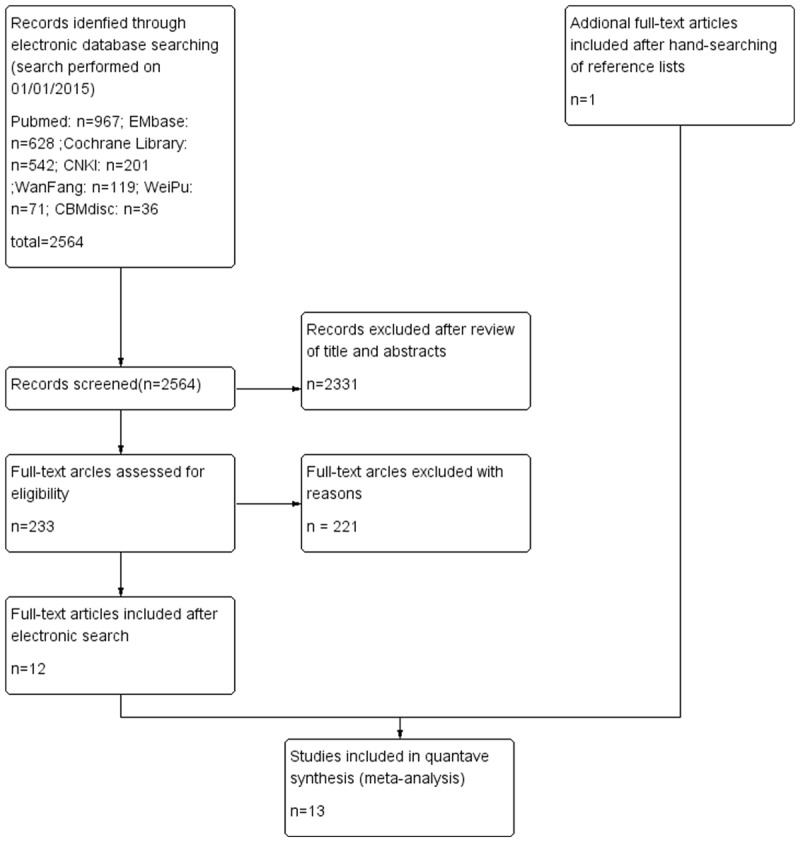
Flow chart providing information about different phases of systematic review.
Table 1.
Summary of included studies
| First author | Year | country | Study period | Study type | No. of patients | No. of TH | No. of TA | Quality |
|---|---|---|---|---|---|---|---|---|
| Graham | 1998 | UK | 1985-1997 | Retrospective | 153 | 32 | 119 | 7 |
| Guan GX | 2011 | China | 1990-2004 | Retrospective | 251 | 123 | 128 | 6 |
| Omloo | 2007 | Netherlands | 1994-2000 | RCT | 220 | 114 | 106 | - |
| Hulscher | 2002 | Netherlands | 1994-2000 | RCT | 220 | 114 | 106 | - |
| Zhou JZ | 2014 | China | 2007-2012 | Retrospective | 334 | 140 | 194 | 7 |
| J. Wayman | 1999 | UK | 1991-1995 | prospective | 40 | 20 | 20 | 6 |
| Kikuo Koufuji | 2005 | Japan | 1991-2000 | Retrospective | 49 | 24 | 25 | 6 |
| Zhang XY | 2014 | China | 2007-2012 | Retrospective | 135 | 57 | 78 | 6 |
| Zhu ZJ | 2006 | China | 2001-2005 | Retrospective | 273 | 175 | 98 | 5 |
| Mitsuru Sasako | 2006 | Japan | 1995-2003 | RCT | 167 | 85 | 82 | - |
| Qin YS | 2012 | China | 2000-2006 | Retrospective | 185 | 133 | 52 | 7 |
| Wu ZY | 2005 | China | 1994-2004 | Retrospective | 351 | 250 | 101 | 5 |
| Zheng B | 2010 | China | 1994-2003 | Retrospective | 331 | 284 | 47 | 7 |
Table 2.
Quality assessment of the included RCTs
| Study | Random allocation | Double-blind | Concealed allocation | Baseline features | Eligibility criteria | Loss to follow-up | Selection bias | Quality |
|---|---|---|---|---|---|---|---|---|
| Mitsuru Sasako | Yes | No | Yes | Yes | yes | Yes | No | Fair |
| Hulscher | Yes | No | Unknown | Yes | yes | Yes | No | Fair |
| Omloo | Yes | No | Unknown | Yes | yes | Yes | No | Fair |
Surgical outcomes
Extent of lymphadenectomy
The number of lymph node dissection was sufficiently reported in 7 studies, which included 2 RCTs [25,34] and 5 Non-RCTs articles [27,29,31-33]. No significant difference between TH and TA group was found in RCTs (WMD=3.72, 95 CI: -18.81~26.26, P=0.75). While in Non-RCTs (WMD=-1.37, 95 CI: -2.51~-0.24, P=0.02) studies, TA a mean of 2 more lymph nodes were retrieved compared to the TH. However, heterogeneity was significant (P<0.001, I2=97%) (Figure 2).
Figure 2.
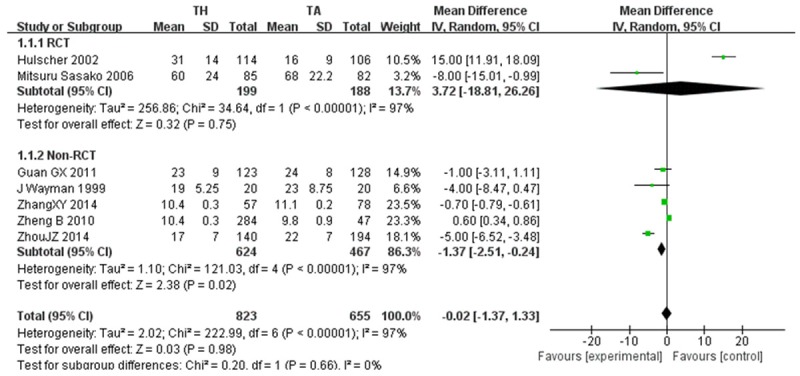
Extent of lymphadenectomy.
Duration of operation and blood loss
Eight articles [24,25,27,29,31-33,36] offered the data about the duration of operation. However, analysis of the data showed no significance between the two approaches (WMD=25.85, 95 CI: -8.70~60.40, P=0.14). Although six studies [25,27,29,31,32,36] provided the data on blood loss, there were no significance between the two approaches (WMD=33.90, 95 CI: -0.56~68.37, P=0.05) (Figure 3).
Figure 3.
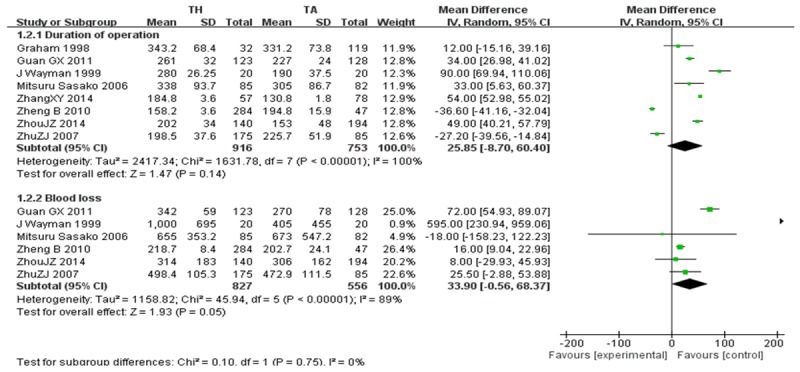
Duration of operation and Blood loss.
Positive of proximal incisal margin
The incidence of positive of proximal incisal margin [25,26,31,33] between the TH group (26 of 398, 6.5%) and the TA group (12 of 340, 3.5%; OR, 1.25; CI, 0.61~2.56; P=0.55) were no significance. Heterogeneity between the studies was not significant (P=0.60; I2=0%) (Figure 4).
Figure 4.
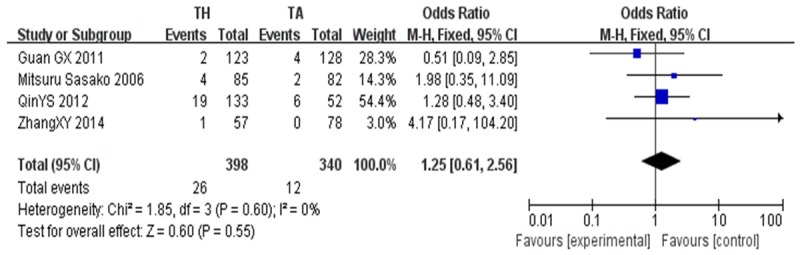
Positive of proximal incisal margin.
Perioperative safety and short-term outcome
Early mortality
Early mortality [24-31,34], reported as either <30 day or in-hospital mortality, was greater for TH approach (32 of 925, 3.5%) as compared to the TA approach (10 of 674, 1.5%; OR, 3.00; CI, 1.45~6.21; P=0.003). Studies did not show any significant heterogeneity (P=0.91; I2=0%) (Figure 5).
Figure 5.
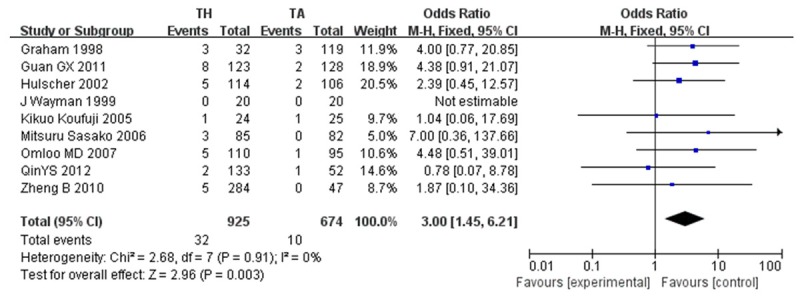
Early mortality.
Anastomotic leak
12 studies [24-27,29-36]reported no significant difference between two approaches (OR, 1.22; CI, 0.82~1.80). Heterogeneity was not significant (P=0.42; I2=3%) (Figure 6).
Figure 6.
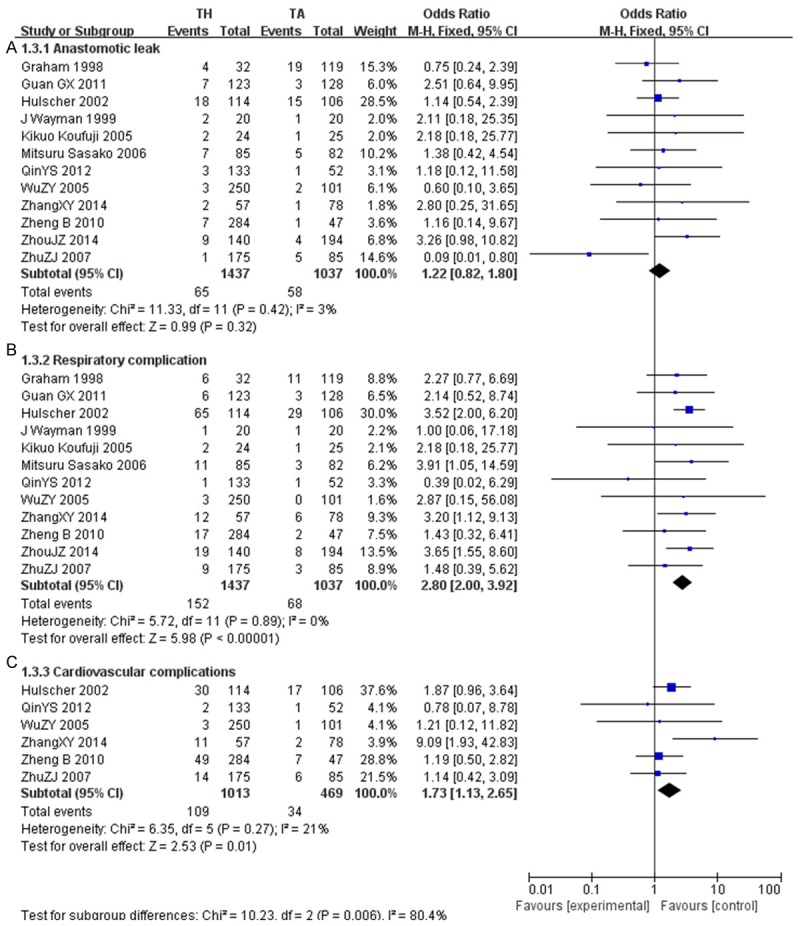
Postoperative complications (A. Anastomotic leak; B. Respiratory complications; C. Cardiovascular complications).
Respiratory and cardiovascular complications
The incidence of overall respiratory complications [24-27,29-36] increased significantly in the TH group (152 of 1437, 10.58%) as compared to the TA group (68 of 1037, 6.55%; OR, 2.80; CI, 2.00~3.92; P<0.001). Heterogeneity between studies was not significant (P=0.89; I2=0%). In addition, rates of cardiovascular complications [26,27,33-36] were significantly lower in TA (34/469, 7.25%) than in TH (109 of 1013, 10.76%; OR, 1.73; CI, 1.13-2.65; P=0.01). Studies did not show significant heterogeneity (P=0.27; I2=21%) (Figure 6).
Hospital stay
The postoperative duration of the hospital stay [27,29,34] for patients who underwent TH was on an average 2 days longer than that for the TA patients (P<0.001; CI, 1.00~3.00). Heterogeneity was not significant for hospital stay (P=0.69; I2=0%) (Figure 7).
Figure 7.
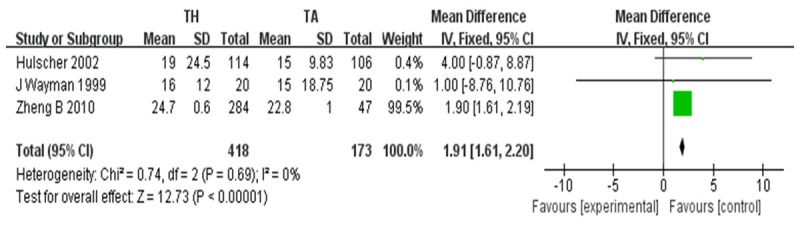
Hospital stay.
Long-term outcomes
1, 3-Year survival
Overall analysis of 1-year survival [26,27] showed no significant difference between the TH group (314 of 417, 75.3%) and TA group (79 of 99, 79.8%; OR, 0.90; CI, 0.52~1.56; P=0.71). No heterogeneity was detected for any other assessed outcomes (P=0.83; I2=0%). Meanwhile, three articles reported a 3-year survival rate [26,27,31], with an overall OR of 0.88, with 95% CI of 0.63-1.23. The study did not show significant difference in 3-year survival rate (P=0.39; I2=0%) (Figure 8).
Figure 8.
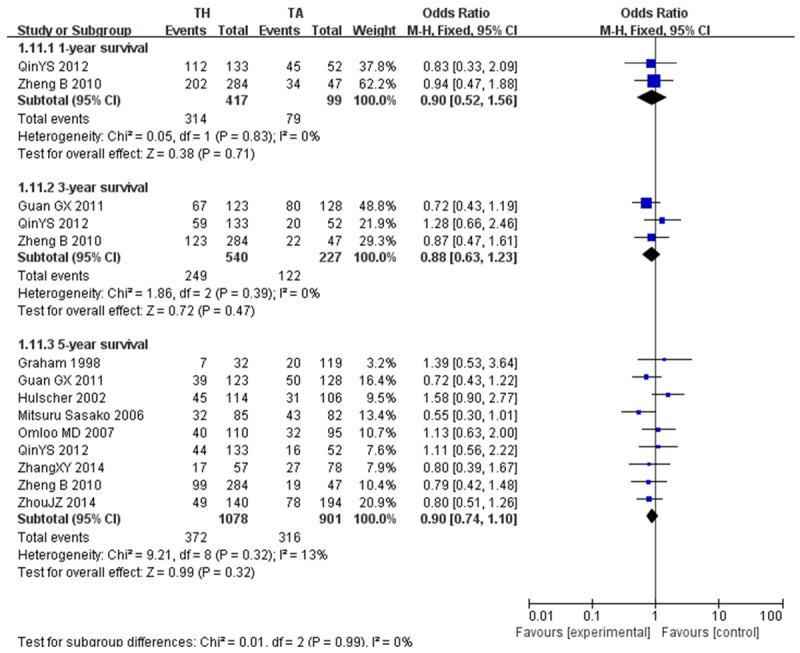
1, 3, 5-year survival.
5-Year survival
The most important outcome after any cancer treatment is the actual 5-year survival rate [24,31,34]. Interestingly this meta-analysis did not show any significant differences in the 5-year survival rate between the TH groups and TA groups (OR, 0.90; CI, 0.74~1.10; P=0.32), and was not subjected to heterogeneity (P=0.32; I2=13%) (Figure 8).
Sensitivity analyses
8 Non-RCTs studies [24,26,27,29-33] received ≥6 points for methodological quality and 3 RCTs studies [25,28,34] of Fair level in sensitivity analysis. Duration of operation, blood loss, positive of proximal incisal margin, anastomotic leak, early mortality, respiratory complication, cardiovascular complications, hospital stay and 1, 3, 5-year survival showed the same outcomes as in the overall analysis. Significant findings specific to high quality sensitivity analysis were: In TA, more lymph nodes were retrieved as compared with TA (OR: -0.57; 95% CI: -0.65~-0.48), although heterogeneity was significant (P<0.00001, I2=97%) (Table 3).
Table 3.
Sensitivity analysis performed for studies comparing TA and TH: high quality studies
| No. of studies | No. of patients | OR/WMD | 95% CI | P-value | HG χ2 P-value | HG I2 | |
|---|---|---|---|---|---|---|---|
| Surgical outcomes | |||||||
| Extent of Lymphadenectomy | 7 | 1478 | -0.57 | -0.65, -0.48 | <0.00001 | <0.00001 | 97 |
| Duration of Operation | 7 | 1409 | 33.53 | -3.03, 70.09 | 0.07 | <0.00001 | 100 |
| Blood Loss | 5 | 1123 | 37.07 | -6.94, 81.07 | 0.10 | <0.00001 | 91 |
| Positive of Proximal margin | 4 | 738 | 1.25 | 0.61, 2.56 | 0.55 | 0.60 | 0 |
| Perioperative Safety and Short-term Outcome | |||||||
| Early Mortality | 9 | 1599 | 3.00 | 1.45, 6.21 | 0.003 | 0.91 | 0 |
| Anastomotic Leak | 10 | 1863 | 1.47 | 0.97, 2.24 | 0.07 | 0.87 | 0 |
| Respiratory complication | 10 | 1863 | 2.93 | 2.06, 4.15 | <0.0001 | 0.85 | 0 |
| Cardiovascular Complications | 4 | 871 | 1.93 | 1.19, 3.13 | 0.008 | 0.13 | 46 |
| Hospital Stay | 3 | 591 | 1.91 | 1.61, 2.20 | <0.00001 | 0.69 | 0 |
| Long-term outcomes | |||||||
| 1-Year Survival | 2 | 516 | 0.90 | 0.52, 1.56 | 0.71 | 0.83 | 0 |
| 3-Year Survival | 3 | 767 | 0.88 | .0.63, 1.23 | 0.47 | 0.39 | 0 |
| 5-Year Survival | 9 | 1979 | 0.90 | 0.74, 1.10 | 0.32 | 0.32 | 13 |
OR, odds ratio; WMD, weighted mean difference; CI, confidence interval; HG, heterogeneity; χ2, Chi squared.
Publication bias
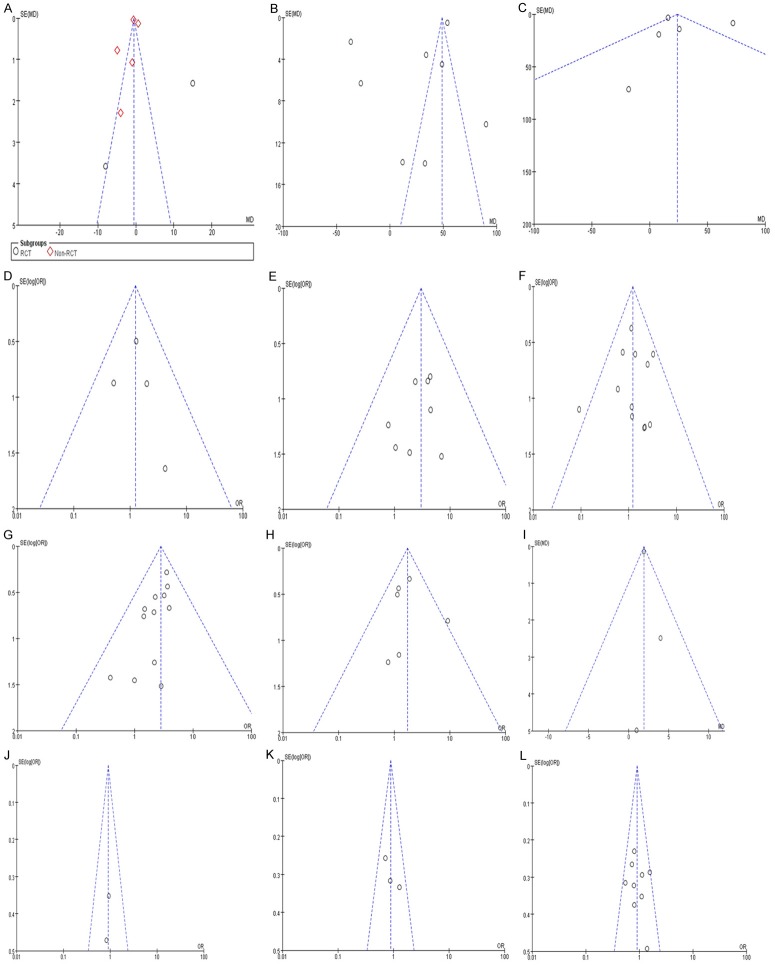
Funnel plots (precision vs. WMD or log OR) demonstrated asymmetry for the extent of lymphadenectomy, blood loss, anastomotic leak and length of hospital stay suggesting presence of publication bias for these outcomes. No points were outside the 95% confidence interval limits for early mortality, duration of operation, respiratory and cardiovascular complications, positive of proximal incisal margin, and 1, 3, 5-year survival suggesting the absence of publication bias for these outcomes (Figure 9).
Figure 9.
Funnel plots demonstrate asymmetry for extent of lymphadenectomy. (A) Blood loss; (C) Anastomotic leak (F) and length of hospital stay (I) suggesting the presence of publication bias. No points fall outside the 95% CI limits for any variables suggesting the absence of publication bias. (A) Extent of lymphadenectomy; (B) Duration of Operation; (C) Blood loss; (D) Positive of proximal margin; (E) Early mortality; (F) Anastomotic leak; (G) Respiratory complication; (H) Cardiovascular complication; (I) Hospital stay; (J) 1-Year survival; (K) 3-Year survival; (L) 5-Year survival.
Discussion
The two major surgical approaches in treating Type II/III AEG are transthoracic (TH) and abdominal-transhiatal (TA) approach and it has been controversial as to which approach is reasonable. Both approaches have their advantages and disadvantages. Our study aimed to evaluate the differences in short and long-term results about the two approaches for providing evidence-based study basis on the treatment of AEG. In this study, we compared the two approaches and the outcomes of the meta-analysis has demonstrated TA to be associated with significantly reduced duration of hospital stays, early mortality and lower rate of postoperative respiratory and cardiovascular complications. Meanwhile, the incidence of proximal positive incisal margin was not increase after TA. Our results showed that there were no significant differences between the two approaches for anastomotic leak, blood loss, duration of operation, the number of lymph node dissection and 1, 3, 5-year survival.
When all data were combined, the number of lymph node dissection did not differ significantly between TH and TA procedures, but the pooled data from Non-RCTs were showed a trend of increasing lymph node accumulation in the TA approach. That may be due to Siewert II/III AEG mainly transfers downward to abdominal lymph node, TA method can be provide direct visualization of the lymph nodes of the abdominal cavity. Because of the location of the tumor between the esophagus and stomach, it is important to determine the complexity of lymph node metastasis, as in the surgery it may be need to clean up the abdominal and thoracic lymph nodes at the same time. Lymph node clearance is regarded as a prognostic factors for the adenocarcinoma of the esophagogastric junction [37]. According to a recent study, the metastasis of posterior mediastinal lymph nodes, paraesophageal lymph nodes and superior phrenic lymph node of AEG accounted for 10%-20% in overall lymph node metastasis. With the change in the tumor location towards the distal, the incidence of pleural metastasis gradually declines. However, we should still adopt the radical operation in order to reduce the postoperative recurrence rate [6,38]. Different subtypes of AEGs mainly transfers downward to abdominal lymph node and may also transfer upward to mediastinal lymph node. The transfer rate of mediastinal lymph node of Siewert type I was the highest, while Type II/III it was lowest and mainly metastasis to posterior and inferior mediastinum. It is reported by Siewert [25] et al that the transfer rate of mediastinum lymph node of II/III AEG were 12% and 6% respectively, of which superior mediastinum rate was only 1%. This shows that the lymph node metastasis of Siewert II/III was more transferred to the abdominal cavity. Our meta-analysis results are similar with the Siewert’s outcomes. In addition, the transfer rate of mediastinal lymph node was associated with the esophagus infiltrating depth and range. The more of the tumor infiltration, the higher of mediastinal lymph node rate and vice versa [39]. Considering the same reason, following consensus has been formed: a) Lymph node dissection of radical resection of II/III AEG should focus on the abdominal cavity. The mediastinal lymph node dissection should be executed based on AEG types, stage, and the esophagus infiltrating degree. b) In principle, Siewert II/III AEG should be in accordance with the operation specification about the upper stomach cancer determined the scope of lymph node cleaning. In case of tumor infiltration, the distal esophagus, resection of the inferior mediastinum lymph node, hiatal lymph nodes and inferior phrenic lymph nodes should be taken into consideration. We did not remove the superior mediastinal lymph nodes in theory. Although, abdominal lymph node metastasis is the major route of Siewert II/III AEG and thoroughly dissection abdominal lymph node is the key way, there is no long-term survival benefit about extended lymph nodes of inferior mediastinum resection [25].
Transthoracic resections resulted in worse postoperative complications than transhiatal resections in our meta-analysis, especially respiratory and cardiovascular complication, but some scholars reported that there was no significantly difference of respiratory complication between TH and TA approaches. Theoretically, TA resections had the deficiency in formal thoracotomy, resulting in a higher frequency of pulmonary and cardiovascular complications [40]. This was confirmed by the present data. The pain after thoracotomy restrained diaphragmatic movement and difficulty to expectorate could interpret why respiratory complications were lower in TA procedure. While cardiovascular complications might be related to myocardial ischemia from pain, hypoxemia, electrolyte disturbance after thoracotomy. However, there was no significantly difference about the incidence of anastomotic leakage. In terms of anastomotic leakage, several articles have reported various results [18,41,42] That is probably due to cough from pulmonary complication that increases the tension of anastomosis; on the other hand, pleural effusion drainage was inadequate and infection persisted, resulting in anastomosis disunion. In addition, after pooling the data, the early morbidity (which is defined as hospital deaths related to special diseases within 30 days after surgery) rates was higher in TH (OR, 3.00; CI, 1.45-6.21). Consequently, TA approach helped patients to recover more quickly and significantly decreased the duration of hospital stay [43].
Meta-analysis on included studies showed that TA had no significant difference from TH on 1, 3, 5-year overall survival rates. This outcome is supported by previously literature [40]. However, a recent article predicted a superior 5-year survival for transhiatal resections, which is consistent with that in one included studies that reported potential higher overall survival rate in TA approach compared with the TH approach in type II tumors [25]. For type III tumor [25], one studies showed potential 5-year survival benefits in the TA approach. Therefore some researchers recommend the TA approach for type II/III AEG. Meanwhile, Mariette [17] et al reported overall 3 or 5 year survival rates were not affected by the choice of surgical approach. The difference in survival rates was associated with R0 resection, pathological node-positive category and tumor differentiation. Some scholars [44] also underlined age as a risk factor for long-term survival. Thus, the long-term survival of patients with Siewert type II/III AEG may also be influenced by tumor stage, complication and completely resection.
There are still some limitations associated with this meta-analysis, though the study was executed according to the Quality of Reporting Meta-Analyses (QUOROM) statements and the Cochrane Handbook (updated March 2011). First of all, the quality of meta-analysis lies on original trials included. In this analysis, 9 original articles were retrospective studies and 1 article was prospective studies. Although they all are the best evidences available to date, the outcomes are less compelling compare to randomized controlled trials (RCT). And there was a potential heterogeneity in several aspects. Meanwhile, RCTs, prospective studies and retrospective studies were assessed using different standards and then pooled together, which may decrease the power of the results. Secondly, the exclusion of original articles published in languages other than English and Chinese is also another potential limitation to this meta-analysis. Thirdly, publication bias was detected on funnel plot analysis for results of extent of lymphadenectomy, blood loss, anastomotic leak and length of hospital stay. This is of concern as the selective publication studies may result in pooled effect sizes obtained from studies exclusively located from the published scientific literature revealing a more significant result in terms of the magnitude of harm or benefit of an intervention than in actual [45,46]. However, it should be noted there are number of limitation to the assessment of publication bias in the meta-analysis. First, the number of articles was inadequate to sensitively detect such a bias [47]. Second, a random effects model of meta-analysis was utilized for this analysis, and this model is known to exaggerate the presence of publication bias due to attributing heavier weighting to smaller studies than the fixed effects model of meta-analysis. Finally, funnel plots are not an ideal method for assessing publication bias due to their inability to differentiate between forms of bias, however they remain the accepted method for assessment of publication bias [48,49]. Then, we used the median to estimate the mean and standard deviation, because the ranges are unstable and unlike other measures of variation, it increase when the sample size increases. They describe the extremes of observed outcomes rather than the average variation. Therefore, ranges have not been recommended to estimate standard deviations, and it may lead to unpersuasive outcomes.
In conclusion, this analysis has shown the TH group has higher incidence of respiratory and cardiovascular complications and early postoperative mortality, so that TH method increased the duration of hospital stay. Furthermore, our results failed to show TA approach bring more advantages to the patients who underwent TH resection on 1, 3, 5-year survival rates. Although two surgical approaches are acceptable, for the elders with poor cardiopulmonary function, we recommended TA approach for treating Siewert II/III AEG in order to achieve better quality of life and reduce early mortality.
Acknowledgements
This study was supported by grants from Capital clinical characteristic application study in Science and Technology Commission of Beijing (NO. Z131107002213046).
Disclosure of conflict of interest
None.
References
- 1.Devesa SS, Blot WJ, Fraumeni JJ. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–9. [PubMed] [Google Scholar]
- 3.Hansen S, Wiig JN, Giercksky KE, Tretli S. Esophageal and gastric carcinoma in Norway 1958-1992: incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer. 1997;71:340–4. doi: 10.1002/(sici)1097-0215(19970502)71:3<340::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TN, Carstens H, Lundell L, Albertsson M. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 5.Falk J, Carstens H, Lundell L, Albertsson M. Incidence of carcinoma of the oesophagus and gastric cardia. Changes over time and geographical differences. Acta Oncol. 2007;46:1070–4. doi: 10.1080/02841860701403046. [DOI] [PubMed] [Google Scholar]
- 6.Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139–46. doi: 10.1002/jso.20218. discussion 146. [DOI] [PubMed] [Google Scholar]
- 7.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009;27:5062–7. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 8.Parshad R, Singh RK, Kumar A, Gupta SD, Chattopadhyay TK. Adenocarcinoma of distal esophagus and gastroesophageal junction: long-term results of surgical treatment in a North Indian Center. World J Surg. 1999;23:277–83. doi: 10.1007/pl00013179. [DOI] [PubMed] [Google Scholar]
- 9.Wijnhoven BP, Siersema PD, Hop WC, van Dekken H, Tilanus HW. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86:529–35. doi: 10.1046/j.1365-2168.1999.01082.x. [DOI] [PubMed] [Google Scholar]
- 10.Kajiyama Y, Tsurumaru M, Udagawa H, Tsutsumi K, Kinoshita Y, Ueno M, Akiyama H. Prognostic factors in adenocarcinoma of the gastric cardia: pathologic stage analysis and multivariate regression analysis. J. Clin. Oncol. 1997;15:2015–21. doi: 10.1200/JCO.1997.15.5.2015. [DOI] [PubMed] [Google Scholar]
- 11.Ellis FJ, Heatley GJ, Krasna MJ, Williamson WA, Balogh K. Esophagogastrectomy for carcinoma of the esophagus and cardia: A comparison of findings and results after standard resection in three consecutive eight-year intervals with improved staging criteria. J Thorac Cardiovasc Surg. 1997;113:836–46. doi: 10.1016/S0022-5223(97)70256-3. discussion 846-8. [DOI] [PubMed] [Google Scholar]
- 12.Steup WH, De Leyn P, Deneffe G, Van Raemdonck D, Coosemans W, Lerut T. Tumors of the esophagogastric junction. Long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J Thorac Cardiovasc Surg. 1996;111:85–94. doi: 10.1016/S0022-5223(96)70404-X. discussion 94-5. [DOI] [PubMed] [Google Scholar]
- 13.Yekebas EF, Schurr PG, Kaifi JT, Link BC, Kutup A, Mann O, Wolfram L, Izbicki JR. Effectiveness of radical en-bloc-esophagectomy compared to transhiatal esophagectomy in squamous cell cancer of the esophagus is influenced by nodal micrometastases. J Surg Oncol. 2006;93:541–9. doi: 10.1002/jso.20544. [DOI] [PubMed] [Google Scholar]
- 14.Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85:424–9. doi: 10.1016/j.athoracsur.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Wong J, Law S. Two approaches to cancer of the cardia. Lancet Oncol. 2006;7:613–5. doi: 10.1016/S1470-2045(06)70770-7. [DOI] [PubMed] [Google Scholar]
- 16.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 17.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545–53. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 18.Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust N Z J Surg. 1999;69:187–94. doi: 10.1046/j.1440-1622.1999.01520.x. [DOI] [PubMed] [Google Scholar]
- 19.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R, Glenville B. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745–53. doi: 10.1016/j.athoracsur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Gertler R, Rosenberg R, Feith M, Schuster T, Friess H. Pouch vs. no pouch following total gastrectomy: Meta-analysis and systematic review. Am J Gastroenterol. 2009;104:2838–51. doi: 10.1038/ajg.2009.456. [DOI] [PubMed] [Google Scholar]
- 24.Graham AJ, Finley RJ, Clifton JC, Evans KG, Fradet G. Surgical management of adenocarcinoma of the cardia. Am J Surg. 1998;175:418–21. doi: 10.1016/S0002-9610(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 25.Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, Nashimoto A, Hiratsuka M Japan Clinical Oncology Group (JCOG9502) Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol. 2006;7:644–51. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 26.Qin YS, Zhuang YZ, Yang JS, Huang CJ, Xu QZ, Huang MS. Surgical treatment of Siewert II adenocarcinoma of the esophagogastric junction. Chin J Gastrointest Surg. 2012;15:910–2. [PubMed] [Google Scholar]
- 27.Zheng B, Chen YB, Hu Y, Wang JY, Zhou ZW, Fu JH. Comparison of transthoracic and transabdominal surgical approaches for the treatment of adenocarcinoma of the cardia. Chin J Cancer. 2010;29:747–51. doi: 10.5732/cjc.009.10748. [DOI] [PubMed] [Google Scholar]
- 28.Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, van Dekken H, Ten Kate FJ, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1000. doi: 10.1097/SLA.0b013e31815c4037. discussion 1000-1. [DOI] [PubMed] [Google Scholar]
- 29.Wayman J, Dresner SM, Raimes SA, Griffin SM. Transhiatal approach to total gastrectomy for adenocarcinoma of the gastric cardia. Br J Surg. 1999;86:536–40. doi: 10.1046/j.1365-2168.1999.01043.x. [DOI] [PubMed] [Google Scholar]
- 30.Koufuji K, Shirouzu K, Aoyagi K, Yano S, Miyagi M, Imaizumi T, Takeda J. Surgery and clinicopathological features of gastric adenocarcinoma involving the esophago-gastric junction. Kurume Med J. 2005;52:73–9. doi: 10.2739/kurumemedj.52.73. [DOI] [PubMed] [Google Scholar]
- 31.Guan GX, et al. Effects of different surgical approaches on Siewert II adenocarcinoma of esophagogastric junction. Chin J Gen Surg. 2011;26:721–5. [Google Scholar]
- 32.Zhou JZ, et al. Comparison of clinical outcome of transthoracic and transabdominal hiatal approaches for the treatment of Siewert type II and III adenocarcinoma of the esophagogastric junction. Chin J Dig Surg. 2014;13:105–9. [Google Scholar]
- 33.Zhang X, Yang J, Ping H, Zuo H, Yang L. Choice of surgical approach for Siewert II and III adenocarcinomas of the esophagogastric junction. Chin J Gastrointest Surg. 2014;17:924–6. [PubMed] [Google Scholar]
- 34.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 35.Wu ZY, et al. Comparison of outcome of transthoracic and transabdominal hiatal resection for the adenocarcinoma of the cardia. Chin J Gastrointest Surg. 2005;8:263–4. [Google Scholar]
- 36.Zhu ZJ, et al. Clinical analysis of transthoracic and transabdominal operation for cardia cancer. Chin J Thorac Cardiovasc Surg. 2007;23:25. [Google Scholar]
- 37.Gronnier C, Piessen G, Mariette C. Diagnosis and treatment of non-metastatic esophagogastric junction adenocarcinoma: what are the current options? J Visc Surg. 2012;149:e23–33. doi: 10.1016/j.jviscsurg.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Ide H, Eguchi R, Ota M, Shimizu S, Isono K. Adenocarcinoma of the esophagogastric junction: A summary of responses to a questionnaire on adenocarcinoma of the esophagus and the esophagogastric junction in Japan. Dis Esophagus. 2002;15:219–25. doi: 10.1046/j.1442-2050.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Baba H, Egashira A, Oki E, Ikebe M, Kakeji Y, Maehara Y. Adenocarcinoma of the esophagogastric junction in Japan. Hepatogastroenterology. 2008;55:103–7. [PubMed] [Google Scholar]
- 40.Chou SH, Chuang HY, Huang MF, Lee CH, Yau HM. A prospective comparison of transthoracic and transhiatal resection for esophageal carcinoma in Asians. Hepatogastroenterology. 2009;56:707–10. [PubMed] [Google Scholar]
- 41.Carboni F, Lorusso R, Santoro R, Lepiane P, Mancini P, Sperduti I, Santoro E. Adenocarcinoma of the esophagogastric junction: the role of abdominal-transhiatal resection. Ann Surg Oncol. 2009;16:304–10. doi: 10.1245/s10434-008-0247-x. [DOI] [PubMed] [Google Scholar]
- 42.Chou SH, Kao EL, Chuang HY, Wang WM, Wu DC, Huang MF. Transthoracic or transhiatal resection for middle- and lower-third esophageal carcinoma? Kaohsiung J Med Sci. 2005;21:9–14. doi: 10.1016/S1607-551X(09)70270-0. [DOI] [PubMed] [Google Scholar]
- 43.Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: A meta-analysis. Ann Surg. 2011;254:894–906. doi: 10.1097/SLA.0b013e3182263781. [DOI] [PubMed] [Google Scholar]
- 44.Jensen LS, Pilegaard HK, Puho E, Pahle E, Melsen NC. Outcome after transthoracic resection of carcinoma of the oesophagus and oesophago-gastric junction. Scand J Surg. 2005;94:191–6. doi: 10.1177/145749690509400303. [DOI] [PubMed] [Google Scholar]
- 45.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 46.Sauerland S, Seiler CM. Role of systematic reviews and meta-analysis in evidence-based medicine. World J Surg. 2005;29:582–7. doi: 10.1007/s00268-005-7917-7. [DOI] [PubMed] [Google Scholar]
- 47.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 48.Greenland S. Invited commentary: A critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140:290–6. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 49.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]