Abstract
This study explored the underlying mechanism of Gingko biloba extract (Ginaton) on dextran sulfate sodium (DSS)-induced acute experimental colitis in mice. 40 male C57BL/6 mice were randomly divided into four groups: normal control group, Ginaton group, Ginaton treatment group, and DSS group. After 7 days administration, mice were sacrificed and colons were collected for H-E staining, immunohistochemistry, real-time PCR and Western blot. By observing clinical disease activity and histological damage, we assessed the effect of Ginaton on DSS-induced acute experimental colitis in mice and observed the effect of Ginaton on normal mice. We also explored the specific mechanism of Ginaton on DSS-induced acute experimental colitis in mice through examining the expression of inflammatory related mediators (gp130, STAT3, p-STAT3, ROR-γt) and cytokines (IL-6, IL-17, IL-23). Ginaton-treated DSS mice showed significant improvement over untreated DSS mice. Specifically, Ginaton improved clinical disease activity (DAI score, weight closs, colon shortening, and bloody stool) and histological damage, and reduced the expression of inflammatory-related mediators (p-STAT3, gp130, ROR-γt) and cytokines (IL-6, IL-17, IL-23). In addition, clinical disease activity, histological damage, the expression of inflammatory related mediators (STAT3, p-STAT3, gp130, ROR-t) and cytokines (IL-6, IL-17, IL-23) in mice of Ginaton group were similar to normal control group. In conclusion, Ginaton ameliorates DSS-induced acute experimental colitis in mice by reducing IL-17 production, which is at least partly involved in inhibiting IL-6/STAT3 signaling pathway and IL-23/IL-17 axis. Moreover, Ginaton itself does not cause inflammatory change in normal mice. These results support that Ginaton can be as a potential clinical treatment for ulcerative colitis (UC).
Keywords: Ginaton, acute experimental colitis, IL6/STAT3, IL-23/IL-17
Introduction
Inflammatory bowel disease (IBD) is a complex set of non-specific intestinal inflammatory diseases with unknown etiology, including ulcerative colitis (UC) and Crohn’s disease (CD). In UC, lesions begin in the rectum, retrograde to the proximal segment, which involve the entire colon and terminal ileum. These lesions are localized in the mucosa and submucosal layers in an uninterrupted pattern [1]. In CD, damage is commonly found in the distal ileum and adjacent colon but can be observed in any part of the gastrointestinal tract from mouth to anus. These lesions involve the whole layer of the bowel wall and occur in a discontinuous pattern [2]. IBD is believed to result from the abnormal interaction among genetic, environmental, microbial, immunological, and infectious factors [3,4]. Experimental evidences are mounting to support immune disorder as a leading factor in the pathogenesis of IBD [5].
Many studies found that IL-6-gp130-STAT3 signaling pathway plays a crucial role in the development of IBD [6-8]. One study conducted by Weaver et al. showed a novel role of the IL-6/STAT3 signaling pathway in Th17 reaction and IL-17 production [9]. Moreover, multiple studies have shown that ROR-γt, which induced by IL-6-gp130-STAT3 signaling pathway, is very important for differentiation of Th17 cells [10-12]. Meanwhile, some researchers have demonstrated that IL-23 participates in generation of Th17 cells and stimulates secretion of IL-17 [13].
Traditional medicine for IBD is divided into three main categories: 5-aminosalicylic acids, glucocorticoid steroids, and immunosuppressive agents. With improved understanding of the pathogenesis of IBD, many effective biological agents have been created, but these biological agents are quite expensive. Furthermore, studies have shown that these biological agents can increase the risks of infection and cancer [14,15]. Thus, identifying new treatments for IBD is a priority.
Ginkgo Biloba extract (EGb) is derived from the leaves of Ginkgo biloba, and the main active constituents are flavonoid glycosides. EGb can remove oxygen free radicals, inhibit lipid peroxidation, inflammation and allergic reaction, modulate immune responses, and promote cell proliferation and apoptosis [16-21]. EGb761 was shown to inhibit the spread of colon cancer cells by up-regulation of P53 and down-regulation of bcl-2 genes [22]. Zhou et al. [23] found EGb to reduce expression of inflammatory factors (e.g. IL-6 and TNF-α) in TNBS-induced colitis. In addition, a study by Kotakadi et al. [24] suggested that EGb could ameliorate DSS-induced acute experimental colitis by promoting apoptosis. But until now, EGb relieves DSS-induced acute experimental colitis whether involves in IL-6/STAT3 signaling pathway and IL-23/IL-17 axis is not elucidated.
In this study, an animal model of acute experimental colitis was induced by freely drinking 3% Dextran Sodium sulfate (DSS) solution for 7 days. Meanwhile, the effect of Ginaton on IL-6/STAT3 signaling pathway and IL-23/IL-17 axis in acute experimental colitis was explored.
Materials and methods
Animal model of acute experimental colitis
Male C57BL/6 mice, aged 6-8 weeks and weighing 22-24 g, were purchased from the experimental animal center of Shengjing Hospital of China Medical University [license number: SCXK (Liao) 2003-0009]. Mice were fed in the SPF laboratory animal room on a 12:12-h light-dark cycles with room temperature 22±2°C and relative humidity 50%-60%. All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the China Medical University Animals Committee.
To induce acute experimental colitis, mice were given ad libitum access to drinking 3% DSS solution [3 g DSS powder (MP Biomedicals, USA, MW 36,000-50,000) in 100 ml drinking water] for 7 days.
Experimental protocol
In a preliminary dose-response experiment, mice were randomly assigned to three groups (n = 8) by different dose of Ginaton (100 mg/kg, 200 mg/kg, 300 mg/kg) administrated, then sacrificed for histological assessment. We found that 300 mg/kg Ginaton has the best therapeutic effect (Figure 1). The dose of 300 mg/kg Ginaton was chose to be used in subsequent experiment.
Figure 1.
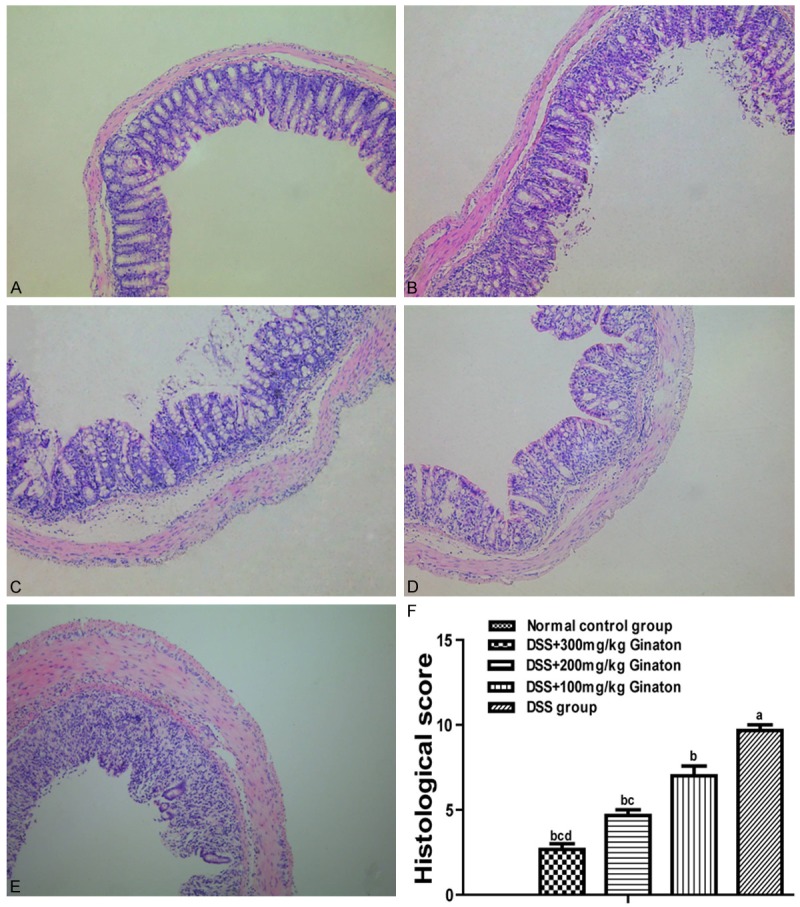
Effect of Ginaton on histological damage in DSS-induced acute experimental colitis. H-E staining of mice colons (× 10) from normal control group (A, saline), DSS mice treated with Ginaton 300 mg/kg (B), 200 mg/kg (C), and 100 mg/kg (D); and DSS group (E, DSS + saline). (F) Histological scores are presented as means ± SEM. aP < 0.01 vs. normal control group, bP < 0.05 vs. DSS group, cP < 0.01 vs. 100 mg/kg Ginaton group, dP < 0.01 vs. 200 mg/kg Ginaton group.
A total of 40 male C57BL/6 mice were randomly divided into four groups (n = 8): Normal control group, 0.8 mL saline was fed everyday by intragastric administration. Ginaton group, 300 mg/kg.d Ginaton (dissolved in 0.8 mL saline) was administrated in the same way. Ginaton treatment group, acute experimental colitis was induced by freely drinking 3% DSS solution for 7 days, while 300 mg/kg.d Ginaton was given by intragastric administration. DSS group, acute experimental colitis was induced as described above, while 0.8 mL saline was fed every day. After Ginaton or saline was administered for 7 days, mice were sacrificed by cervical dislocation and colons were collected. Colons were washed 2-3 times with pre-cooling saline, then 0.5-1.0 cm segments which is 2 cm away from the anus were collected and fixed in 10% neutral buffered formalin for H-E staining and immunohistochemistry. The remaining colon tissue of each mouse was divided into two sections and stored at 80°C for real-time PCR and Western blot.
Evaluation of acute experimental colitis
Acute experimental colitis was assessed by disease activity index (DAI) score [25-27] (Table 1), weight change, colon length, histological damage, and histological score [28] (Table 2). Histological score of colons was completed independently by two pathologists, and the average score in each group was calculated.
Table 1.
DAI score chart
| Score | Body loss (%) | Stool-consistency | Occult/gross bleeding |
|---|---|---|---|
| 0 | 0 | Normal | Normal |
| 1 | 1-5 | ||
| 2 | 5-10 | Loose | Occult bleeding |
| 3 | 10-15 | ||
| 4 | > 15 | Diarrhea | Gross bleeding |
The disease activity index = (combined score of weight loss, stool consistency and bleeding)/3. Normal stool, shaped stool; loose Pasty, unformed stools which do not adhered to the anus; Diarrhea stool, Watery stools which adhered to the anus.
Table 2.
Histological score chart
| Integral | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Inflammation | None | Mild | Moderate | Severe | |
| Depth of the lesion | None | Mucous layer | Submucosa | Muscularis and serosa | |
| Crypt damage | None | 1/3 | 2/3 | 100% | 100% with epithelium loss |
| Pathological change range | None | 0%-25% | 26%-50% | 51%-75% | 76%-100% |
RNA isolation and quantitative real-time PCR
Total RNA was extracted from colon tissue with Trizol. Next, 500 ng RNA was reverse transcribed using 200 U M-MLV (Promega Corporation, Madison, WI, USA), and PCR was performed using a real-time PCR system (Applied Biosystems, Forster, CA, USA). All PCR reactions were done in triplicate, using the gene GAPDH as an endogenous control. The primer sequences used for cDNA amplification were as follows: GAPDH, 5’-ACTCCACTCACGGCAAATTC-3’ and 5’-TCTCCATGGTGGTGAAGACA-3’; IL-6, 5’-AGAAATCTGCAGCTCCCACC-3’ and 5’-CTGTGCTCAGTGACCGAGTT-3’; gp130, 5’-TAACTCCCGTATTCGCCACG-3’ and 5’-TTTGTCCGAACAGTCGGTCC-3’; STAT3, 5’-CCCGTACCTGAAGACCAAGT-3’ and 5’-TCCATGTCAAACGTGAGCGA-3’; ROR-γt, 5’-GGAGCTCTGCCAGAATGACC-3’ and 5’-CAAGGCTCGAAACAGCTCCAC-3’; IL-23, 5’-ACCTGCTGGACTCGGACAT-3’ and 5’-GGCGAGGCATCTGTTGAT-3’; and IL-17A, 5’-TCCACCGCAATGAAGACCCTGA-3’ and 5’-TCCAGCTTTCCCTCCGCATTGA-3’.
Western blot analysis
The protein levels of p-STAT3 and STAT3 in colon tissue were quantified by Western blot. Colon tissues of mice were added into RIPA lysis buffer. Then, these tissue samples were homogenized at 4°C, centrifuged at 12,000 × g for 30 min, and supernatant was retained. After degeneration, 50 μg samples were separated by SDS-PAGE and transferred to Polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat milk (5 g non-fat dry milk powder in 100 ml of TBST) at room temperature for 2 hours, then incubated with specific anti-phospho-STAT3 antibody (1:1000) and anti-STAT3 antibody (1:1000) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Finally, membranes were incubated with peroxidase-conjugated secondary antibody (1:5000) at room temperature for 1 hour. Protein levels were quantified by gray values, assessed by professional software (Software Total Lab Dynamics Ltd, Phoretix, Newcastle, UK).
Immunohistochemical assay
Expression of IL-6, IL-17, and IL-23 in mouse colon tissue was determined by immunohistochemistry. Colons were fixed in 10% neutral buffered formalin for one week, then paraffin-embedded, sliced, dehydrated, and retrieved of antigen. Sections were treated with 3% hydrogen peroxide at room temperature for 5 min, then incubated with antibody IL-17 (1:500), IL-23 (1:500), and IL-6 (1:500) (all from Santa Cruz Biotechonology, Santa Cruz, CA, USA) respectively. After washing with PBS, the sections were incubated with secondary antibody and DAB substrate. The color reaction was stopped with distilled water, and sections were counterstained with Hematoxylin. Sections incubated with PBS instead of primary antibody served as negative controls. For immunohistochemistry analysis, data were expressed as optical density.
Statistical analysis
All analyses were carried out using SPSS 18.0 (SPSS Inc, Chicago, IL, USA). Data are expressed as mean ± SEM. Statistical analysis for significant differences was conducted by use of one-way ANOVA (equal variances assumed) or Tamhane’s T2 tests (equal variances not assumed). P-values less than 0.05 were considered to be statistically significant.
Results
Clinical disease activity
Ginaton exhibited striking improvements in DSS-induced acute experimental colitis, as shown by reducing DAI score, inhibiting body loss and colon shortening (Figure 2). The DAI scores of normal control group and Ginaton group were 0. Compared with the DSS group, mice of Ginaton treatment group showed a reduced DAI score beginning at day 4 (P < 0.05). Body weight of mice in normal control group and Ginaton group increased gradually during the experiment. Compared with the DSS group, mice of Ginaton treatment group had slower weight loss starting at day 4 (P < 0.05). Similarly, mice of Ginaton treatment group showed significantly longer colons than DSS group (P < 0.01). Bloody stool around the anus was observed on day 7 (Figure 3).
Figure 2.
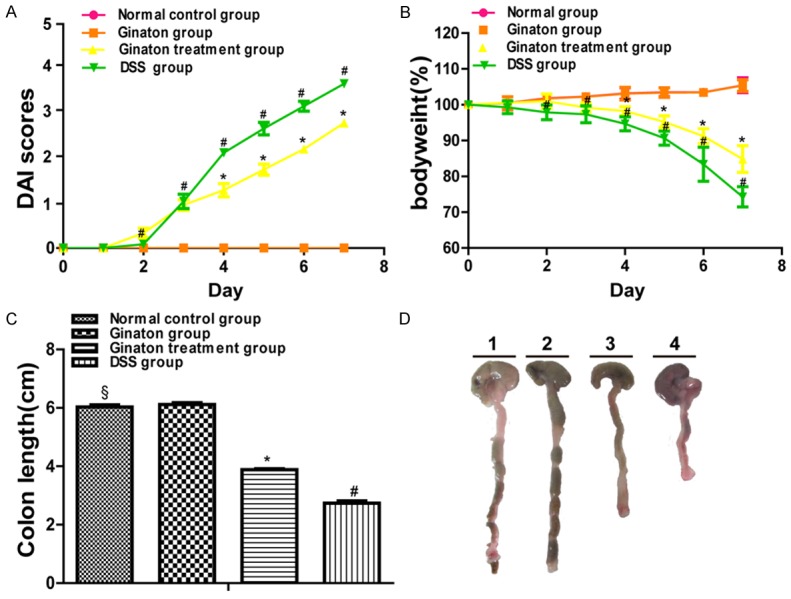
Effect of Ginaton on clinical disease activity in DSS-induced acute experimental colitis, assessed by DAI score (A), weight change (B), and colon length (C). (D) Representative colon of each group (Line 1, normal control group; Line 2, Ginaton group; Line 3, Ginaton treatment group; Line 4, DSS group).
Figure 3.
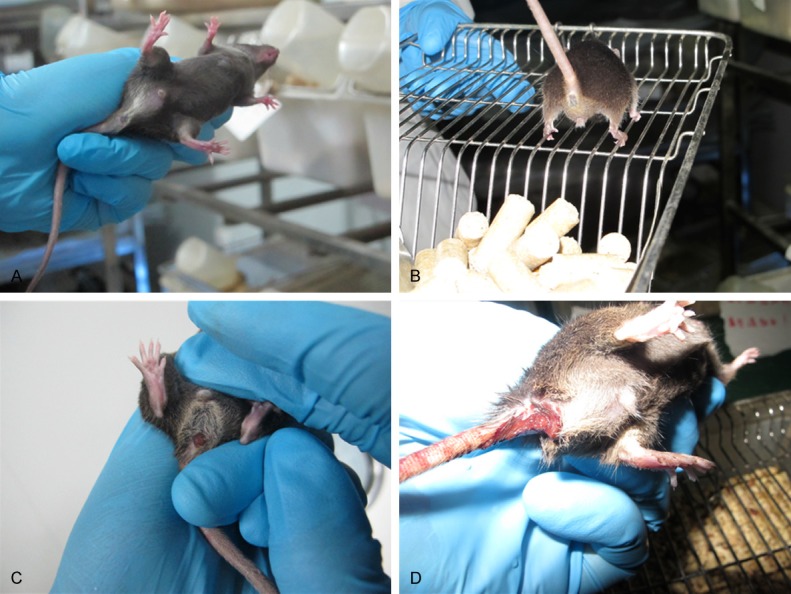
Effect of Ginaton on bloody stool in DSS-induced acute experimental colitis. Normal control group (A, saline), Ginaton group (B, Ginaton), Ginaton treatment group (C, DSS + 300 mg/kg Ginaton) and DSS group (D, DSS + saline). Mice of normal control group and Ginaton group had no bloody around anus. There were lots of bloody stool adhering to the anus of mice in DSS group, but little bloody stool was found around the anus of mice in Ginaton treatment group. These pictures showed that Ginaton could improve the degree of bloody stool in DSS-induced acute experimental colitis.
Histology
Colons in normal control group and Ginaton group had intact membrane structure. In contrast, the membrane structures of colons in DSS group were disarranged. Specifically, glands had disappeared, and inflammatory cells had infiltrated into mucosa and submucosa. Colons of Ginaton treatment group showed damage in only part of membrane structure and reduced inflammatory cells infiltration. Histological score was significantly reduced in Ginaton treatment group compared with DSS group (P < 0.05) (Figure 4).
Figure 4.
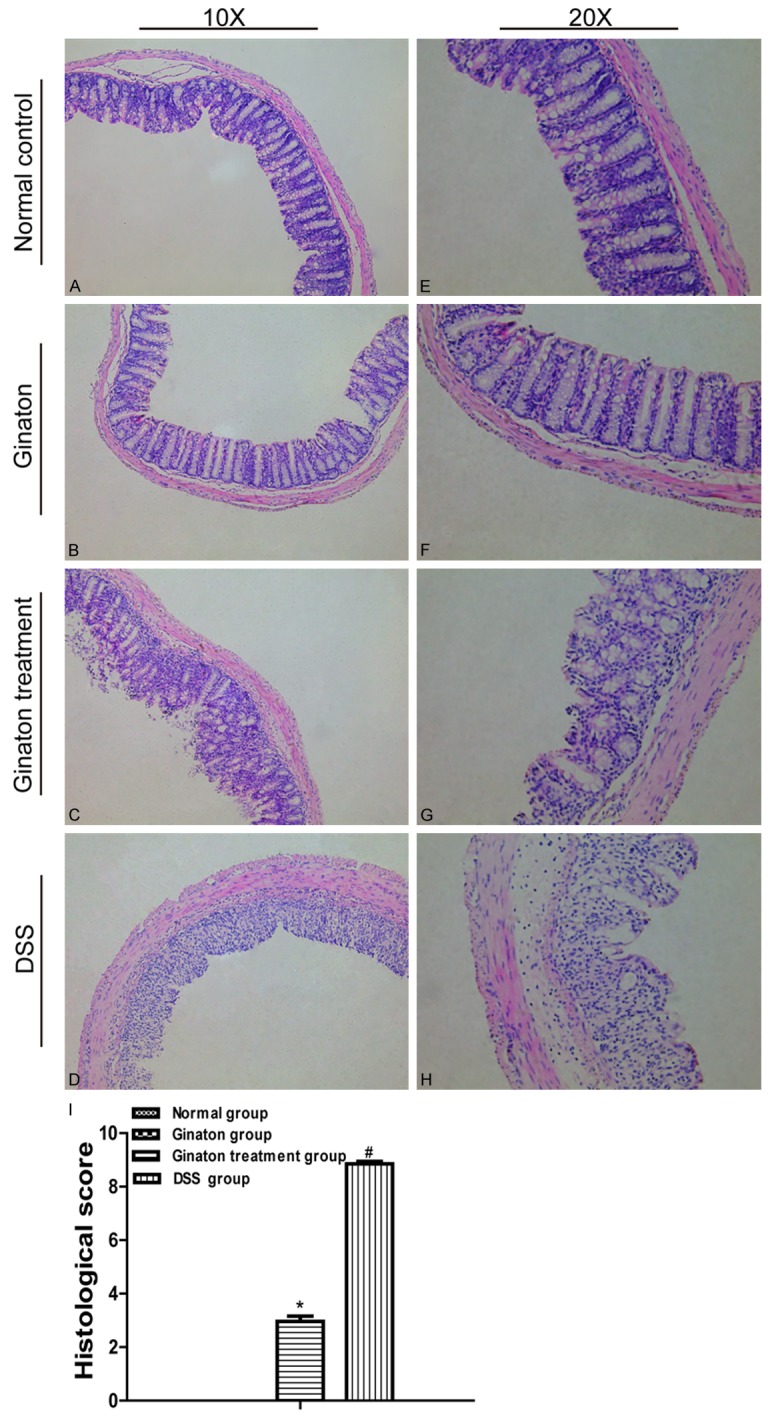
Effect of Ginaton on histological damage in DSS-induced acute experimental colitis. (H-E) staining of mice colons (× 10) (A-D) and (× 20) (E-H) from normal control group (A and E), Ginaton group (B and F), Ginaton treatment group (C and G), and DSS group (D and H). (I) Histological scores are presented as means ± SEM. #P < 0.01 vs. normal control group; *P < 0.05 vs. DSS group.
The mRNA expression of IL-6, gp130, STAT3, ROR-γt, IL-17, and IL-23
In contrast to normal control group, mice of DSS group exhibited elevated mRNA expression of IL-6, gp130, STAT3, ROR-γt, IL-17, and IL-23 mRNA (P < 0.05). Compared with DSS group, mRNA expression of these factors significantly reduced in Ginaton treatment group (P < 0.05). IL-6, gp130, STAT3, ROR-γt, IL-17 and IL-23 mRNA expressions in Ginaton group were similar to normal control group (P > 0.05) (Figure 5).
Figure 5.
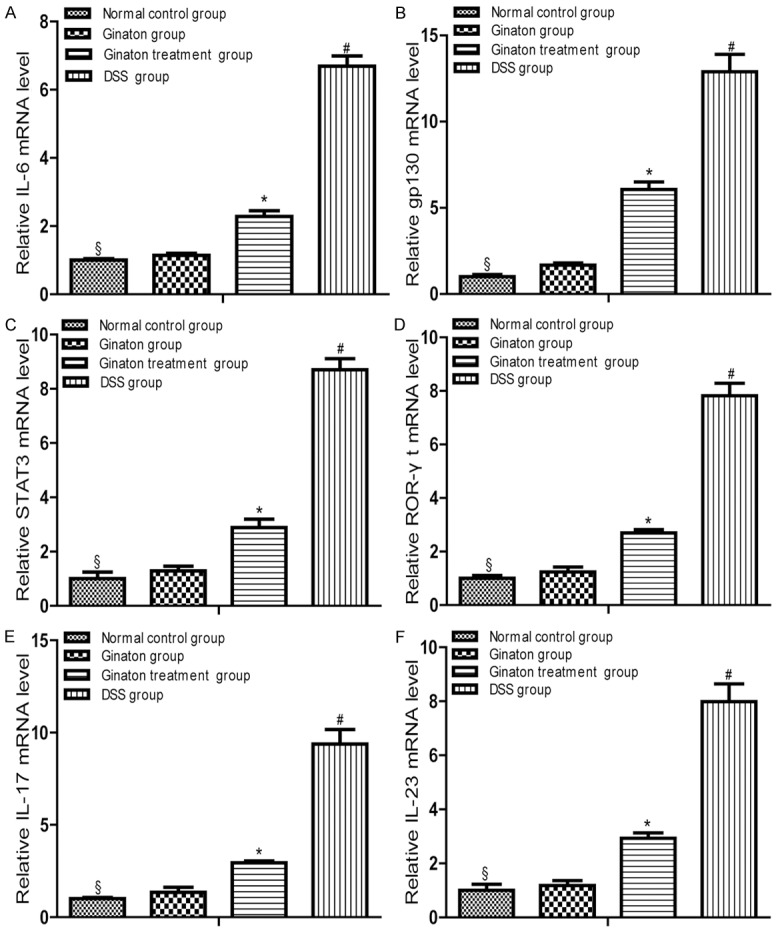
Effect of Ginaton on relative mRNA expressions of IL-6 (A), gp130 (B), STAT3 (C), ROR-γt (D), IL-17 (E) and IL-23 (F) in DSS-induced acute experimental colitis. Treatment with Ginaton could effectively reduced mRNA expressions of IL-6, gp130, STAT3, ROR-γt, IL-17 and IL-23. #P < 0.01 vs normal control group; *P < 0.05 vs. DSS group; §P > 0.05 vs. Ginaton group.
The protein expression of p-STAT3 and STAT3
Protein expression levels of p-STAT3 and STAT3 were quantified by Western blot (Figure 6). Compared to normal control group, mice of DSS group showed increased p-STAT3 protein expression in the colon (P < 0.05). p-STAT3 protein expressions in colons of Ginaton treatment group were significantly reduced in comparison with the DSS group (P < 0.05). The protein expression of STAT3 in colons of each group had no statistical difference (P > 0.05).
Figure 6.
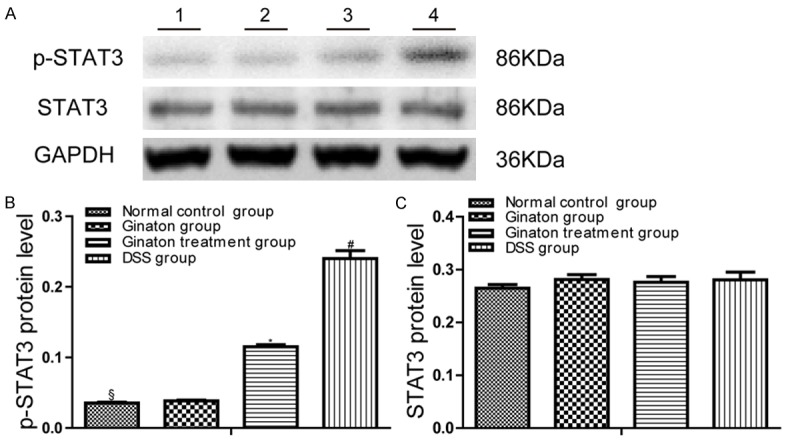
Effect of Ginaton on protein expressions of p-STAT3 and STAT3 in DSS-induced acute experimental colitis. (A) p-STAT3 and STAT3 protein expression was observed in each group mice (Line 1, normal control group; Line 2, Ginaton group; Line 3, Ginaton treatment group; Line 4, DSS group). Protein level of p-STAT3 (B) and STAT3 (C) in each group mice were expressed as gray value. #P < 0.05 vs. normal control group; *P < 0.01 vs. DSS group; §P > 0.05 vs. Ginaton group.
The protein expression of IL-6, IL-17 and IL-23
The protein expressions of IL-6, IL-17 and IL-23 in mice were examined by immunohistochemistry. By observing immunohistochemistry films, we found that IL-6, IL-17 and IL-23 were mainly distributed in the mucosa and submucosa layer of colons (Figure 7). As shown in Figure 8, protein expressions of IL-6, IL-17 and IL-23 in DSS group were significantly increased compared with normal control group (P < 0.05). Conversely, protein expressions of these factors were significantly reduced in Ginaton treatment group (P < 0.05). IL-6, IL-17 and IL-23 protein expressions in Ginaton group were similar to normal control group (P > 0.05).
Figure 7.
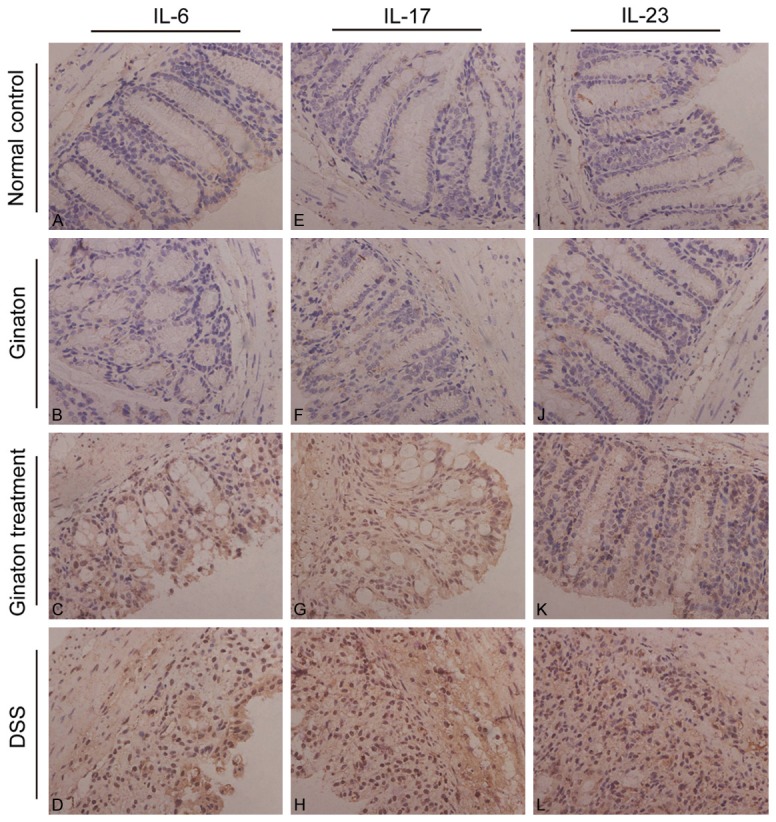
Effect of Ginaton on expressions of IL-6 (A-D), IL-17 (E-H), and IL-23 (I-L) in normal control group (A, E, I), Ginaton group (B, F, J), Ginaton treatment group (C, G, K), and DSS group (D, H, L). IL-6, IL-17 and IL-23 were mainly distributed in the mucosa and submucosa layer of the colon. A large number of brown granules were seen in DSS group in contrast to normal control group. Compared with DSS group, less brown granules were found in Ginaton treatment group.
Figure 8.
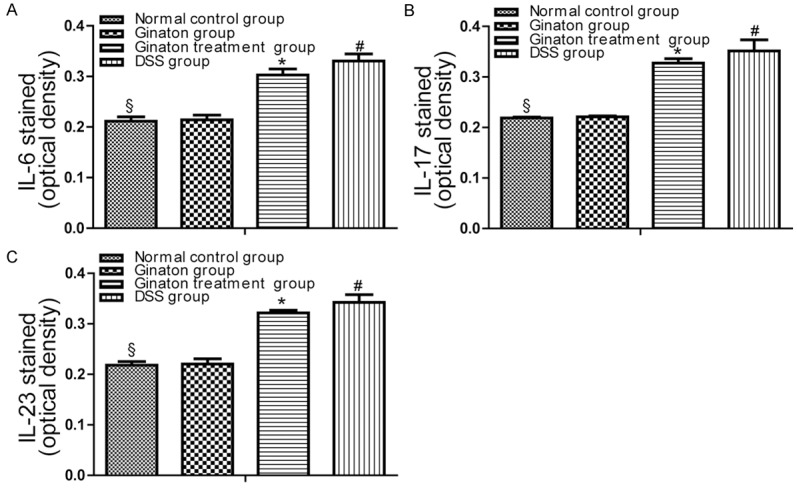
The results of Immunohistochemistry were expressed as optical density (OD). Data of optical density in each group were presented as means ± SEM. #P < 0.01 vs. normal control group; *P < 0.05 vs. DSS group; §P > 0.05 vs. Ginaton group.
Discussion
Our study suggested that Ginaton can improve clinical disease activity (DAI score, weight loss, colon shortening, and bloody stool), reduce histological injury, and decrease expressions of inflammatory related mediators (gp130, p-STAT3, ROR-γt) and cytokines (IL-6, IL-17, IL-23) in colon tissues of mice with DSS-induced acute experimental colitis.
TNBS, DSS, and acetic acid are used to build animal models of colitis. But now, oral administration of DSS is the most common method to induce experimental colitis. DSS-induced experimental colitis has similar pathological and clinical manifestations with UC [26,29-31]. The underlying mechanisms of DSS-induced acute experimental colitis are unclear. Some researchers found that the mechanism involves the activation of intestinal macrophages [30,32,33]. However, recent studies found that the inflammatory process caused by DSS is related to immune disorders [34,35]. In our study, mice were provided with 3% DSS instead of drinking water for one week to induced acute experimental colitis. Mice exposed to DSS were found with gross bloody stool, weight loss, colon shorten and histological injury compared with the normal control group.
Il-6 produced by hyper-activation immune cells (e.g., monocytes and macrophages) involved in the pathogenesis of UC [36,37]. Accumulating evidences suggest that IL-6 plays a crucial role in IBD [38]. A study by Feng et al. [39] demonstrated that serum IL-6 level was consistently elevated in patients with UC, indicating the pathogenesis of UC is closely related to IL-6. In another study, anti-IL-6R monoclonal antibody effectively reduced T-cell expansion and decreased adhesion molecules and inflammatory response in the CD45RBhighSCID (sever combined immuno-deficient) adaptive transfer model of colitis [8,40]. It proved that IL-6 couples with its receptor (sIL-6R) to form a complex which activates gp130-positive cells, inducing the initiation of signaling by activator of STAT3 [41,42]. Moreover, studies reported that STAT3 can be phosphorylated after activating by IL-6, and then transported into the nucleus to regulate transcription of downstream target genes [8,43,44]. The protein level of P-STAT3 was always related to disease activity in IBD patients and in some animal models of experimental colitis [45]. Our study revealed that the expressions of IL-6, gp130, and p-STAT3 were significantly increased in mice of DSS group but reduced in Ginaton treatment group. There exists widespread controversy about whether STAT3 expression is enhanced in experimental colitis. However, we found that no difference in STAT3 expression between normal control group and DSS group, consistent with the result of Liu et al. [46]. In addition, our results showed that protein expression of STAT3 was not consistent with mRNA expression, which may result from post-translational modification.
IL-17 which is secreted by Th17 cells derived from CD4+ T cells plays an important role in the pathogenesis of UC [47]. IL-17 can effectively mediate neutrophil mobilization and pro-inflammatory responses that ultimately lead to inflammatory cell infiltration and tissue damage [48]. One study conducted by Weaver et al. [9] demonstrated a novel role of IL-6/STAT3 signaling pathway in Th17 reaction and IL-17 production. In addition, another study revealed that inhibition of IL-6/STAT3 pathway by triptolide could strikingly reduce the production of IL-17 [7]. Multiple studies have shown that ROR-γt which induced by IL-6-gp130-STAT3 signaling pathway, is very important for differentiation of Th17 cells [10-12]. Moreover, ROR-γt is necessary to transcription of IL-17 [49]. In this study, expressions of ROR-γt and IL-17 were consistent with IL-6, gp130, and p-STAT3 among the four groups. In conclusion, Ginaton alleviates DSS-induced acute experimental colitis in mice by reducing IL-17 production, which is at least partly involved in inhibiting IL-6/STAT3 signaling pathway and IL-23/IL-17 axis.
IL-23 mainly activated immune cells, to induce a large number of inflammatory cytokines which ultimately leads to tissue damage in colitis [50]. Moreover, recent studies suggested that IL-23 involves in the pathogenesis of UC by increasing IL-17 secretion [51,52]. Both animal and human studies have confirmed a critical role of the IL-23/IL-17 axis in the pathogenesis of IBD [47,49,50]. In our study, the expression of IL-23 was significantly enhanced in DSS group relative to normal control group. In contrast to DSS group, the expression of IL-23 was significantly reduced in mice of Ginaton treatment group. The result implies that Ginaton ameliorates acute experimental colitis may involves in restraining IL-23/IL-17 axis.
In conclusion, Ginaton ameliorates DSS-induced acute experimental colitis in mice by reducing IL-17 production, which is at least partly involved in inhibiting IL-6/STAT3 signaling pathway and IL-23/IL-17 axis. Meanwhile, Ginaton itself does not cause inflammatory change in colons of normal mice. These results support that Ginaton can be as a potential clinical treatment for ulcerative colitis. In addition, future research should be conducted to investigate other inflammatory pathways which may be involved in reducing the production of IL-17 in Ginaton-treated DSS mice.
Disclosure of conflict of interest
None.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye L, Cao Q, Cheng J. Review of inflammatory bowel disease in China. ScientificWorldJournal. 2013;2013:296470. doi: 10.1155/2013/296470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo K, Shiga H, Kinouchi Y, Shimosegawa T. Inflammatory bowel disease: IBD. Rinsho Byori. 2009;57:527–32. [PubMed] [Google Scholar]
- 4.Matricon J. Immunopathogenesis of inflammatory bowel disease. Med Sci. 2010;26:405–10. doi: 10.1051/medsci/2010264405. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto-Furusho JK, Podolsky DK. Innate immunity in inflammatory bowel disease. World J Gastroenterol. 2007;13:5577–80. doi: 10.3748/wjg.v13.i42.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets. 2008;9:369–74. doi: 10.2174/138945008784221116. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N, Li JS. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol. 2010;47:2467–74. doi: 10.1016/j.molimm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuyama K, Matsumoto S, Masuda J, Yamasakii H, Kuwaki K, Takedatsu H, Sats M. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–56. [PubMed] [Google Scholar]
- 9.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Tetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abreu C, Rocha-Pereira N, Sarmento A, Magro F. Nocardia infections among immunomodulated inflammatory bowel disease patients: A review. World J Gastroenterol. 2015;21:6491–8. doi: 10.3748/wjg.v21.i21.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang Q, Tandon R, Goh KL, Pan GZ, Fock KM, Fiocchi C, Lam SK, Xiao SD. Management consensus of inflammatory bowel disease for the Asia-Pacific region. J Gastroenterol Hepatol. 2006;21:1772–82. doi: 10.1111/j.1440-1746.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 16.Pehlivan M, Dalbeler Y, Hazinedaroglu S, Arikan Y, Erkek AB, Gunal O, Türkçapar N, Türkçapar AG. An assessment of the effect of Ginkgo Biloba EGb 761 on ischemia reperfusion injury of intestine. Hepatogastroenterology. 2002;49:201–4. [PubMed] [Google Scholar]
- 17.Kusmic C, Basta G, Lazzerini G, Vesentini N, Barsacchi R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: a link between vitamin E preservation and prostaglandin biosynthesis. J Cardiovasc Pharmacol. 2004;44:356–62. doi: 10.1097/01.fjc.0000137164.99487.42. [DOI] [PubMed] [Google Scholar]
- 18.Zeybek N, Gorgulu S, Yagci G, Serdar M, Simsek A, Kaymakcioglu N, Devesi S, Dzcelik H, Tufan T. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. J Surg Res. 2003;115:286–93. doi: 10.1016/s0022-4804(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res. 2006;53:324–30. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) In Rats. Phytother Res. 2001;15:449–51. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 21.Harputluoglu MM, Demirel U, Yucel N, Karadağ N, Temel I, Firat S, Aladağ M, Karincaoğlu M, Hilmioğlu F. The effects of Gingko biloba extract on acetic acid-induced colitis in Rats. Turk J Gastroenterol. 2006;17:177–82. [PubMed] [Google Scholar]
- 22.Chen XH, Miao YX, Wang XJ, Yu Z, Geng MY, Han YT, Wnag LX. Effects of Ginkgo biloba extract EGb761 on human colon adenocarcinoma cells. Cell Physiol Biochem. 2011;27:227–32. doi: 10.1159/000327948. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, An P, Liu SQ, Yu HG. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006:92642. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotakadi VS, Jin Y, Hofseth AB, Ying L, Cui X, Volate S, Chumanevich A, Wood PA, Price RL, McNeal A, Singh UP, Singh NP, Naqakatti M, Nagakatti PS, Matesic LE, Auclair K, Warqovich MJ, Hofeth LJ. Ginkgo biloba extract EGb 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis. 2008;29:1799–806. doi: 10.1093/carcin/bgn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 26.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 27.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–8. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorck S, Jennische E, Dahlstrom A, Ahlman H. Influence of topical rectal application of drugs on dextran sulfate-induced colitis in rats. Dig Dis Sci. 1997;42:824–32. doi: 10.1023/a:1018880501437. [DOI] [PubMed] [Google Scholar]
- 30.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Carilli R. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–75. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- 32.Maltby S, Wohlfarth C, Gold M, Zbytnuik L, Hughes MR, McNagny KM. CD34 is required for infiltration of eosinophils into the colon and pathology associated with DSS-induced ulcerative colitis. Am J Pathol. 2010;177:1244–54. doi: 10.2353/ajpath.2010.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 34.Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol. 2011;2011:342637. doi: 10.1155/2011/342637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Siqnori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sang LX, Chang B, Dai C, Gao N, Liu WX, Jiang M. Heat-killed VSL#3 ameliorates dextran sulfate sodium (DSS)-induced acute experimental colitis in rats. Int J Mol Sci. 2013;15:15–28. doi: 10.3390/ijms15010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuyama K, Sata M, Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn. 1991;26:20–8. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- 39.Feng JS, Yang Z, Zhu YZ, Liu Z, Guo CC, Zheng XB. Serum IL-17 and IL-6 increased accompany with TGF-beta and IL-13 respectively in ulcerative colitis patients. Int J Clin Exp Med. 2014;7:5498–504. [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 41.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009;15:2095–103. doi: 10.2174/138161209788489140. [DOI] [PubMed] [Google Scholar]
- 42.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–9. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 43.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–35. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 44.Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, Fiocchi C, Rizzetto M, Peqoraro L, Sateqna-Guidetti C, Brizzi MF. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91–8. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Liu YL, Liu GX, Chen X, Yang K, Yang YX, Xie Q, Gan HK, Huang XL, Gan HT. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol. 2013;17:314–20. doi: 10.1016/j.intimp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–13. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 48.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 50.Catana CS, Berindan Neagoe I, Cozma V, Magdas C, Tabaran F, Dumitrascu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823–30. doi: 10.3748/wjg.v21.i19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouyang W, Kolls JK, Zheng Y. The biological functions of T h elper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


