Abstract
Purpose: Rhabdomyosarcoma (RMS) is the most frequent soft tissue sarcoma in children. We have retrospectively explored the treatment results of childhood RMS and identified prognostic factors in multicenter in China, in order to lay the foundation for further multicenter study. Methods: This retrospective study was carried out analyzing the medical records of 161 patients with the pathological diagnosis of RMS from January, 2001 to February, 2014 at 5 large cancer centers in China. The data was reviewed clinico-epidemiological factors. Age, gender, histology type, primary site, tumor size, intergroup rhabdomyosarcoma study (IRS) group and results of treatments were evaluated. Patients were followed up to Dec 31, 2014. Results: The median age of our patients was 51 months. 10.5% of our patients were infants. The genitourinary system was the most common primary site of tumor (43.5%). The proportion of primary site of head and neck except parameningeal, at 28.2% (42 cases), while the proportion of parameningeal region was 4.6% (7 cases). The histological findings were as follows: 130 cases (80.7%) with embryonal, 19 cases (11.9%) with alveolar and 5 cases (3.1%) with botryoid type. According to the classification system of the IRS group, 1 case (0.6%) was group I, 54 cases (33.5%) were group II, 46 cases (28.6%) were group III and 60 cases (37.3 %) were group IV. 149 patients were treated and followed-up regularly, Patients in Beijing children’s hospital (n=95) were enrolled in IRS-II/COG-D9803, D9802 protocols. while the other patients (n=54) started on treatment according to Chinese Anti-cancer Association protocol. There were median time of 51 months for following up, 60 occurred event. The ten-year event free survival rate was 53.4±5.1%, overall survival was 65.3±6.3%. The relations between outcome and age (0.046), primary site (0.022), pathologic subtype (0.013), tumor size (0.008) and IRS group (P=0.000) were associated significantly with event free survival. Among the variables, age (P=0.028) and IRS group (P=0.000) were associated significantly with overall survival. Multivariate analysis showed that overall survival for RMS was dependent on IRS group (P=0.026). Conclusions: The epidemiological characteristics of our patients are quite similarly to the worldwide data. Except for the higher prevalence of group IV in our patients and the higher percentage of patients with primary tumor site in the genitourinary system, this study showed that overall survival for RMS is depended on disease group.
Keywords: Childhood RMS, multicenter, China
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in childhood, accounting for approximately 6.5% of pediatric tumors in China [1]. Recently, multimodality diagnosis and therapy were used to increased survival rate in China. Unfortunately, the information about the prognosis for patients and progression-free survival of RMS are absence in multicenter in China. Because of the disappointing results, as well as the limited enrollment of patients, we discuss the final results and detailed analysis of prognostic factors in clinical characteristics and prognosis of childhood RMS at 5 larger cancer centers was reported, from 2001 to 2014, onto study of Chinese Anti-cancer Association (CAA).
Patients and methods
A retrospective analysis was performed on 161 medical records of children with RMS who were admitted, treated, and followed up at the hematology/oncology wards of pediatric departments in Beijing Children’s Hospital (BCH), Beijing Tongren Hospital, Beijing Shijitan Hospital, Wuhan Children’s Hospital and Peking University First Hospital during the period from January, 2001 to February, 2014. This retrospective study was approved by the hospital ethics committee, and informed consents were provided to all the patients. Patients were followed up to Dec 31, 2014. The medical records were reviewed for:
1) Personal data for example, name, age, gender, nationality and residence. 2) Presenting symptoms and signs, primary site of the tumor, pathologic subtype characters of the tumor. 3) Routine laboratory investigations at presentation and during treatment, for example, complete blood count, liver function tests, kidney function tests, serum electrolytes, LDH, bone marrow aspirate. 4) Imaging studies: ultrasound, CT, and MRI, on primary site and other common metastatic sites. 5) Risk stratification for patients treated according to IRS-II/COG D9803 and D9802 for BCH; CAA protocol for other hospitals. 6) Treatment protocols including surgery, radiotherapy, and chemotherapy, patients’ outcome.
Treatment regimens
According to the IRSG-II, most patients with RMS in BCH treated by surgery, radiotherapy and chemotherapy consisted of vincristine, actinomycin and cyclophosphamide (VAC) over two years. Since 2009, VAC was used for patients with low-risk RMS, VAC alternating with vincristine, topotecan and cyclophosphamide (VTC) was used for patients with intermediate and high-risk RMS for 14-16 cycles in modified COG D9803 and D9802 [2,3].
For CAA protocols [4,5], the chemotherapy consisted of vincristine, cyclophosphamide and cisplatin (VCP) alternating for etoposide, ifosfamide and vincristine (IEV), alternating for vincristine, cyclophosphamide, cisplatin and doxorubicin (AVCP), alternating for actinomycin. etoposide and vincristine (DEV). After completing remission, got 6 cycles of chemotherapy and stopped treatment. And surgery, radiotherapy was performed after 5-6 cycles of chemotherapy for all protocols.
Statistical methods
The Kaplan-Meier method was used to estimate the event free survival (EFS) and overall survival (OS) distributions. Differences between survival curves were analyzed by the log-rank test. Cox proportional hazards regression models were used to describe the association between the risk of failure and some status while accounting for potential confounding factors. The distributions of categorical patients’ characteristics were compared between the groups by using a Fisher’s exact test. P<0.05 was considered statistically significant, unless otherwise indicated. The site of first recurrence was defined as local if the tumor recurred at the primary site only; or regional if regional lymph nodes were involved, with or without local recurrence; or distant if any metastatic disease was present.
An early failure was defined as follows: First, a failure caused by progressive diseases, second: death as a result of progressive disease or other causes, which lasting less than 120 days, from the start of the study resulting in discontinuation of study protocol.
Results
Patient demographics
Total 161 children with RMS, the median age of our patients was 51 months (age 3-191 months) with 83.2% of patients were below the age of 10 years, 17 infants with RMS (10.5%). The male (110) to female (51) ratio was 2.15:1. Genitourinary system was the most common affected primary site of tumor followed by head and neck, then extremities, retroperitoneum and lastly others, such as thoracic cavity, axillary region, sacral region and biliary tract (Table 1).
Table 1.
Demographic characteristics and the primary tumor sites of patients
| N=161 | % | |
|---|---|---|
| Age (months) | (3-191) | |
| X ± SD | ||
| Range | 49.06±3.86 | |
| <10 | 135 | 83.2 |
| ≥10 | 26 | 16.8 |
| Gender | ||
| Male | 110 | 68.3 |
| Female | 51 | 31.7 |
| Primary site | ||
| Genitourinary | 70 | 43.5 |
| Head and neck | 50 | 31.1 |
| Extremities | 18 | 11.2 |
| Retroperitoneum | 10 | 6.2 |
| Others | 13 | 8.1 |
Others: thoracic cavity, axillary region, sacral region and biliary tract.
The embryonal RMS was the most common pathologic subtype, 130 (80.7%), followed by alveolar 19 (11.7%), and lastly the botryoid 5 (3.1%), spindle 4 (2.5%), and others subtypes (mixed and anaplastic RMS) in 3 (1.9%) (Table 2). According to IRS postsurgical grouping classification, Group IV was the most common group of our patients (37.3%), followed by group II (33.5%), then group III (28.6%), and lastly group I (0.6%) (Table 3). The proportion of tumor size larger than 5 cm was 47.2%. 19.3% of patients had lymph node involvement and 34.2% of patients had metastasis at the time of diagnosis (Table 4) and lung (10 cases) was most common metastasis site of tumor.
Table 2.
Pathologic subtype of tumors
| Pathologic subtype | N=161 | % |
|---|---|---|
| Embryoyonal | 130 | 80.7 |
| Alveolar | 19 | 11.8 |
| Botryoid | 5 | 3.1 |
| Spindle cell | 4 | 2.5 |
| Others | 3 | 1.9 |
Others subtypes: mixed and anaplastic RMS.
Table 3.
Intergroup rhabdomyosarcoma study (IRS) group
| Stage | N=161 | % |
|---|---|---|
| 1 | 1 | 0.6 |
| 2 | 54 | 33.5 |
| 3 | 46 | 28.6 |
| 4 | 60 | 37.3 |
Table 4.
Tumor size, lymph node and distant metastasis of patients
| Tumor size | N=161 | % |
|
| ||
| <5 | 67 | 41.6 |
| 5-10 | 59 | 36.6 |
| >10 | 17 | 10.6 |
| Unknown | 18 | 11.2 |
|
| ||
| Lymph node | N=161 | % |
|
| ||
| -ve | 130 | 80.7 |
| +ve | 31 | 19.3 |
|
| ||
| Distant metastasis | N=161 | % |
|
| ||
| -ve | 101 | 62.7 |
| +ve | 60 | 37.3 |
Pre-treatment prognostic factors
There was a significant relationship among pathologic subtypes of tumor, primary site of tumor and metastasis, 54.5% of patients with alveolar subtype and others subtype had metastasis at the time of diagnosis, while only 30.9% of embryonal, botryoid and spindle cell subtype had metastasis (P=0.034). 60% of retroperitoneum and 61.5% of others had metastasis at the time of diagnosis (P=0.009) (Table 5). There was no significant statistical relationship between pathologic subtypes and primary site of tumor (P=0.47) (Table 6).
Table 5.
Relationship between primary site, pathologic subtype and metastasis
| Site | |||||||
|
|
|||||||
| Metastasis | Total n (%) | Head/neck n (%) | Genit n (%) | Extremities n (%) | Getrop n (%) | Others n (%) | P value |
|
| |||||||
| -ve | 101 (62.7) | 38 (76.0) | 42 (60.0) | 13 (72.2) | 4 (40.0) | 4 (30.8) | 0.014 |
| +ve | 60 (37.3) | 12 (24.0) | 28 (40.0) | 5 (27.8) | 6 (60.0) | 9 (69.2) | |
| Total | 161 (100) | 50 (31.1) | 70 (43.5) | 18 (11.2) | 10 (6.2) | 13 (8.1) | |
|
| |||||||
| Pathologic subtype | |||||||
|
|
|||||||
| Metastasis | Total n (%) | Embryoyonal + Botryoid + Spindle cell + Others n (%) | Alveolar n (%) | P value | |||
|
| |||||||
| -ve | 106 (65.8) | 95 (66.9) | 6 (31.6.1) | 0.004 | |||
| +ve | 55 (34.2) | 44 (33.1) | 13 (68.4) | ||||
Retro: retroperitoneum; Genit: genitourinary.
Table 6.
Relationship between pathologic subtype and primary site of tumor
| Site | P value | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Head/neck | Genit | Extremities | Retrop | Others | ||
| Embryoyonal | 43/33.1% | 57/43.8% | 12/9.2% | 8/6.2% | 10/7.7% | 0.47 |
| Alveolar | 5/26.3% | 6/31.6% | 5/26.3% | 1/5.3% | 2/10.5% | |
| Botryoid | 0/.0% | 4/80.0% | 1/20.0% | 0/.0% | 0/.0% | |
| Spindle cell | 1/25.0% | 2/50.0% | 0/.0% | 1/25.0% | 0/.0% | |
| Others | 1/33.3% | 1/33.3% | 0/.0% | 0/.0% | 1/33.3% | |
| Total | 50/31.1% | 70/43.5% | 18/11.2% | 10/6.2% | 13/8.1% | |
Outcome and survival
149 patients were treated and followed up regularly at 5 large cancer center, IRSG IRS-II/IV pilot BCH (n=95), CAA protocol for other hospitals (n=54). Regarding 60 occurred events, the median time of followed up period is 51 months. Of 149 patients, 7 (4.7%) were found to have an early disease progression and did not receive planned protocol. The median failure time was 60 days (range, 10 to 120 days). 2 patients suffered from severe infection and bleeding at initial chemotherapy, additional early failures resulted from treatment-related death without progression. 1 patient had second primary malignancy (primitive neuroectodermal tumor) after 70 months, but he was followed up another 130 months and got event free survival. 48 patients were local progression and 9 were distant metastasis. The genitourinary system, head and neck and others were prone to failure (Table 7).
Table 7.
Types and sites of failure
| No of failure | |
|---|---|
| Type of failure | |
| Local | 48 |
| Distant | 9 |
| Second primary malignancy | 1 |
| Severe infection and bleeding | 2 |
| Failure of site | |
| Head and neck | 15 |
| Extremities | 5 |
| Genitourinary | 27 |
| Retroperitoneum | 3 |
| Others | 10 |
| Failure of time | |
| 0-120 days | 7 |
| 4-12 months | 38 |
| >12 months | 15 |
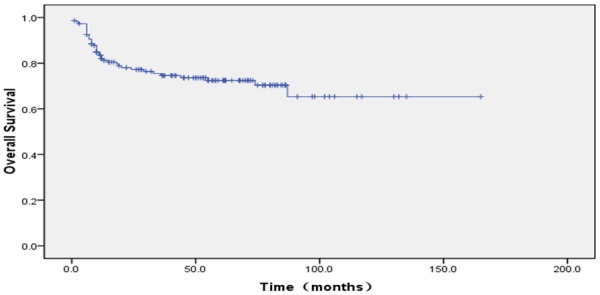
The proportion of ten year OS was 65.3±6.3%. The proportion of ten year EFS was 53.4±5.1% (Figures 1, 2). According to protocols of treatment, the estimated EFS rate was 50.1%±6.1 and 59.9%±8.3 for patients on BCH and CAA protocol, while mean OS time was 37.9±30.9 months and 50.7±37.7 months, respectively. The estimated OS rate was 62.3%±9.3 and 69.1%±6.5 for patients on BCH and CAA protocol, while mean OS time was 43.3±33.4 months and 51.2±37.0 months for patients on BCH and CAA protocol, respectively (Figures 3, 4). According to protocols of treatment for patients on BCH, the estimated 5 year EFS rate was 51.1%±9.1 and 54.3.2%±7.1 and the estimated 5 year OS rate was 66.4%±8.8 and 77.2%±6.1 before and after 2008, respectively (Figures 5, 6).
Figure 1.
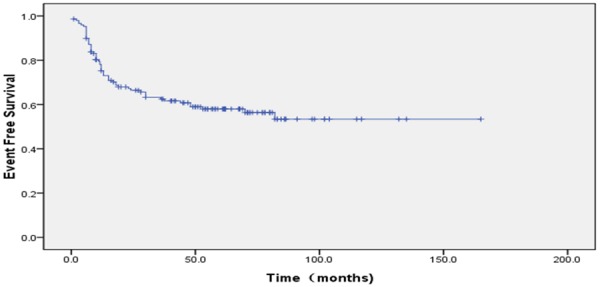
60 patients who developed progression. Ten year EFS was 53.4±5.1%. (95% CI 85.25%~111.41%), while mean EFS time was 40.0±33.8 months.
Figure 2.
39 patients were died. Ten year OS was 65.3±6.3%. (95% CI 104.26%~131.42%), while mean OS time was 45.0±34.8 months.
Figure 3.
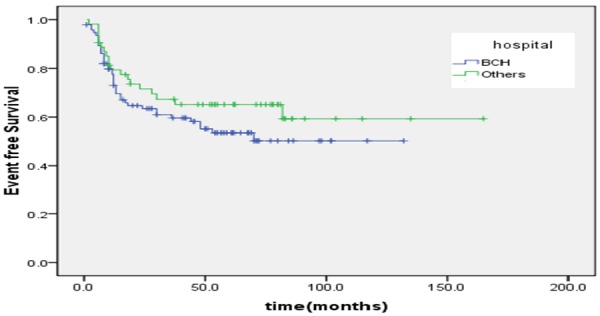
According to protocols of treatment, the estimated EFS rate was 50.1%±6.1 and 59.9%±8.3 for patients on BCH and CAA protocol, respectively, while mean EFS time was 37.9±30.9 months and 50.7±37.7 months for patients on BCH and CAA protocol, respectively (X=1.5, P=0.20).
Figure 4.
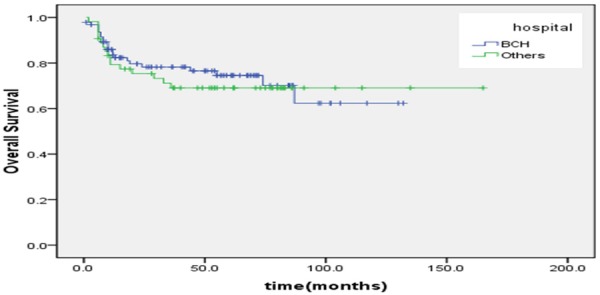
According to protocols of treatment, the estimated OS rate was 62.3%±9.3 and 69.1%±6.5 for patients on BCH and CAA protocol, respectively, while mean OS time was 43.3±33.4 months and 51.2±37.0 months for patients on BCH and CAA protocol, respectively (X=0.2, P=0.65).
Figure 5.
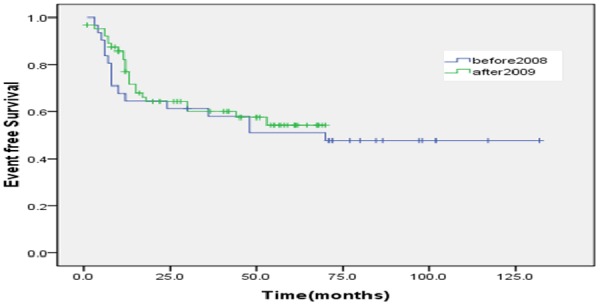
According to protocols of treatment for patients on BCH, the estimated 5 year EFS rate was 51.1%±9.1 and 54.3.2%±7.1 before and after 2008, respectively.
Figure 6.
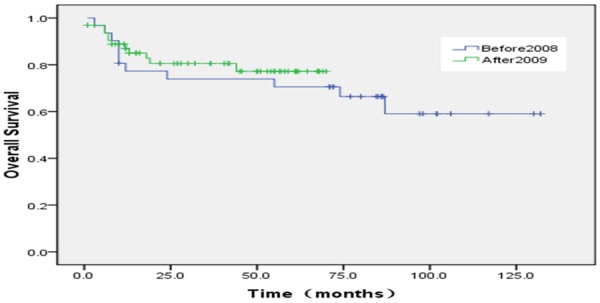
According to protocols of treatment for patients on BCH, the estimated 5 year OS rate was 66.4%±8.8 and 77.2%±6.1 before and after 2008, respectively.
Prognostic factors
The relations between outcome and each of age, gender, primary site, pathologic subtype, metastasis, IRS postsurgical grouping are shown in (Tables 8, 9). The relations between outcome and age (0.046), primary site (0.022), pathologic subtype (0.013), tumor size (0.008) and IRS group (P=0.000) were associated significantly with event free survival. Among the variables, Age (P=0.028) and IRS group (P=0.000) were associated significantly with overall survival. The estimated ten-year OS rate for group 1, 2, 3, 4 was 100%, 88.6±4.4, 65.2±13.2 and 23.1±16.9, respectively. The estimated ten-year EFS rate for group 1, 2, 3, 4 was 100%, 80.9±7.1, 44.1±12.1 and 27.8±6.9 respectively (Figures 7, 8). Bone marrow metastasis in 4 cases, 3 cases was died for 6, 9, 12 and 22 months after diagnosis, 1 case relapsed after stopping treatment 2 months and now tumor stable. The primary site of head and neck nonparameningial in 42 cases (28.2%), parameningial region in 7 cases (4.6%). The estimated ten-year OS rate was 77.8±28.6, 28.6±23.7 for respectively (X=3.55, P=0.06) (Figure 9). Multivariate analysis showed that overall survival for RMS is just depend on IRS group (P=0.026) (Table 10).
Table 8.
Relation between outcome EFS and clinical characteristics
| Survival | Event | χ2 | P value | ||
|---|---|---|---|---|---|
| Age | 3.99 | 0.046 | |||
| <10 | 79 | 44 | |||
| ≥10 | 10 | 16 | |||
| Gender | |||||
| Male | 57 | 45 | 1.75 | 0.186 | |
| Female | 32 | 15 | |||
| Primarysite | |||||
| Head and neck | 34 | 15 | 11.48 | 0.022 | |
| Genitourinary | 36 | 27 | |||
| Extremities | 11 | 5 | |||
| Retroperitoneum | 5 | 3 | |||
| Others | 3 | 10 | |||
| Pathologictype | 12.61 | 0.013 | |||
| Embryoyonal | 72 | 46 | |||
| Alveolar | 6 | 13 | |||
| Botryoid | 5 | 0 | |||
| Spindle cell | 3 | 1 | |||
| Others | 3 | 0 | |||
| Metastasis | |||||
| -ve | 70 | 24 | 26.9 | 0.000 | |
| +ve | 19 | 36 | |||
| Size | 9.59 | 0.008 | |||
| <5 | 45 | 18 | |||
| 5-10 | 32 | 22 | |||
| >10 | 6 | 10 | |||
Table 9.
Relation between outcome OS and clinical characteristics
| Survival | Dead | χ2 | P value | ||
|---|---|---|---|---|---|
| Age | 4.82 | 0.028 | |||
| <10 | 96 | 27 | |||
| ≥10 | 14 | 12 | |||
| Gender | |||||
| Male | 72 | 30 | 1.75 | 0.209 | |
| Female | 38 | 9 | |||
| Primarysite | |||||
| Head and neck | 37 | 12 | 9.43 | 0.051 | |
| Genitourinary | 49 | 14 | |||
| Extremities | 13 | 3 | |||
| Retroperitoneum | 5 | 3 | |||
| Others | 7 | 7 | |||
| Pathologic type | 4.97 | 0.29 | |||
| Embryoyonal | 88 | 30 | |||
| Alveolar | 11 | 8 | |||
| Botryoid | 5 | 0 | |||
| Spindle cell | 3 | 1 | |||
| Metastasis | |||||
| -ve | 80 | 14 | 20.1 | 0.000 | |
| +ve | 30 | 25 | |||
| Size | 3.60 | 0.165 | |||
| <5 | 49 | 14 | |||
| 5-10 | 43 | 11 | |||
| >10 | 10 | 6 | |||
Figure 7.
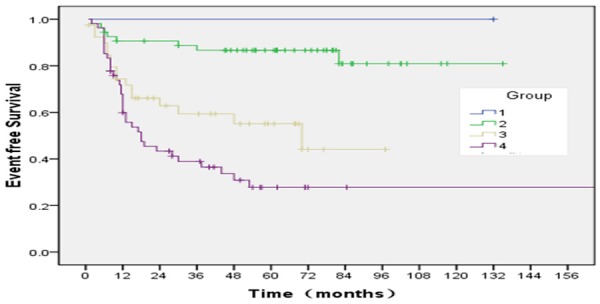
According to protocols of treatment, the estimated ten-year OS rate for group 1, 2, 3, 4, was 100%, 80.9±7.1, 44.1±12.1 and 27.8±6.9, respectively (X=32.30, P=0.000).
Figure 8.
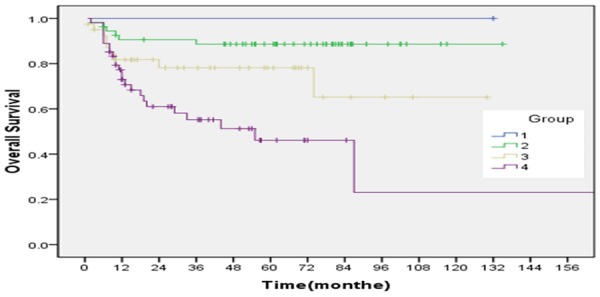
According to protocols of treatment, the estimated ten-year OS rate for group 1, 2, 3, 4, W 100%, 88.6±4.4; 65.2±13.2 and 23.1±16.9, respectively (X=20.02, P=0.000).
Figure 9.
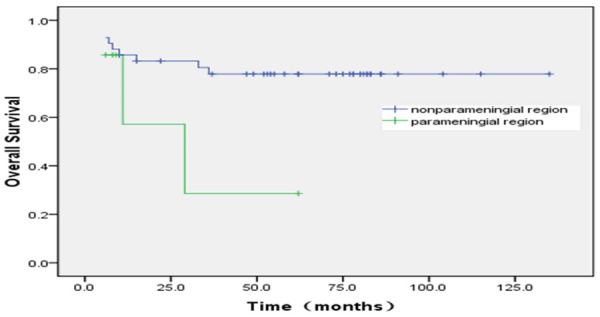
The primary site of head and neck nonparameningial in 42 cases (28.2%), parameningial region in 7 cases (4.6%). The estimated ten-year OS rate was 77.8±28.6; 28.6±23.7 for respectively (X=3.55, P=0.06).
Table 10.
Clinical factors that may affect its prognosis for single factor and multivariate analysis
| Clinical factors | EFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Single factor | HR | 95% CI | P | Single factor | HR | 95% CI | P | |
| Age | 0.046 | 3.39 | 0.237-1.046 | 0.065 | 0.028 | N1 | N1 | |
| Primary site | 0.186 | N1 | N1 | 0.051 | N1 | N1 | ||
| pathologic type | 0.022 | N1 | N1 | 0.29 | ||||
| Group | 0.000 | 13.93 | 0.060-0.417 | 0.003 | 0.000 | 9.22 | 0.006-0.549 | 0.026 |
| Size | 0.008 | N1 | N1 | 0.165 | N1 | N1 | ||
N1: not in the final Cox model.
Discussion
RMS is a highly malignant childhood cancer [1,6,7]. It is the most common form of soft tissue sarcoma in children. The median age of our patients was 51 months with 83.2% of patients below the age of 10 years. These results are similar to the IRS IV report [8] that the median age of patients was 60 months, with 72% of patients below the age of 10 years. But in our study, 68.3% of patients were males and 31.7% were females, the ratio of male to female was 2.15:1, higher than IRS IV’s report (1.6:1).
In our study, genitourinary system was the most common affected primary site of tumor (43.5%), followed by head and neck (31.1%), then extremities (11.2%), and lastly retroperitoneum in (6.2%). These results are different from the IRS IV report, which found that head and neck was the most common affected primary site of tumor (41%), followed by the genitourinary system site (31%), then extremities (13%), and retroperitoneum (7%). This difference can be explained by the small sample size of our patients compared to these studies.
In our study, embryonal RMS was the most common pathologic subtype (80.7%) while alveolar RMS represents 11.8% of patients. Hessissen [9] found that embryonal subtype represents 73% while alveolar subtype represents 13% of patients and Abd El-Aal [10] found that embryonal and alveolar subtypes represent 87.3% and 12.7% of patients, respectively, and this is similar to our results. These results are different from the IRS IV reported that the embryonal subtype represent 70% including the botryoid and spindle cell variants, if the botryoid and spindle cell variants were added to embryonal subtype (71.7%). In our study, 47.2% of patients’ tumor sizes were larger than 5 cm at the time of diagnosis while 41.6% were smaller than 5 cm. These results are similar to the report by IRS IV that 55% of tumor size is larger than 5 cm, but different from a Japanese study (75%) conducted by Hosoi [11].
In our study, 18% of patients had lymph node involvement. These results are similar to the Hosoi showed that 19% of patients had LN involvement. 37.3% of patients had metastasis at the time of diagnosis. According to IRS postsurgical grouping classification, group IV was the most frequent group (37.3%), followed by group II (33.5%), then group III (28.6%), and lastly group I (0.6%). High percentage of patients with metastasis at the time of diagnosis, group IV in our patients can be explained by the primary health care physicians’ unawareness about early presenting symptoms and signs of the disease, together with the unavailability of diagnostic facilities which can allow earlier detection of cases with localized disease.
There was a significant relationship among pathologic subtypes of tumor, primary site of tumor and metastasis, 68.4% of patients with alveolar subtype and others subtype had metastasis at the time of diagnosis while only 33.1% of embryonal, botryoid and spindle cell subtype had metastasis (P=0.004). 60% of retroperitoneum and 69.2% of others had metastasis at the time of diagnosis (P=0.014). There was no significant statistical relationship between pathologic subtypes and primary site of tumor (P=0.47). Wiener found that head and neck RMS are characterized by embryonal histology in most cases. On the other hand, extremities RMS are more likely to have an alveolar subtype and nearly 80% of genitourinary system RMS was embryonal in nature. Lawrence et al. reported that head and neck RMS are most common embryonal subtype. Mandell et al. reported that nearly 50% of extremities RMS are alveolar subtype.
In our study, there was a significant relationship (P=0.028) between age and outcome of patients. Forty six percent of patients more than 10-year-old died while about 78% of patients less than 10-year-old survived. Punyko et al found that patients aged 1~9 years at the time of diagnosis showed good prognosis, while those below 1 year and 10~19 years showed poor prognosis. On the other hand, our results revealed no significant statistical relationship between primary site of tumor and OS (P=0.051). These results are not matched with the study reported by Crist et al that primary sites with more favorable prognosis include the orbit and nonparameningeal head and neck, paratestis, vulva, vagina, uterus, and biliary tract. In our study, there was no significant relationship between pathologic subtype of tumor and OS (P>0.05). But our results revealed that there was a significant statistical relationship between primary site of tumor and EFS (P=0.022). And there was also a significant relationship between pathologic subtype of tumor, size and EFS (P=0.013, 0.008 respectively).
Our study reported that there was a significant relationship (P=0.000) between metastasis and outcome, 77.4% of patients who had metastasis at the time of diagnosis died while 82.7% of patients without metastasis survived. Breneman et al found [12] that children with metastatic disease at diagnosis have the poorest prognosis and the prognostic significance of metastatic disease is modified by tumor histology (embryonal is more favorable than alveolar) and by the number of metastatic sites.
In our study, the percentage of 10-year-old OS was 65.3%. Ten year EFS was 53.4%. This result is similar to that reported by Abd El-Aal et al. Among the variables, age (P=0.028) and IRS group (P=0.000) were significantly associated with overall survival. The relations between outcome and age (0.046), primary site (0.022), pathologic subtype (0.013), tumor size (0.008) and IRS group (P=0.000) were associated significantly with event free survival. Multivariate analysis showed that overall survival for RMS depends on IRS group (P=0.026).
In relation to the IRS pre-surgical staging classification, our results showed that the highest estimated EFS was group I and II (90.6%) followed by group III (66.9%), then group IV with (22.2%). Our results showed that the estimated EFS in relation to pathologic subtype was higher in embryonal subtype than in alveolar subtype (58.1% versus 15.5%). In relation to the IRS pre-surgical staging classification, our results showed that the highest estimated EFS was group I and II (83.1%) followed by group III (45.2%), then group IV with (26.7%). These results are similar to Aaron R. Weiss found that [13] the estimated OS rate was 62.3% and 69.1% for patients on BCH and CAA protocol, respectively. While mean OS time was 43.3 months and 51.2 months for patients on BCH and CAA protocol, respectively.
Neither BCH nor other hospitals shows significant statistical relationship between protocol of treatment and outcome (36% of patients received CAA protocol versus 64% of those received IRSG protocol in BCH). The estimated EFS rate was 50.1% and 59.9% for patients on IRSG and CAA protocol, respectively. It’s possible that, in other hospitals, the primary sites of tumor were mainly head and neck, the majority of group was II or III, while, genitourinary system was the most common affected primary site of tumor in BCH (89.8% vs. 93.4%, P=0.000). Since 2009, COG D 9803, COG D 9802 protocol was selected for treatment of RMS in BCH. Our study showed that there was no significant statistical relationship between protocol of treatment and outcome.
Conclusion
Apart from the higher prevalence of group IV in our patients and the higher percentage of patients with primary tumor site in the genitourinary system, the epidemiological characteristics of our patients are quite similar to the worldwide data. This study showed that overall survival and event free survival for RMS depend on disease groups.
Acknowledgements
Thanks for the support of Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support, code ZY201404.
Disclosure of conflict of interest
None.
References
- 1.Li PJ. Pediatric tumor pathology. 2nd edition. Beijing Publishing House; 2001. The constituent ratio of pediatric tumor; p. 6. [Google Scholar]
- 2.Minn AY, Lyden ER, Anderson JR, Million L, Arndt CA, Brown K, Hawkins DS, Donaldson SS. Early Treatment Failure in Intermediate-Risk Rhabdomyosarcoma: results from IRS-IV and D9803-a Report from the Children’s Oncology Group. J. Clin. Oncol. 2010;27:4228–4232. doi: 10.1200/JCO.2010.29.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta AA, Anderson JR, Pappo AS, Spunt SL, Dasgupta R, Indelicato DJ, Hawkins DS. Patterns of Chemotherapy-Induced Toxicities in Younger Children and Adolescents with Rhabdomyosarcoma: a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Cancer. 2012;118:1130–1137. doi: 10.1002/cncr.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang JY. The chemotherapy of common solid tumors with children. Pediatric oncology professional committee Chinese Anticancer Association in June 16. 2008 [Google Scholar]
- 5.Huang DS, Zhang Y. Children’s rhabdomyosarcoma of the diagnosis and treatment. J Clin Pediatr. 2012;30:404–440. [Google Scholar]
- 6.Wexler L, Helman L. Rhabdomyosarcoma and the undifferentiated sarcomas. In: Pizzo P, Poplack D, editors. Principles and Practice of Pediatric Oncology. 3rd edition. Philadelphia, Pa, USA: Lippincott-Raven; 1997. pp. 799–829. [Google Scholar]
- 7.Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975-2005. Cancer. 2009;115:4218–4226. doi: 10.1002/cncr.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J. Clin. Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 9.Hessissen L, Kanouni L, Kili A, Nachef MN, El Khorassani M, Benjaafar N, Khattab M, El Gueddari Bel K. Pediatric rhabdomyosarcoma in Morocco. Pediatr Blood Cancer. 2010;54:25–28. doi: 10.1002/pbc.22173. [DOI] [PubMed] [Google Scholar]
- 10.Abd El-Aal HH, Habib EE, Mishrif MM. Rhabdomyosarcoma: the experience of the pediatric unit of Kasr El-Aini Center of Radiation Oncology and Nuclear Medicine (NEMROCK) (from January 1992 to January 2001) J Egypt Natl Canc Inst. 2006;18:51–60. [PubMed] [Google Scholar]
- 11.Hosoi H, Teramukai S, Matsumoto Y, Tsuchiya K, Iehara T, Hara J, Mitsui T, Kaneko M, Hatae Y, Hayashi Y, Mabuchi O, Adachi N, Morikawa Y, Nishimura S, Kumagai M, Takamatsu H, Sawada T, Sugimoto T. A review of 331 rhabdomyosarcoma cases in patients treated between 1991 and 2002 in Japan. Int J Clin Oncol. 2007;12:137–145. doi: 10.1007/s10147-006-0638-6. [DOI] [PubMed] [Google Scholar]
- 12.Breneman JC, Lyden E, Pappo AS. Prognosticfactors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma--a report from the Intergroup Rhabdomyosarcoma Study IV. J. Clin. Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 13.Weiss AR, Lyden ER, Anderson JR, Hawkins DS, Spunt SL, Walterhouse DO, Wolden SL, Parham DM, Rodeberg DA, Kao SC, Womer RB. Histologic and clinical characteristics can guide staging evaluations for children and adolescents with rhabdomyosarcoma: a report from the children’s oncology group soft tissue sarcoma committee. J. Clin. Oncol. 2013;31:3226–3232. doi: 10.1200/JCO.2012.44.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]


