Abstract
Aim: We investigated the role of human cytomegalovirus (HCMV) and its mechanism in extravillous cytotrophoblast (EVT) proliferation and invasion in vitro. Methods: Differential enzymatic digestion combined with gradient centrifugation, was used to isolate primary EVT from human chorionic villi collected from early placentae of healthy pregnant women. HCMV infection was determined by immunofluorescence staining of HCMVpp65 antigen expression. An MTT assay was used to examine the role of HCMV in the proliferation of EVT. Quantitative real-time polymerase chain reaction (qRT-PCR), immunocytochemical staining and Western blots were carried out in a control group (EVT) and a virus group (EVT+HCMV) to examine the expression of major genes and protein in TGF-β/Smad signaling pathways in EVT 48 h after inoculation with HCMV. An in vitro cell invasion assay was performed to analyze the influence of HCMV on EVT invasion. Results: HCMV significantly inhibited the proliferation of EVT 48 h after viral infection (P < 0.05). The expression of TGF-β1, Smad1, Smad2, Smad3, Smad4, and Smad5 genes was significantly increased (P < 0.05), but that of TGF-β2, TGF-β3, TGFβRI, TGFβRII, Smad7, MMP2, and MMP9 was significantly decreased in the virus group 48 h after HCMV infection (P < 0.05). Smad7, MMP-2 and MMP-9 protein levels were significantly decreased and the TGF-β1 protein level was significantly increased in infected EVT (all P < 0.05). Conclusions: HCMV may act on multiple steps of the TGF-β/Smad signaling pathway to impede EVT proliferation and invasion.
Keywords: Human cytomegalovirus, extravillous cytotrophoblast, TGF-β1, Smad7, invasion, proliferation
Introduction
Human cytomegalovirus (HCMV) is the most common pathogen that causes intrauterine infection. In the United States and Europe, the neonatal HCMV infection rate is about 2% [1]. In large cities of China, the active HCMV infection rate in pregnant women is about 6.97%, and newborn babies are diagnosed with HCMV in 37.32% cases [2]. HCMV infection during pregnancy can lead to miscarriage, stillbirth, retardation of fetal growth and brain [3]. However, the underlying mechanism(s) have not been fully elucidated.
During normal pregnancy, cytotrophoblasts (CTs) differentiate into villous trophoblasts (VTs) and extravillous cytotrophoblasts (EVTs) after implantation of the late blastocyst. VTs fuse to form syncytiotrophoblasts (STs), which are involved in endocrine, immune, defense and placental exchange. EVTs proliferate to form multilayered columns of cells and then invade the endometrium and the uterine spiral arteries in order to anchor chorionic villi in the uterus and remodel the uterine arterioles. EVTs are formed about three weeks after fertilization and then gradually invade the decidua and superficial muscle, replacing the endothelial cells of the uterine spiral arteries, and remodel the spiral arteries for successful pregnancy [4]. Therefore, dysfunction of EVT invasion often leads to poor remodeling of spiral arteries and insufficient blood supply to the placenta, which are important factors in the pathology of pregnancy-related complications such as miscarriage, stillbirth, fetal growth retardation and pregnancy-induced hypertension [5]. In our previous study, we found that HCMV reduced the invasion of EVT [2].
During pregnancy, transforming growth factor-β (TGF-β) promotes endometrial decidualization, inhibits trophoblast cell proliferation, migration, and invasion and regulates the formation and function of placenta [6]. TGF-β1 plays an important role in trophoblast cell differentiation and EVT invasion. TGF-β acts via Smad transcriptional factors to regulate cell proliferation, differentiation, migration and apoptosis. Smad7 is expressed in human chorionic villi CTs and STs and antagonizes TGF-β/Smad signaling, in which it is a downstream target gene and its expression indicates TGF-β/Smad signaling pathway activation [7,8]. Smad7 inhibits cell invasion and metastasis by inducing apoptosis and reducing the expression and activity of tissue matrix metalloproteinases-2 (MMP-2) and -9 (MMP-9) [9-11]. Matrix metalloproteinases (MMPs), especially MMP-2 and MMP-9, are the rate-limiting enzymes, which mediate EVT invasion and placenta formation [12].
In the current study, an in vitro culture of primary EVTs was generated and successfully infected with HCMV. This model was used to investigate the effect of HCMV on EVT proliferation and invasion. The possible molecular mechanisms underlying HCMV-induced miscarriage, stillbirth, and fetal growth retardation were also briefly examined.
Materials and methods
Materials
Tissue samples and virus
Placenta samples were obtained from healthy pregnant women (peripheral HCMV IgM negative) who voluntarily chose to terminate their pregnancies during the first trimester (5-10 weeks). Samples were collected between July 2012 and July 2014 in the Obstetrics and Gynecology department of the Central Hospital of Taian. The Medical Ethics Committee of the Central Hospital of Taian approved all experimental procedures and patients signed informed consent. The virus strain, HCMV AD169, was provided by the Hubei Provincial Institute of Virology and the TCID50 was 10-5/0.1 ml.
Reagents
The following materials were used: DMEM/Ham’s F 12 medium (Gibco, Life Technologies, Carlsbad, CA, USA); fetal bovine serum (FBS, Hyclone, GE Healthcare, Little Chalfont, UK); DNase I and type I rat tail collagen (Sigma-Aldrich, St. Louis, MO, USA); Percoll (Amersham Pharmacia, GE Healthcare, Little Chalfont, UK); mouse anti-human cytokeratin 7 (CK7) monoclonal antibody, anti-vimentin (Vim) monoclonal antibody, rabbit anti-HCMV pp65 polyclonal antibody, rabbit anti-TGF-β1 polyclonal antibody, rabbit anti-β-actin polyclonal antibody, rabbit anti-MMP-2 polyclonal antibody, rabbit anti-MMP-9 polyclonal antibody, rabbit anti-Smad7 polyclonal antibody (Santa Cruz Biotech, Dallas, Texas, USA); and total protein extraction kit (BestBio, Shanghai, China).
Methods
Isolation and culture of primary EVTs
EVT isolation and primary culture was carried out according to a modified protocol of Handschuh et al [13]. The human chorionic villi were thoroughly washed in sterile D-Hank’s to remove the decidua and matrix. The remaining tissue was cut into 1 mm3 pieces and digested in enzyme solution (0.125% trypsin, 4.2 mM MgSO4, 25 mM Hepes and 20 U/ml DNase I) at 37°C, with gentle shaking, for 50 min. The supernatant was collected and filtered through an 80 mesh (177 micron) and 300 metal mesh. The filtrate was centrifuged at 500× g for 10 min and the supernatant was discarded. The pellets were suspended in DMEM/Ham’s F-12 medium and the cell suspension was supplemented with 35%, 40%, 45% or 50% Percoll gradient solution and centrifuged at 1000× g for 25 min. The cell suspension in 40~45% Percoll gradient was transferred to another centrifuge tube and washed twice in DMEM/Ham’s F-12 medium, supplemented with 10% FBS. The cells were suspended in DMEM/Ham’s F12 medium, supplemented with 10% FBS, seeded at a density of 50000 cells/cm2 in 48 well-culture plates coated with type I rat tail collagen (0.006 mmol/L acetic acid solution of rat tail collagen) and cultured in a 37°C, 5% CO2 incubator for 24 h. The cultures were then washed 3 times with sterile D-Hank’s and fresh medium was added.
EVT infection by HCMV
EVTs were seeded in 6-well plates at a density of 50 000/cm2 and cultured at 37°C and 5% CO2 for 24 h. The EVT culture medium was replaced with the HCMV culture medium. The virus group was inculated with 4 μL HCMV (TCID50:10-5.46/0.1 mL) and the normal group was treated with PBS. Two hours later, the HCMV medium was discarded, and EVTs were washed with sterile D-Hank’s 3 times. The EVT culture medium was added, and the EVTs were cultured at 37°C and 5% CO2 for 12, 24, 48, and 72 h. The HCMVpp65 antigen was detected with immunofluorescence staining, HCMVpp65 expression results were analyzed using a HMIAS-2000 high-resolution color medical image analysis system. Ten high magnification photomicrographs were randomly selected. The mean optical density of positive cells was determined and used for semi-quantitative statistical analysis.
MTT assay
Cells were seeded in 96-well plates at a density of 103/ml. Each well contained 200 μL of cell solution and a blank control was included in each plate. The cells were divided into control and virus groups. The viral group was further divided into 7 subgroups and 10, 20, 30, 40, 50, 70 or 100 μL of HCMV solution was added to each subgroup. A 20 μL volume of MTT solution was added to each well at 12, 24, 48 and 72 h and the plate was incubated for another 4 h. The plate was removed and the medium was discarded. After addition of 150 μL DMSO to each well the plate was shaken for 10 min. The OD value at 490 nm was measured by enzyme-linked immunosorbent assay (ELISA) and the results were used to analyze the effects of HCMV on EVT proliferation. The minimum viral titer with a significant effect on cell proliferation was considered to be the optimal viral titer for inoculation.
Grouping
The virus group was inoculated with HCMV (by volume, 100 TCID50 HCMV: DMEM/Ham’s F-12 containing 3% FBS = 1:6); the control group received the same volume of PBS. After 2 h, the supernatant was removed. The cells were washed 3 times with PBS and then cultured in DMEM/ Ham’s F-12 medium containing 10% FBS, for 48 h. Cells were collected for immunocytochemistry and Western blotting analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Two groups of EVTs were treated for 48 h and EVT was used for extracting total RNA with an RNA extraction kit (Takara, Japan). RNA was transcribed into cDNA using a reverse transcription kit (Takara, Japan) for the following experiments. According to the sequences of major genes in TGF-β/Smad signaling pathways, real-time fluorescent quantitative PCR primers were designed, and the amplified length was 200 bp. β-actin was designed as the internal standard. Primer sequences are listed in Table 1. The PCR reaction system was prepared using 10 μL 2*SYBR Green general qPCR Master Mix (TaKaRa, Japan), 0.6 μL upstream/downstream primer, respectively (10 μmmol•L-1), and 8.8 μL 1:100 diluted cDNA. The total reaction volume was 20 μL. The reaction mixture was precipitated following centrifugation at 1500 rpm/min. The PCR was conducted under the following reaction conditions: pre-degenerated for 30 s at 95°C; degeneration for 3 s at 95°C; and annealing and extension for 30 s at 60°C. The dissolution curve was constructed. Finally, the data were directly read off the real-time fluorescence quantitative PCR system (Applied Biosystems, Foster City, CA, USA).
Table 1.
Primer sequences information
| mRNA | Primers (5’-3’) | |
|---|---|---|
| TGF-β1 | Forward | GGGACTATCCACCTGCAAGA |
| Reverse | CCTCCTTGGCGTAGTAGTCA | |
| TGF-β2 | Forward | CGCCAAGGAGGTTTACAAAA |
| Reverse | CTCCATTGCTGAGACGTCAA | |
| TGF-β3 | Forward | GAGATGTTGTGTGGCAATGG |
| Reverse | TTGCCCTTAATCCCAGACAG | |
| TGFβRI | Forward | GATGGGCTCTGCTTTGTCTC |
| Reverse | CAAGGCCAGGTGATGACTTT | |
| TGFβRII | Forward | GGGGAAACAATACTGGCTGA |
| Reverse | GAGCTCTTGAGGTCCCTGTG | |
| Smad1 | Forward | CTACCCTCACTCTCCCACCA |
| Reverse | GCACCAGTGTTTTGGTTCCT | |
| Smad2 | Forward | CGAAATGCCACGGTAGAAAT |
| Reverse | CCAGAAGAGCAGCAAATTCC | |
| Smad3 | Forward | CCCCAGAGCAATATTCCAGA |
| Reverse | GGCTCGCAGTAGGTAACTGG | |
| Smad4 | Forward | CCATTTCCAATCATCCTGCT |
| Reverse | ACCTTTGCCTATGTGCAACC | |
| Smad5 | Forward | AACCTGAGCCACAATGAACC |
| Reverse | GTGGCATATAGGCAGGAGGA | |
| Smad6 | Forward | ACGGTGACCTGCTGTCTCTT |
| Reverse | ACGTGACGGTTTTGAGTTCC | |
| Smad7 | Forward | TCCTGCTGTGCAAAGTGTTC |
| Reverse | TCTGGACAGTCTGCAGTTGG | |
| MMP2 | Forward | ATGACAGCTGCACCACTGAG |
| Reverse | ATTTGTTGCCCAGGAAAGTG | |
| MMP9 | Forward | TTGACAGCGACAAGAAGTGG |
| Reverse | GCCATTCACGTCGTCCTTAT | |
| β-action | Forward | CATCCGTAAAGACCTCTATGCCAAC |
| Reverse | ATGGAGCCACCGATCCACA | |
Immunocytochemistry (fluorescence) staining
Forty-eight h after HCMV infection, the cells were fixed for 30 min in a methanol-acetone (1:1) solution at room temperature. SP immunocytochemical staining was used to detect CK7, Vim and c-erbB-2 in order to determine purity. HCMV infection was determined by HCMV pp65 antigen staining and EVT cell invasion was indirectly estimated by analysis of TGF-β1, Smad7, and MMP-2 and MMP-9 expression levels. Mouse anti-human CK7, anti-Vim monoclonal antibody, rabbit anti-HCMVpp65, TGF-β1, Smad7, MMP-2, and MMP-9 polyclonal antibodies were diluted 1:100. TGF-β1, Smad7, MMP-2 and MMP-9 expression results were analyzed as mentioned before.
Western blot
TGF-β1, Smad7, MMP-2 and MMP-9 protein levels were determined by Western blot. The total protein was extracted and the concentration was measured using a total protein extraction kit (BestBio), according to the manufacturer’s instructions. Fifty μg of total protein from each sample was separated on a 10% SDS-PAGE gel by electrophoresis and transferred to a PVDF membrane. The membrane was blocked in 5% skim milk at room temperature for 2 h and then incubated with rabbit anti-TGF-β1, Smad7, MMP-2 or MM-P9 polyclonal antibody (1:500 dilution) overnight at 4°C. The membrane was then washed in TBST and incubated with the secondary antibody at room temperature for 2 h. The membrane was washed again in TBST, incubated with ECL reagent and protein bands were visualized under a gel imager. Gel image Quantity One analysis software was used to measure the absorbance values of the target protein and the internal control β-actin protein. The mean absorbance of the target protein over that of the internal control was used as the relative expression level of the target protein.
In vitro invasion assay
In vitro invasion assay was used for determining the invasive potential of EVTs. The Transwell chamber coated with Matrigel was placed in 24-well culture plates and 400 μL medium containing conditioned medium and complete medium (1:1) was infused into well out of chamber. In the HCMV group, EVT at 1×105/mL and 100 TCID50 HCMV 14.29 μL (with a total amount of 100 μL) was used in the Transwell chamber. In the control group, an identical volume of PBS was used instead of HCMV solution. Each group had 4 duplicate samples. After incubation for 24 h, the sample was fixed with formaldehyde, hematoxylin-stained and observed under an inverted microscope to count the number of cells migrating through the micropore membrane. For each sample, 10 randomly selected high power fields were counted.
Statistical analysis
All experiments were performed 3 times. The SPSS 18.0 software package was used for statistical analysis and data were expressed as mean ± standard deviation (x̅±s). The significance was assessed by ANOVA. A P < 0.05 was considered to be statistically significant.
Results
Purity of isolated EVTs
Immunocytochemistry showed that the majority of the isolated cells contained a single nucleus and were triangular and irregular in shape. More than 96% of the cells were positively stained for CK7 and c-erbB-2, and occasionally for Vim, suggesting that the primary EVT isolated from human chorionic villi were of high purity (Figure 1).
Figure 1.
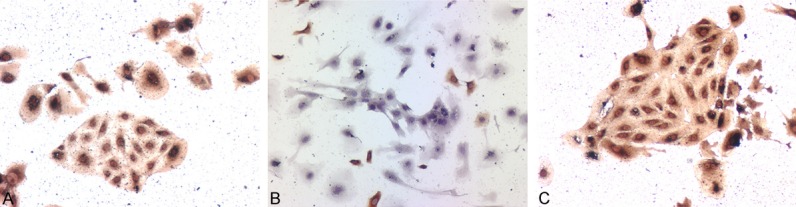
Identification of primary EVT by CK7, Vim and c-erbB-2 antigens. A-C. CK7, Vim and c-erbB-2 antigens were detected, respectively, by immunocytochemistry ×100.
In vitro HCMV infection of EVT
As shown by Figure 2, HCMV-infected EVT showed strong HCMVpp65 staining (red signal), while non-infected EVT showed no detectable HCMVpp65 expression, suggesting that HCMV infected and proliferated in host EVT. Using immunofluorescence staining quantitative analysis, we found that HCMVpp65 expression in EVT at 48 h and 72 h was significantly higher than that of 12 h and 24 h (P < 0.05), but was significantly different between 48 h and 72 h (P > 0.05). This phenomenon may be caused by excessive proliferation of the virus resulting in a higher number of EVT deaths.
Figure 2.
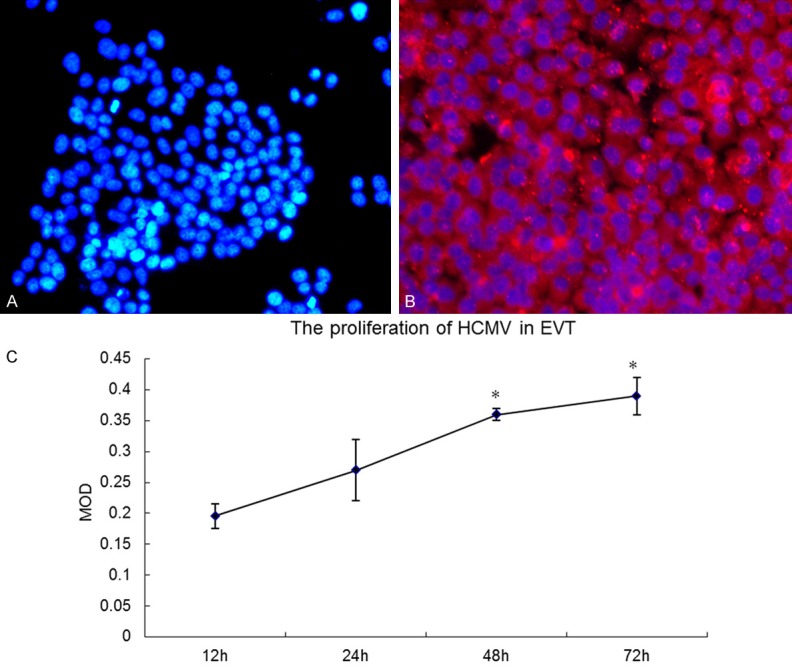
Immunofluorescence staining of EVT. A. Control group (Nucleus stained blue; no red signal); B. Virus group (HCMV pp65 antigen stained red; nucleus stained blue) ×200; C. Immunofluorescence staining quantitative analysis: HCMVpp65 expression in EVT at 48 h and 72 h was significantly higher than at 12 h and 24 h (P < 0.05), but was significantly different between 48 h and 72 h (P > 0.05).
Effect of HCMV on EVT proliferation
Compared with the control group, infection with 10 μL to 100 μL virus solution for 12 h had little effect on EVT proliferation (P > 0.05). However, EVT proliferation was significantly inhibited after infection with 70 μL to 100 μL virus solution for 24 h; 20 μL, 30 μL, 40 μL or 50 μL virus solution for 48 h; or 10 μL virus solution for 72 h (P < 0.05). No significant difference was observed in the inhibition of EVT cell proliferation at 48 h or 72 h after infection between the two groups of 20 μL, 30 μL or 40 μL virus solutions (P > 0.05). Based on these results, the 20 μL solution containing 100TCID50 HCMV, diluted in 120 μL medium, was selected for subsequent experiments (Figures 3 and 4).
Figure 3.
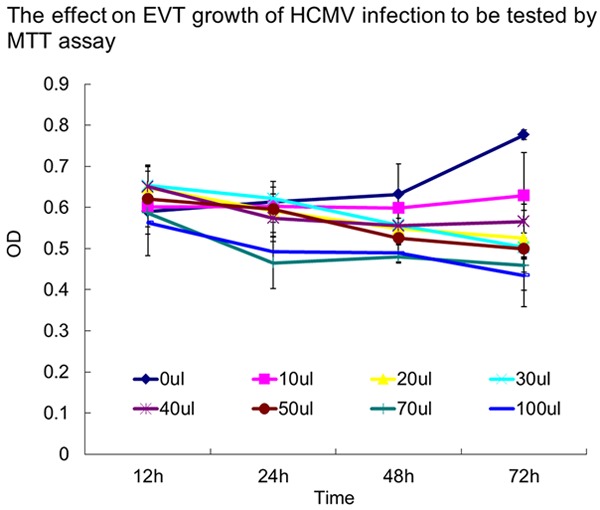
Effect of HCMV on EVT tested by MTT. Compared with the control group, infection with 10 μl-100 μL virus solution for 12 h had little effect on EVT proliferation (P > 0.05). However, EVT proliferation was significantly inhibited (P < 0.05) after infection with 70 μL and 100 μL virus solution for 24 h; 20, 30, 40 or 50 μL virus solution for 48 h; or 10 μL virus solution for 72 h. There was no significant difference in the inhibition of EVT cell proliferation at 48 h or 72 h after infection with 20 μL, 30 μL or 40 μL virus solution (P > 0.05). These experiments above were performed in triplicate.
Figure 4.
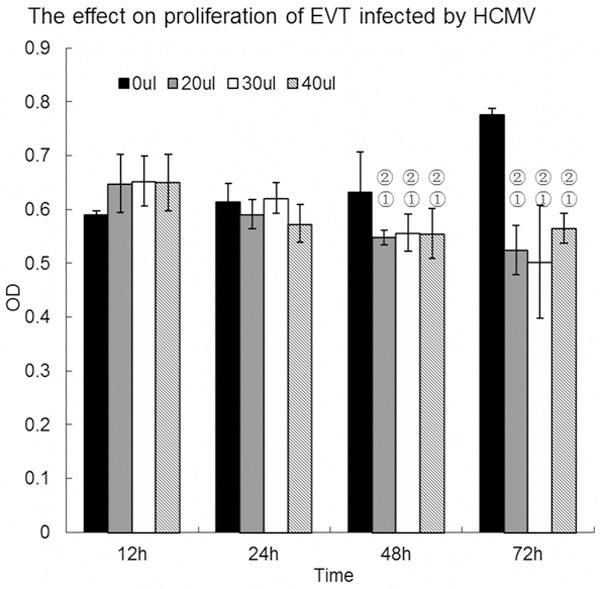
EVT proliferation following HCMV infection with 20 μL, 30 μL and 40 μL virus solution. Compared with the control group, EVT proliferation was significantly inhibited (P < 0.05) after infection with 20 μL, 30 μL or 40 μL virus solution for 48 h and 72 h, without any significant difference in inhibition (P > 0.05). The experiments were performed in triplicate.
Effect of HCMV on expression of major genes in TGF-β/Smad signaling pathways in EVT
The results of qRT-PCR showed that the gene expression of TGF-β1, Smad1, Smad2, Smad3, Smad4, and Smad5 was significantly increased (P < 0.05), but that of TGF-β2, TGF-β3, TGFβRI, TGFβRII, and Smad7 was significantly decreased in the virus group 48 h after HCMV infection (P < 0.05). Immunocytochemistry and Western blot results showed that the control and virus groups expressed the TGF-β1 and Smad7 protein. The TGF-β1 protein level was significantly elevated in the virus group 48 h after HCMV infection (P < 0.05) (Figure 5) whereas the Smad7 protein level was significantly decreased in the virus group 48 h after HCMV infection (P < 0.05) (Figure 6).
Figure 5.
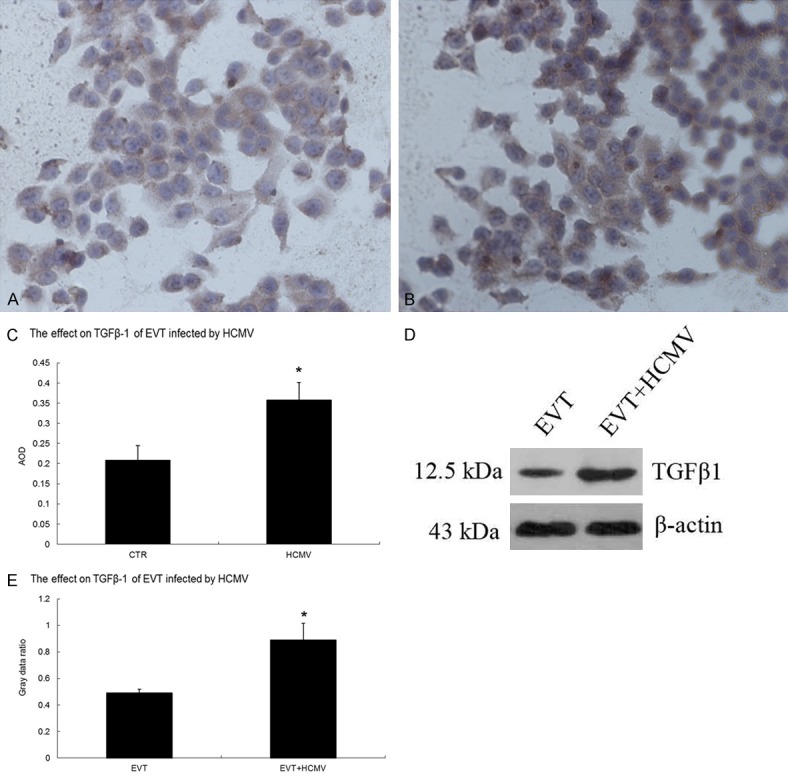
Effect of HCMV on TGF-β1 protein expression in EVT. A. TGF-β1 immunocytochemical staining in the EVT control group (200×); B. TGF-β1 immunocytochemistry in HCMV-infected EVT (200×); C. Comparison of TGF-β1 immunocytochemistry results between the control and virus groups; D. TGF-β1 Western blots in the control and virus groups; E. Comparison of TGF-β1 Western blots between the control and virus groups. The experiments were performed in triplicate.
Figure 6.
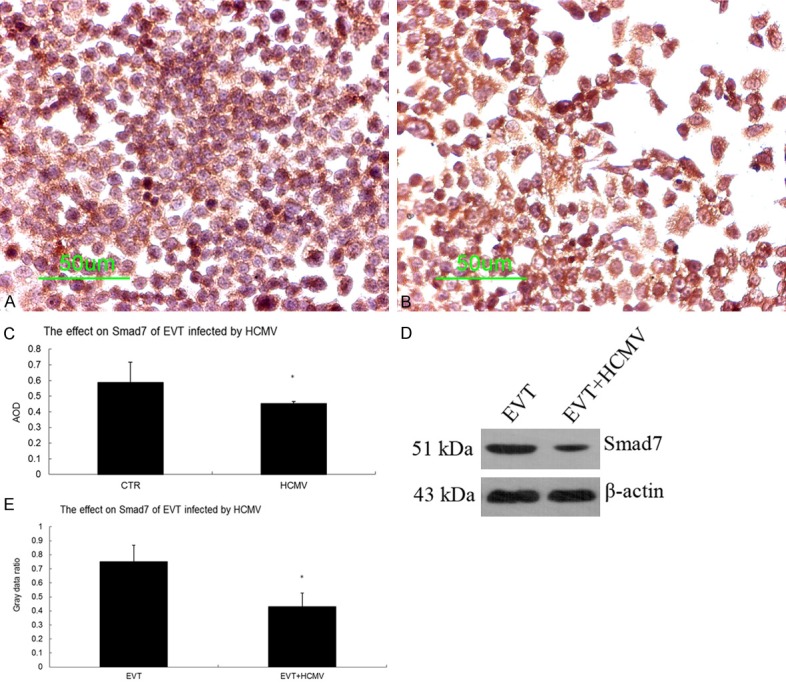
Effect of HCMV on Smad7 protein expression in EVT. A. Smad7 immunocytochemical staining in the EVT control group (200×); B. Smad7 immunocytochemical staining in HCMV-infected EVT (200×); C. Comparison of Smad7 immunocytochemistry between the control and virus groups; D. Smad7 Western blots in the control and virus groups; E. Comparison of Smad7 Western blots between the control and virus groups. These experiments above were performed in triplicate.
Effect of HCMV on expression of MMP-2 and MMP-9 in EVT
The results of qRT-PCR showed that the gene expression of MMP2 and MMP9 was significantly decreased in the virus group 48 h after HCMV infection (P < 0.05). Immunocytochemistry and Western blot results showed that both the control and virus groups expressed the MMP-2 and MMP-9 proteins. The MMP-2 and MMP-9 protein levels were decreased in the virus group 48 h after HCMV infection (P < 0.05) (Figures 7 and 8).
Figure 7.
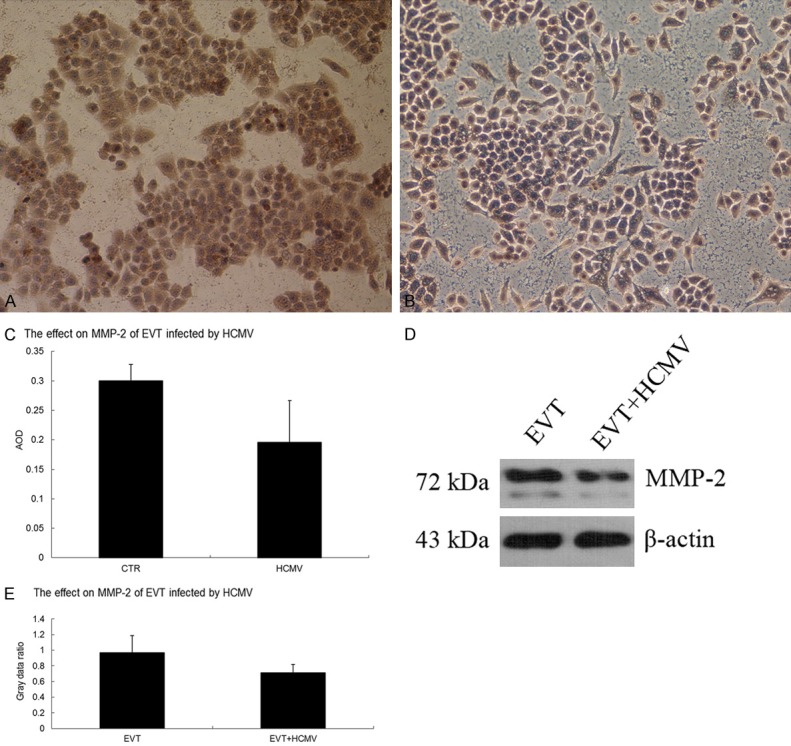
Effect of HCMV on MMP-2 protein expression in EVT. A. MMP-2 immunocytochemistry in the EVT control group (200×); B. MMP-2 immunocytochemistry in HCMV-infected EVT (200×); C. Comparison of MMP-2 immunocytochemistry between the control and virus groups; D. MMP-2 Western blots in the control and virus groups; E. Comparison of MMP-2 Western blots between the control and virus groups. The experiments were performed in triplicate.
Figure 8.
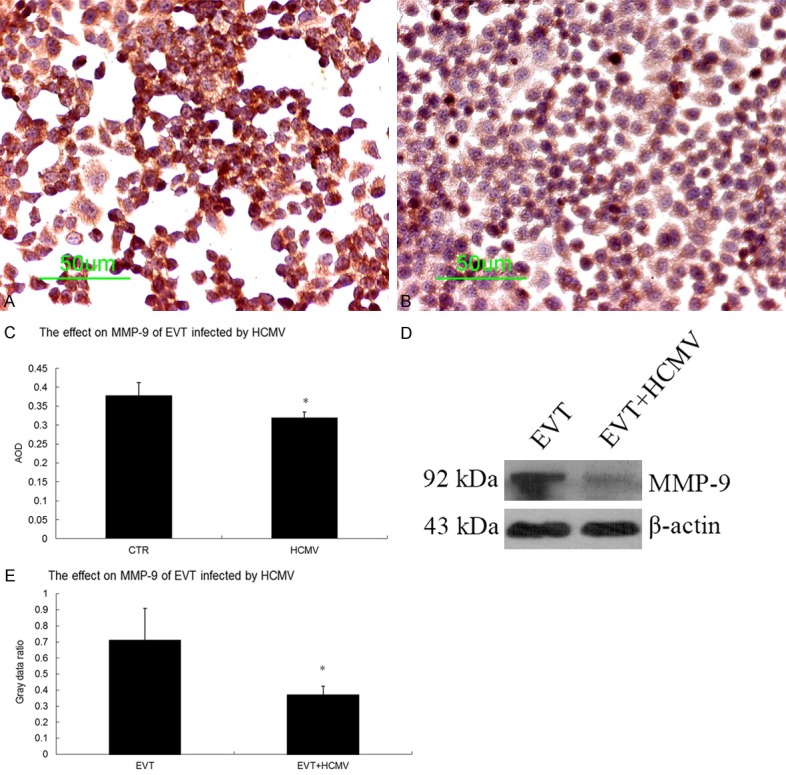
Effect of HCMV on MMP-9 protein expression in EVT. A. MMP-9 immunocytochemical staining in the EVT control group (200×); B. MMP-9 immunocytochemical staining in HCMV-infected EVT (200×); C. Comparison of MMP-9 immunocytochemistry between the control and virus groups; D. MMP-9 Western blot results in the control and virus groups; E. Comparison of MMP-9 Western blots between the control and virus groups. These experiments above were performed in triplicate.
Effect of HCMV on EVT invasion
EVT invaded the Matrigel. The number of EVT penetrating the Matrigel was 57.0 ± 3.61 in the control group and 49.2 ± 3.27 in the virus group (P < 0.05), suggesting that HCMV reduced EVT invasion.
Discussion
HCMV is distributed worldwide and humans are the only host. Several HCMV strains have been identified based on differences in antigen expression. The HCMV ADl69 strain is the standard laboratory strain. The HCMVpp65 antigen is an early indicator of active HCMV infection since it is expressed in vascular endothelial cells, peripheral blood lymphocytes, monocytes and polymorphonuclear leukocytes within 6-24 h of infection [14,15]. Therefore, the HCMV pp65 antigen was selected as an infection marker in this study.
Currently the mechanism(s) underlying intrauterine HCMV infection is not clear, though trophoblast cells are considered the primary site of intrauterine HCMV infection. HCMV infects different types of trophoblast cells [1,16-20], including CTs, STs, VTs and EVT, leading to cell damage and death, poor differentiation of CTs, impaired VT fusion, diminished numbers of and immune secretion dysfunction in STs, and reduced EVT proliferation and invasion [1].
EVT invasion mainly depends on two pathways: MMPs and the urokinase plasminogen activator (uPA) system [21]. EVT infiltration occurs along the spiral arteries at around 10 weeks, reaching the decidua at around 12 weeks and invading a third of the myometrium within 20 weeks of pregnancy. Therefore, EVT invasion of the uterine decidua and superficial myometrium, in addition to physiological remodeling of uterine spiral arteries, are prerequisites for a successful pregnancy. EVT proliferation and invasion dysfunction can result in poor remodeling of spiral arteries and poor placental substance exchange, which is an important pathophysiological mechanism in miscarriage, stillbirth, intrauterine growth retardation, premature birth and pregnancy-induced hypertension [22,23].
Primary EVT culture provides an appropriate tool to study the mechanisms related to the aforementioned conditions. CK is a cytoskeletal protein of epithelial cells, c-erbB-2 is an epidermal growth factor receptor with tyrosine kinase activity and can be used as a specific EVT marker and Vim is a marker of endothelial and stromal cells but is not expressed in EVTs [24]. In the current study, healthy early chorionic villi and isolated primary EVTs were collected. Immunocytochemistry staining showed that the isolated primary EVTs were mononuclear, that the majority of cells were triangular or irregular in shape and that almost all of the cells were CK7 and c-erbB-2 positive, and occasionally Vim positive. This suggests that a sufficient number of highly pure primary EVTs were isolated, thus laying the foundation for future study. In this study, HCMV viremia during pregnancy was modeled by infecting primary EVTs with HCMV. Immunofluorescence staining showed that the HCMVpp65 signal appeared within the infected primary EVTs, but not in the control group, indicating that HCMV infected and replicated within primary cultured EVTs that served as HCMV host cells [25].
During the normal course of pregnancy, TGF-β promotes endometrial decidualization, inhibits trophoblast cell proliferation, migration, and invasion, stimulates trimester and later trophoblast cells to form multinucleated cells and regulates the formation and function of the placenta [26]. In early pregnancy, low TGF-β1 expression leads to inadequate maternal immune tolerance and embryonic implantation disorders, while high TGF-β1 expression affects the normal invasion of trophoblast cells into endometrial tissue, also resulting in embryonic implantation disorders [27,28]. TGF-β1 regulates trophoblast cell differentiation and invasion as well as EVT invasion in a dose-dependent manner [29]. TGF-β1 expression peaks at 17 and 34 weeks of pregnancy, which is consistent with the completion of EVT invasion into the decidua and placenta at 18 weeks and the conclusion of fetal growth at 35 weeks of pregnancy [30,31]. Results of the current study showed that the primary EVT expressed TGF-β1, which was significantly higher after HCMV infection.
The Smad proteins are the only confirmed substrates of the TGF-β receptor [32] thus far. They are functionally divided into three categories: (i) receptor-regulated Smads (R-Smads), including Smad1, Smad2, Smad3, Smad5 and Smad8; (ii) common mediator SMAD (Co-Smads), including Smad4 and Smad10, which are the common mediators of TGF-β signaling; and (iii) inhibitory Smads (I-Smads), including Smad6 and Smad7. Smad6 mainly inhibits the BMP signaling and Smad7 mainly acts on the TGF-β signaling pathway.
Smads are key mediators of the TGF-β signal transduction pathway, as well as cell proliferation, differentiation, migration and apoptosis. Binding of biologically active TGF-β to TGFβRII activates TGFβRI, resulting in the phosphorylation of C-terminal serine residues and activation of receptor-specific Smad proteins (Smad1/Smad5 or Smad2/Smad3). Activated R-Smads associate with the Co-Smads (Smad4) to form heterodimers and translocate to the nucleus, where they directly bind to the DNA sequence in conjunction with other positive or negative transcription factors to stimulate transcription. Smad6 competes with Smad4 for phosphorylated Smad1 or directly binds to activated TGFβRI to prevent phosphorylation of R-Smads and Smad7 directly binds to activated receptors, thus both function as negative regulators of the TGF-β/Smad signaling pathway [33]. Smad7 expression is a major indicator of TGF-β pathway activation since TGF-β signaling induces its expression [7,8].
Smad7 induces apoptosis, reduces the expression and activity of MMP-2, MMP-9 and uPA, inhibits cell invasion and metastasis [9-11] and negatively regulates TGF-β1 receptors, thus blocking TGF-β1 signal transduction and reducing ECM synthesis to inhibit fibrosis [34]. In the placenta, Smad7 is mainly located in the cytoplasm of villi CTs and STs. In the TGF-β/Smad signaling pathway, TGF-β stimulates the Smad7 transcription as a negative feedback to decrease TGF-β signaling [35], thus regulating EVT invasion temporally and spatially. Our results suggest that the primary EVT expressed Smad7 and after HCMV infection, and Smad7 expression in primary EVT was significantly reduced.
MMPs are closely related to blastocyst implantation, placental formation and vascular remodeling [36] and function as a rate-limiting biochemical mediator and marker of trophoblast invasion [37]. MMP-2 and MMP-9 are considered to be the rate-limiting enzymes involved in EVT invasion and placenta formation [12]. During placenta formation, the physical environment of the maternal-fetal interface changes, including the ECM components. A variety of hormones, growth factors and cytokines are also produced by the placenta or maternal decidua, all of which directly or indirectly affect the MMPs/tissue inhibitor of metalloproteinases (TIMPs) system to regulate EVT invasion [38]. TGF-β induces fibronectin, plasminogen activator inhibitor and TIMP-1 expression to inhibit MMPs activation [39]. Under normal circumstances, MMPs, TIMPs and TGF-β1 are temporally and spatially coordinated to regulate the synthesis and degradation of collagen type IV in ECM. MMPs/TIMPs dysregulation and abnormal EVT invasion cause miscarriage, intrauterine growth restriction, hypertensive disorders in pregnancy and trophoblastic disease [40,41]. MMP-9 secretion by trophoblast cells is negatively correlated with exogenous TGF-β1 levels. TGF-β1 significantly enhances the adhesion between the trophoblast cells, as well as inhibits MMP-2 and MMP-9 expression, indicating that TGF-β1 may promote adhesion between trophoblasts, inhibit the secretion of MMP-2 and MMP-9 and regulate EVT invasion during normal pregnancy [42,43].
Results of the current study confirmed that primary EVTs express MMP-2 and MMP-9 with enzymatic activity and that HCMV infection reduced the expression level and activity of both enzymes. In addition, it was found that HCMV infection reduced EVT invasion.
In short, in EVTs and villi, HCMV infection boosted the expression of TGF-β1. It also reduced the expression of Smad7, the expression and activity of MMP-2 and MMP-9 and the invasion of EVT. This suggests that HCMV damages EVT, resulting in increased secretion of TGF-β1. Meanwhile, HCMV inhibits Smad7 expression, which suppresses the negative feedback, leading to a further increase in TGF-β1, which binds to receptors on trophoblast cells to excessively inhibit the secretion of MMP-2 and MMP-9. Therefore, abnormal TGF-β1 expression disrupts the balance between the inhibition and stimulation of trophoblast cell differentiation and invasion, leading to excessive suppression of invasion and differentiation of trophoblast cells, leading to shallow the EVT implantation that prevents the formation of uterine spiral artery endothelial cells.
HCMV acts on multiple steps of the TGF-β/Smad signaling pathway to impede EVT proliferation and invasion, leading to narrow spiral arteries, high blood impedance, decreased placental blood perfusion and substance exchange, resulting in poor pregnancy outcomes. Additional studies will be conducted using whole animal experiments.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (NO 30672243), Natural Science Foundation of Hubei province (NO 2009CDB216), Natural Science Foundation of Shandong province (ZR2012HL15) and Tai’an Science and Technology Development Plan (NO 20123023). Special thanks are due to our present and former lab members for their contributions over the years.
Disclosure of conflict of interest
None.
Abbreviations
- HCMV
Human cytomegalovirus
- CT
cytotrophoblast
- VT
villous trophoblast
- EVT
extravillous cytotrophoblast
- ST
syncytiotrophoblast
- TGF-β
transforming growth factor-β
- MMP
Matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
- CK7
cytokeratin 7
- Vim
vimentin
- uPA
urokinase plasminogen activator
- ECM
extracellular matrix
References
- 1.LaMarca HL, Nelson AB, Scandurro AB, Whitley GS, Morris CA. Human cytomegalovirus-induced inhibition of cytotrophoblast invasion in a first trimester extravillous cytotrophoblast cell line. Placenta. 2006;27:137–147. doi: 10.1016/j.placenta.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Liu T, Zheng X, Chen J, Wang N, Xiao J, Zhang D, Yin Z, Li W, Chen S. Effect of human cytomegalovirus on invasive capability of early pregnant extravillous cytotrophoblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31:819–823. doi: 10.1007/s11596-011-0683-x. [DOI] [PubMed] [Google Scholar]
- 3.Warner JA, Zwezdaryk KJ, Day B, Sullivan DE, Pridjian G, Morris CA. Human cytomegalovirus infection inhibits CXCL12- mediated migration and invasion of human extravillous cytotrophoblasts. Virol J. 2012;9:255. doi: 10.1186/1743-422X-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto-Tabata T, McDonagh S, Chang HT, Fisher S, Pereira L. Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J Virol. 2004;78:2831–2840. doi: 10.1128/JVI.78.6.2831-2840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzi T, Marzioni D, Giannubilo S, Quaranta A, Crescimanno C, De Luca A, Baldi A, Todros T, Tranquilli AL, Castellucci M. Expression patterns of two serine protease HtrA1 forms in human placentas complicated by preeclampsia with and without intrauterine growth restriction. Placenta. 2009;30:35–40. doi: 10.1016/j.placenta.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Prossler J, Chen Q, Chamley L, James JL. The relationship between TGFbeta, low oxygen and the outgrowth of extravillous trophoblasts from anchoring villi during the first trimester of pregnancy. Cytokine. 2014;68:9–15. doi: 10.1016/j.cyto.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Meoli EM, Oh U, Grant CW, Jacobson S. TGF-beta signaling is altered in the peripheral blood of subjects with multiple sclerosis. J Neuroimmunol. 2011;230:164–168. doi: 10.1016/j.jneuroim.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant C, Oh U, Yao K, Yamano Y, Jacobson S. Dysregulation of TGF-beta signaling and regulatory and effector T-cell function in virus-induced neuroinflammatory disease. Blood. 2008;111:5601–5609. doi: 10.1182/blood-2007-11-123430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA, Strutz F. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int. 2005;68:96–109. doi: 10.1111/j.1523-1755.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Fan J, Chen Z, Meng ZQ, Luo JM, Lin JH, Zhou ZH, Chen H, Wang K, Xu ZD, Liu LM. Low-level expression of Smad7 correlates with lymph node metastasis and poor prognosis in patients with pancreatic cancer. Ann Surg Oncol. 2009;16:826–835. doi: 10.1245/s10434-008-0284-5. [DOI] [PubMed] [Google Scholar]
- 11.Kuga H, Morisaki T, Nakamura K, Onishi H, Noshiro H, Uchiyama A, Tanaka M, Katano M. Interferon-gamma suppresses transforming growth factor-beta-induced invasion of gastric carcinoma cells through cross-talk of Smad pathway in a three-dimensional culture model. Oncogene. 2003;22:7838–7847. doi: 10.1038/sj.onc.1207046. [DOI] [PubMed] [Google Scholar]
- 12.Pollheimer J, Fock V, Knofler M. Review: the ADAM metalloproteinases-novel regulators of trophoblast invasion? Placenta. 2014;35(Suppl):S57–63. doi: 10.1016/j.placenta.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28:175–184. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Wang D. Clinical value of human cytomegalovirus phosphoprotein 65 in the diagnosis of cytomegalovirus disease. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:263–265. [PubMed] [Google Scholar]
- 15.Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J Virol. 2004;78:10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauwel B, Mariame B, Martin H, Nielsen R, Allart S, Pipy B, Mandrup S, Devignes MD, Evain-Brion D, Fournier T, Davrinche C. Activation of peroxisome proliferator-activated receptor gamma by human cytomegalovirus for de novo replication impairs migration and invasiveness of cytotrophoblasts from early placentas. J Virol. 2010;84:2946–2954. doi: 10.1128/JVI.01779-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210–1226. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lashmit P, Wang S, Li H, Isomura H, Stinski MF. The CREB site in the proximal enhancer is critical for cooperative interaction with the other transcription factor binding sites to enhance transcription of the major intermediate-early genes in human cytomegalovirus-infected cells. J Virol. 2009;83:8893–8904. doi: 10.1128/JVI.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonagh S, Maidji E, Chang HT, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol. 2006;35:210–215. doi: 10.1016/j.jcv.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Seeho SK, Park JH, Rowe J, Morris JM, Gallery ED. Villous explant culture using early gestation tissue from ongoing pregnancies with known normal outcomes: the effect of oxygen on trophoblast outgrowth and migration. Hum Reprod. 2008;23:1170–1179. doi: 10.1093/humrep/den066. [DOI] [PubMed] [Google Scholar]
- 21.Plaisier M, Koolwijk P, Willems F, Helmerhorst FM, van Hinsbergh VW. Pericellular-acting proteases in human first trimester decidua. Mol Hum Reprod. 2008;14:41–51. doi: 10.1093/molehr/gam085. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Tan R, MacCalman CD, Eastabrook G, Park SH, Dutz JP, von Dadelszen P. IFN-gamma-mediated extravillous trophoblast outgrowth inhibition in first trimester explant culture: a role for insulin-like growth factors. Mol Hum Reprod. 2008;14:281–289. doi: 10.1093/molehr/gan018. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Q, Basak A, Mbikay M, Tsang BK, Gruslin A. Role of pro-IGF-II processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci U S A. 2005;102:11047–11052. doi: 10.1073/pnas.0502357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malassine A, Handschuh K, Tsatsaris V, Gerbaud P, Cheynet V, Oriol G, Mallet F, Evain-Brion D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta. 2005;26:556–562. doi: 10.1016/j.placenta.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Halwachs-Baumann G, Weihrauch G, Gruber HJ, Desoye G, Sinzger C. hCMV induced IL-6 release in trophoblast and trophoblast like cells. J Clin Virol. 2006;37:91–97. doi: 10.1016/j.jcv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Nadeem L, Munir S, Fu G, Dunk C, Baczyk D, Caniggia I, Lye S, Peng C. Nodal signals through activin receptor-like kinase 7 to inhibit trophoblast migration and invasion: implication in the pathogenesis of preeclampsia. Am J Pathol. 2011;178:1177–1189. doi: 10.1016/j.ajpath.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 28.Xavier MB, Libonati RM, Unger D, Oliveira C, Corbett CE, de Brito AC, Quaresma JA. Macrophage and TGF-beta immunohistochemical expression in Jorge Lobo’s disease. Hum Pathol. 2008;39:269–274. doi: 10.1016/j.humpath.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2011;589:7–20. doi: 10.1113/jphysiol.2010.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lysiak JJ, Hunt J, Pringle GA, Lala PK. Localization of transforming growth factor beta and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta. 1995;16:221–231. doi: 10.1016/0143-4004(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 31.Xu G, Chakraborty C, Lala PK. Reconstitution of Smad3 restores TGF-beta response of tissue inhibitor of metalloprotease-1 upregulation in human choriocarcinoma cells. Biochem Biophys Res Commun. 2003;300:383–390. doi: 10.1016/s0006-291x(02)02845-0. [DOI] [PubMed] [Google Scholar]
- 32.Huang CH, Tseng WY, Yao CC, Jeng JH, Young TH, Chen YJ. Glucosamine promotes osteogenic differentiation of dental pulp stem cells through modulating the level of the transforming growth factor-beta type I receptor. J Cell Physiol. 2010;225:140–151. doi: 10.1002/jcp.22206. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Halder SK, Kashikar ND, Cho YJ, Datta A, Gorden DL, Datta PK. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology. 2010;138:969–80. e1–3. doi: 10.1053/j.gastro.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez AV, Le Bellego F, Ludwig MS. Imbalance of receptor-regulated and inhibitory Smads in lung fibroblasts from bleomycin-exposed rats. Am J Respir Cell Mol Biol. 2007;36:206–212. doi: 10.1165/rcmb.2006-0132OC. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Luo S, Wang Y, Zhuang L, Chen Y, Peng C. Smads in human trophoblast cells: expression, regulation and role in TGF-beta-induced transcriptional activity. Mol Cell Endocrinol. 2001;175:111–121. doi: 10.1016/s0303-7207(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 36.Husslein H, Haider S, Meinhardt G, Prast J, Sonderegger S, Knofler M. Expression, regulation and functional characterization of matrix metalloproteinase-3 of human trophoblast. Placenta. 2009;30:284–291. doi: 10.1016/j.placenta.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46:266–273. doi: 10.1111/j.1479-828X.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 38.Tao L, Suhua C, Juanjuan C, Zongzhi Y, Juan X, Dandan Z. In vitro study on human cytomegalovirus affecting early pregnancy villous EVT’s invasion function. Virol J. 2011;8:114. doi: 10.1186/1743-422X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AN, Carter QL, Kniss DA, Brown TL. Characterization of a TGFbeta-responsive human trophoblast-derived cell line. Placenta. 2001;22:425–431. doi: 10.1053/plac.2001.0653. [DOI] [PubMed] [Google Scholar]
- 40.Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78:1064–1072. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ioannidis I, Dimo B, Karameris A, Vilaras G, Gakiopoulou H, Patsouris E, Lazaris AC. Comparative study of the immunohistochemical expression of metalloproteinases 2, 7and 9between clearly invasive carcinomas and “in situ” trophoblast invasion. Neoplasma. 2010;57:20–28. doi: 10.4149/neo_2010_01_020. [DOI] [PubMed] [Google Scholar]
- 42.Lin HY, Yang Q, Wang HM, Qi JG, Zhang H, Wang HX, Tsang BK, Zhu C. Involvement of SMAD4, but not of SMAD2, in transforming growth factor-beta1-induced trophoblast expression of matrix metalloproteinase-2. Front Biosci. 2006;11:637–646. doi: 10.2741/1823. [DOI] [PubMed] [Google Scholar]
- 43.Zhao MR, Qiu W, Li YX, Zhang ZB, Li D, Wang YL. Dual effect of transforming growth factor beta1 on cell adhesion and invasion in human placenta trophoblast cells. Reproduction. 2006;132:333–341. doi: 10.1530/rep.1.01112. [DOI] [PubMed] [Google Scholar]


