Abstract
As a kind of α2 adrenergic receptor agonists, dexmedetomidine generates sedation, anti-anxiety and anesthesia effects by hyperpolarizing noradrenergic nerve cells in locus coeruleus. This study was designed to investigate the neuroprotective of dexmedetomidine attenuates isoflurane-induced cognitive impairment, and the possible underlying mechanism in aging rat. Firstly, we used isoflurane-induced aging rat model to analyze the therapeutical effect of dexmedetomidine on cognitive impairment. Next, commercial ELISA kits were used to analyze tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), methane dicarboxylic aldehyde (MDA) and superoxide dismutase (SOD) and caspase-3 levels. In addition, Western blotting was used to detect the protein expression of P38 MAPK, PTEN and phosphorylation-Akt (p-Akt) expression. Our results showed that the neuroprotective of dexmedetomidine significantly attenuates isoflurane-induced cognitive impairment in aging rat. Moreover, dexmedetomidine significantly inhibited these TNF-α, IL-1β, MDA, SOD and caspase-3 activities in isoflurane-induced aging rat. Meanwhile, the neuroprotective effects of dexmedetomidine on isoflurane-induced cognitive impairment significantly suppressed Bcl-xL/Bad rate, P38 MAPK and PTEN protein expression and activated p-Akt protein expression in aging rat. Collectively, neuroprotective effect of dexmedetomidine attenuates isoflurane-induced cognitive impairment through antioxidant, anti-inflammatory and anti-apoptosis in aging rat.
Keywords: Dexmedetomidine, isoflurane-induced cognitive impairment, antioxidant, anti-inflammatory and anti-apoptosis
Introduction
With a 170-year history of clinical application, inhalation anesthetics are widely used in clinical anesthesia because of their strong anesthesia efficiency, excellent security, and easy adjustment of anesthesia depth. Inhalation anesthesia plays an enormous role in completing operations smoothly, securely and successfully, for the more than 40 million person-times of patients who received general anesthesia in America every year [1-3]. What’s more, the protection against ischemic injuries of vital organs such as heart, brain and kidney has lead to broader clinical application prospects of inhalation anesthetics in recent years [4]. However, it is unclear that how do inhalation anesthetics produce anesthesia reactions such as unconsciousness, amnesia, immobilization, analgesia and so on [5]. As a result, there appears a widely shared concern for adverse reactions of anesthetics as follows: intraoperative awareness, postoperative agitation, nausea and vomit, adult postoperative cognitive dysfunction (POCD, with an 10-62% incidence rate), influences on infant development of intelligences and central nervous system (CNS) [6]. Therefore, elucidating mechanisms of issues above has a far-reaching significance to revealing general anesthesia mechanism, instructing clinical medication, improving security of clinical anesthesia, developing new general anesthetics and narcotic antagonist [7]. Besides, it contributes to exploring advanced brain functions such as memory, perception, movement, sleep and awakening in neuroscience field. The impacts of general anesthetics such as isoflurane on cognitive function are likely to continue for some time after anesthesia recovery, some of which manifest as postoperative cognitive dysfunction [8].
Dexmedetomidine performs very well in anti-anxiety, sedation and analgesia so that it can reduce stress reaction, maintain stable function of cardiovascular system and protect organs in some ways [2]. During anesthesia recovery, ICU sedation and decannulation, dexmedetomidine awaken patients with a continuous calming effect and good respiratory function [2,9]. As a result, it can provide patients who need anesthesia, intensive care and auxiliary examination with safe and effective sedation and analgesia [3]. In the present study, we aim to evaluate the neuroprotective of dexmedetomidine on cognitive impairment after isoflurane-induced aging rat. Moreover, our results demonstrated that dexmedetomidine revealed antioxidant, anti-inflammatory and anti-apoptosis effects, and in related pathways isoflurane-induced aging rat.
Materials and methods
Animals
Nineteen-month-old male Sprague-Dawley rats from Institute of Experimental Animal of Capital Medical University, Beijing, China, were used to execute this study. The animal protocol was approved by the Standing Committee on Animals at Capital Medical University. All Sprague-Dawley rats were 12 hours/12 hours light/dark cycle, bred under temperature: 22 ± 2°C; humidity: 55 ± 5% and available ad libitum.
Anesthetic exposures and grouping
Fifty Sprague-Dawley rats were allowed to acclimate to their new environment for one week and randomly separated into three groups: (1) anesthesia group (n = 20); (2) treated group (n = 20); and (3) control group (n = 10). In anesthesia group and treated group, all rats received 1.4% isoflurane (BeastBio, Shanghai, China) in 100% oxygen (BeastBio, Shanghai, China) for 2 hours in an anesthetizing chamber. All rats were maintained the rectal temperature at 37 ± 0.5°C. In treated group, all rats were received with 1.0 mg/kg of dexmedetomidine for 6 hours. In anesthesia group and control group, all rats were received with same amount of normal saline.
Cognitive function
The Morris Water Maze test was performed using a round pool (diameter, 150 cm; depth, 50 cm) at 24°C. A video tracking system was recorded to analyze swimming motions using motion detection software for the Morris Water Maze. All rats were returned its regular cage with free access to food, dry and kept warm after every trial. After isoflurane exposure for four days, the place trials were performed on the 15th day to determine the rats’ ability to obtain spatial information. In each of the four quadrants of the swimming pool, all rats received 4 trials every day. Rats were putted into the swimming pool facing the wall and after rats allotted 120 sec to find the platform in the third quadrant upon placed in a fixed position, in which they sat for 20 sec before being removed from the pool. The rat was gently guided to the platform and allowed to remain there for 20 sec, if a rat did not find the platform within 120 sec. The less time it took a rat to reach the platform, the better the learning ability. After the four-day period to evaluate memory retention capabilities, probe trials were conducted immediately. The probe trials were removed submerged platform from the pool and allowing the rats to swim for 120 sec.
Enzyme-linked immunosorbent assay (ELISA) of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), methane dicarboxylic aldehyde (MDA) and superoxide dismutase (SOD) levels
The tissues were homogenized in 70% formic acid, then the homogenates were centrifuged at 12 000 g for 5 hour. The protein concentration was measured using Coomassie blue fast staining solution as instructed (BeastBio, Shanghai, China). Monoclonal antibody was seed into 96-well separable micro-plates and captured A-beta. The absorbance was then measured at 450 nm.
Western blot analysis
Hippocampi were immediately putted into ice and homogenized in epoxydicarboxylic fatty acid buffer with a handheld homogenizer. The homogenates were centrifuged for 10 minutes at 13,000 g at 4°C. The protein concentration was measured using Coomassie blue fast staining solution as instructed (Beyotime, Nanjing, China). 50 mg protein samples were separated using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred into a nitrocellulose membrane (Wuhan Boster Biological Technology., Ltd, Wuhan, China). After membrane blocked with 5% skimmed milk in TBST for 2 h, membrane was incubated with primary antibody against P38 MAPK (1:1000, Santa Cruz Biotechnology, USA), PTEN (1:1000, Santa Cruz Biotechnology, USA), Akt (1:1000, Santa Cruz Biotechnology, USA), p-Akt (1:1000, Santa Cruz Biotechnology, USA) and β-actin (1:1000, Santa Cruz Biotechnology, USA) overnight 4°C. Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies and ECL kit (Beyotime, Nanjing, China).
Statistical analysis
All data were presented as mean ± S.E.M and analyzed by one-way ANOVA. All statistical data were analyzed using SPSS 12 for windows. All P-values were corrected. Statistical significance was accepted at P<0.05.
Results
Neuroprotective of dexmedetomidine increase isoflurane-induced cognitive function in aging rat
After isoflurane exposure, as shown in Figure 1A, the escape latency in isoflurane-induced anesthesia rat is higher than that of control group. However, treatment with dexmedetomidine significantly decreased the isoflurane-induced escape latency in rat (Figure 1A). In the probe test, there was an increase in path length of isoflurane-induced anesthesia group, compared with those in the control group (Figure 1B). Moreover, pretreatment with dexmedetomidine significantly weakened the isoflurane-induced path length in rat (Figure 1B). Isoflurane exposure dramatically inhibited the time spent in target quadrant and times of crossing platform after isoflurane exposure compared with the control (Figure 1C, 1D). In addition, dexmedetomidine treatment could elevate the inhibition the time spent in target quadrant and times of crossing platform after isoflurane exposure in rat (Figure 1C, 1D). Swimming speeds were also analyzed during place trials, and there was no significant difference among the three groups (Figure 1E).
Figure 1.
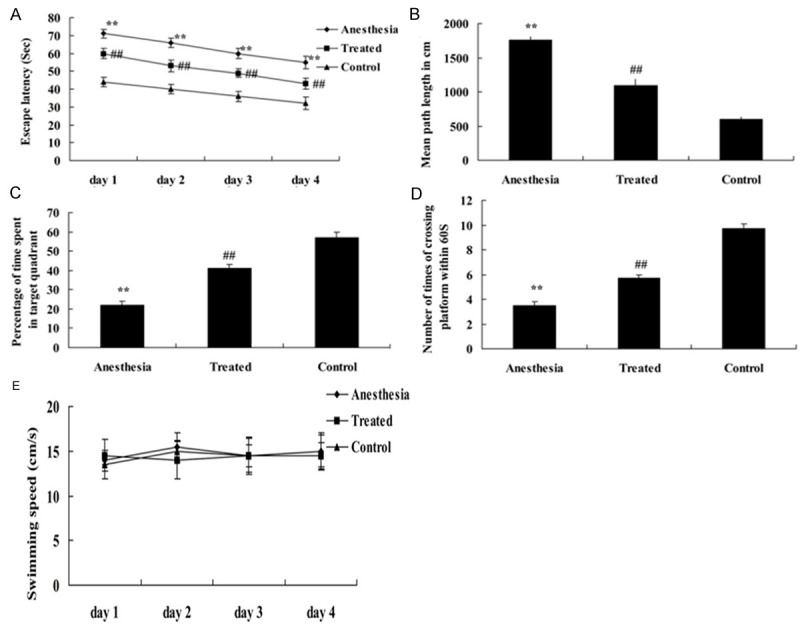
Neuroprotective of dexmedetomidine increase isoflurane-induced cognitive function in aging rat. Neuroprotective of dexmedetomidine on escape latency (A), path length (B), the time spent in target quadrant (C) times of crossing platform (D) and swimming speeds (E) in isoflurane-induced aging rat. **P<0.01 compared with Control group; #P<0.05 compared with anesthesia group.
Antioxidant effect of dexmedetomidine in isoflurane-induced aging rat
As shown in Figure 2A, 2B, there were an efficient increase in the MDA level and an efficient decrease in the SOD level after isoflurane exposure in rat compared with the control. Administrate of dexmedetomidine efficiently reversed the isoflurane-induced MDA and SOD levels in rat following isoflurane exposure (Figure 2A, 2B).
Figure 2.
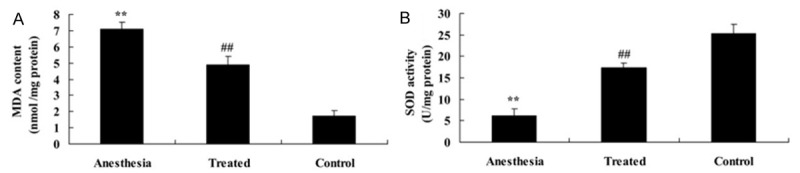
Antioxidant effect of dexmedetomidine in isoflurane-induced aging rat. Antioxidant effect of dexmedetomidine on MDA (A) SOD (B) activities in isoflurane-induced aging rat. **P<0.01 compared with Control group; #P<0.05 compared with anesthesia group.
Anti-inflammatory effect of dexmedetomidine in isoflurane-induced aging rat
As shown in Figure 3A, 3B, rats in isoflurane-induced group showed a rapid advance in TNF-α and IL-1β levels compared with the control. Treat-ment with dexmedetomidine quickly inhibited the advance of TNF-α and IL-1β levels in isoflurane-induced rat.
Figure 3.
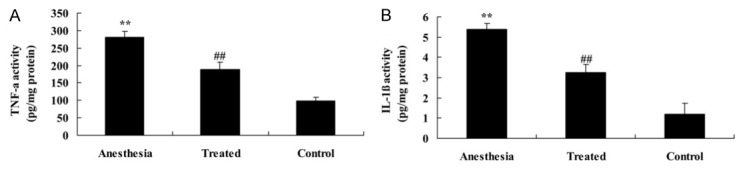
Anti-inflammatory effect of dexmedetomidine in isoflurane-induced aging rat. Anti-inflammatory effect of dexmedetomidine on the TNF-α (A) and IL-1β (B) levels in isoflurane-induced aging rat. **P<0.01 compared with control group; #P<0.05 compared with anesthesia group.
Anti-apoptosis effect of dexmedetomidine on caspase-3 activity in isoflurane-induced aging rat
As shown in Figure 4, the elevation of cell apoptosis and caspase-3 activity of isoflurane-induced aging rat were observed compared with the control. The elevation of caspase-3 activity of isoflurane-induced aging rat was promptly receded by treatment with dexmedetomidine (Figure 4).
Figure 4.
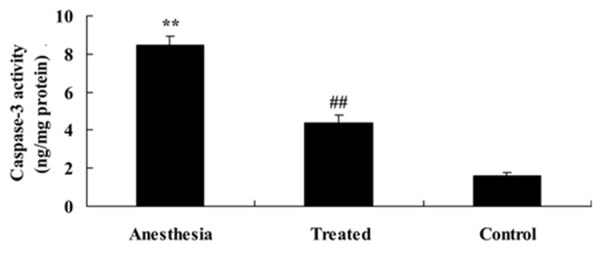
Anti-apoptosis effect of dexmedetomidine on caspase-3 activity in isoflurane-induced aging rat. **P<0.01 compared with Control group; #P<0.05 compared with anesthesia group.
Anti-apoptosis effect of dexmedetomidine on Bcl-xL/Bad in isoflurane-induced aging rat
As shown in Figure 5, Bcl-xL/Bad rate of isoflurane-induced aging rat was memorably de-creased compared with the control. Pretreatment with dexmedetomidine memorably promoted the inhibition of Bcl-xL/Bad rate of isoflurane-induced aging rat (Figure 5).
Figure 5.
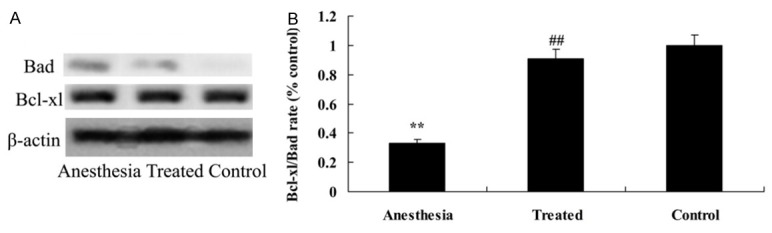
Anti-apoptosis effect of dexmedetomidine on Bcl-xL/Bad in isoflurane-induced aging rat. Anti-apoptosis effect of dexmedetomidine on Bcl-xL/Bad using Western blotting assays (A) and statistical analysis of Bcl-xL/Bad (B) in isoflurane-induced aging rat. **P<0.01 compared with control group; #P<0.05 compared with anesthesia group.
Neuroprotective of dexmedetomidine on P38 MAPK in isoflurane-induced aging rat
To investigate the mechanism effect of dexmedetomidine on cognitive impairment induced by isoflurane anesthesia, the P38 MAPK protein expression was measured using western blot analysis. Compared with control group, the P38 MAPK protein expression was remarkably increased in isoflurane anesthesia group (Figure 6). These impairments were attenuated by dexmedetomidine application in isoflurane anesthesia rat (Figure 6).
Figure 6.
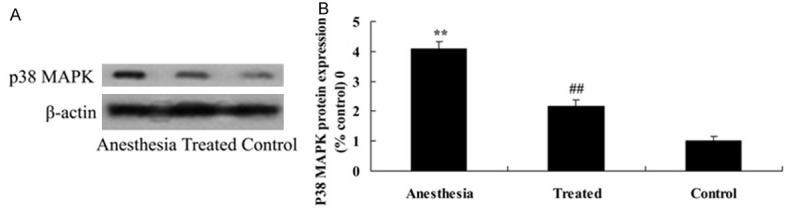
Neuroprotective effect of dexmedetomidine on P38 MAPK in isoflurane-induced aging rat. Anti-apoptosis effect of dexmedetomidine on P38 MAPK using western blotting assays (A) and statistical analysis of P38 MAPK (B) in isoflurane-induced aging rat. **P<0.01 compared with control group; #P<0.05 compared with anesthesia group.
Neuroprotective of dexmedetomidine decrease PTEN expression in isoflurane-induced aging rat
To check into the mechanism effect of dexmedetomidine on cognitive impairment in isoflurane-induced rat, hippocampal region was take out and measured the PTEN protein expression of isoflurane-induced rat. Isoflurane exposure dramatically enhanced the levels of PTEN protein expression in the hippocampal after isoflurane exposure compared with the control (Figure 7A, 7B). The elevation of PTEN protein expression was attenuated by dexmedetomidine application (Figure 7A, 7B).
Figure 7.
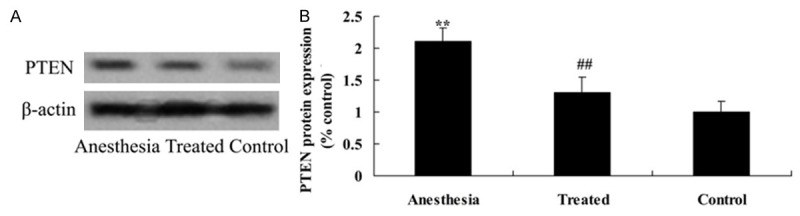
Neuroprotective of dexmedetomidine decrease PTEN expression isoflurane-induced cognitive function in aging rat. Anti-apoptosis effect of dexmedetomidine on PTEN using western blotting assays (A) and statistical analysis of PTEN (B) in isoflurane-induced aging rat. **P<0.01 compared with control group; #P<0.05 compared with anesthesia group.
Neuroprotective of dexmedetomidine increase p-AKT/Akt expression in isoflurane-induced aging rat
To research the mechanism effect of dexmede-tomidine on isoflurane-induced cognitive im-pairment in rat, Akt pathway was analyzed in every group. Akt pathway of isoflurane-induced rat was effectively suppressed compared with the control (Figure 8A, 8B). However, treatment with dexmedetomidine effectively activated the suppression of Akt pathway in isoflurane-induced rat (Figure 8A, 8B).
Figure 8.

Neuroprotective of dexmedetomidine increase p-AKT/Akt expression in isoflurane-induced aging rat. Anti-apoptosis effect of dexmedetomidine on p-AKT/Akt using western blotting assays (A) and statistical analysis of p-AKT/Akt (B) in isoflurane-induced aging rat. **P<0.01 compared with Control group; #P<0.05 compared with anesthesia group.
Discussion
As a CNS complication caused by multiple factors like operation and anesthesia, POCD have clinical manifestations such as insanity, anxiety, personality changes and memory impairment, seriously impacting patients’ life qualities and bringing heavy burden to families and society [10]. Several factors as follows are considered at present to be related to POCD occurrence: anesthesia methods, anesthetic, operation type, basic health condition, complication, hypoxia, hypotension, age, basic cognitive difference and so on. Several anesthetics widely used in clinical practice, including inhalation anesthetics such as isoflurane, sevoflurane and enflurane, and intravenous anesthetics such as ketamine and diprivan, can lead to POCD, the effect of which can be promoted by operations and anesthetic complications. Enjoying the enormous popularity in clinical application, isoflurane significantly influences postoperative cognitive decline. The present study demonstrated that the neuroprotective of dexmedetomidine significantly increase isoflurane-induced cognitive function in aging rat. It is possible that dexmedetomidine may be a potential drug target for isoflurane-induced cognitive function in aging rat.
SOD is a kind of oxygen radical scavenger existed in organisms natively, the activity of which reacts the ability to scavenge free radicals [3]. MDA, with cytotoxicity, is the major degradation product of lipid peroxidation caused by free radicals, the content of which reacts the severity of free radicals attack [3]. It is shown by researches that there were oxidative injuries of animal models’ serums and brain tissues, with higher SOD activity and lower MDA activity [10]. In present study, we found that dexmedetomidine efficiently decrease the MDA level and increase the MDA level isoflurane-induced aging rat. Cakir et al. reported that dexmedetomidine prevent against oxidative damage in rat with renal ischemia reperfusion [11]. Dong et al. found that dexmedetomidine protects against ischemia-reperfusion injury through SOD and MDA in rat with skeletal muscle [12]. Opinions vary on POCD’s complicated pathogenesis. It is proved by basic and clinical researches that isoflurane can induce cognition impairment. Present evidence shows that IL-1, especially IL-1β can lead to reduced learning and memory capacity, and infusing IL-1 receptor antagonist into ventricle can improve global brain ischemia, the reason of which may be related to hippocampal inflammation [13]. According to several researches, increased expressions of hippocampal inflammation cytokines can lead to cognition impairment. It is proved by TNF-α and IL-1β receptors knock-out mice that IL-1β mediated hippocampal inflammation, which is triggered by autoimmune response induced by peripheral operations, is related to memory impairment [14]. The continuous overexpression of IL-1β in mice hippocampi can lead to contextual and spatial memory impairments [15]. In this study, dexmedetomidine significantly decrease the TNF-α and IL-1β levels in isoflurane-induced aging rat. Moreover, Zhang et al. elaborated dexmedetomidine suppressed TNF-α and IL-1β activities in lipopolysaccharide-stimulated astrocytes.
According to recent researches, during neuron death caused by POCD, especially delayed neuronal death along with apoptosis, there occur changes of apoptosis-related genes and their expression products, leading to apoptosis [9]. Participated in pathological processes of neuron damage after cerebral ischemia, Caspase-3 and Bcl-xL/Bad plays an important role in cerebral ischemia [4]. We found that treatment with dexmedetomidine significantly inhibited the elevation of caspase-3 activity and promoted the inhibition of Bcl-xL/Bad rate of isoflurane-induced aging rat. Besides, Li et al. expounded that dexmedetomidine attenuates neurotoxicity opinions through inhibited propofol-induced caspase-3 activation [16]. Li et al. also reported that dexmedetomidine could reduce isoflurane-induced neuroapoptosis of neonatal rats through reducing the ratio of Bcl-xL/Bad and PI3K/Akt pathway [17].
It is found by recent researches that MAPKs signal pathways, especially p38MAPK signal pathway, play important roles in the descent of learning and memory capacity [18]. As a member of MAPKs which can be induced by many factors such as TNF-α and IL-1β, p38MAPK participates in cell proliferation, apoptosis and differentiation, playing an important role in apoptosis [19]. In our study, dexmedetomidine attenuated the p38MAPK protein expression in isoflurane anesthesia aging rat. Tanabe et al. suggested that the effect of dexmedetomidine suppresses IL-1β-induced IL-6 synthesis through P38 MAPK in rat glial cells. Zhou et al. reported that the effects of dexmedetomidine on p38-MAPK in spinal microglia in rats with spared nerve injury [20].
As one of important intracellular transduction signal pathways, PI3K/Akt pathway has classical effects of anti-apoptosis and survival-promoting so that it plays an important role in protection from POCD, new vessels formation and anti-apoptosis. PTEN gene, encoding PTEN protein, is the first highly conservative tumor suppressor gene with dual specificity phosphatase activity. PIP3 is dephosphorylated by PTEN protein specifically to antagonize PI3K/Akt signal pathway so that cell growth signals are perturbed, inducing cell death and cell cycle arrest. In a word, PTEN gene has a negative regulation of cell survival and proliferation [21]. Our results showed that dexmedetomidine suppressed PTEN/PI3K/Akt signal pathway in isoflurane-induced aging rat. Li et al. reported that dexmedetomidine could reduces isoflurane-induced neuroapoptosis of neonatal rats through PI3K/Akt pathway [17].
In conclusion, the neuroprotective effect of dexmedetomidine attenuates isoflurane-induced cognitive impairment through antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Nevertheless, dexmedetomidine is commonly used clinically for anesthesia-induced cognitive impairment in aging rat, this effect of dexmedetomidine may have great translational potential drug if a clinical injury from anesthetics is proven in patients.
Disclosure of conflict of interest
None.
References
- 1.Li SY, Xia LX, Zhao YL, Yang L, Chen YL, Wang JT, Luo AL. Minocycline mitigates isoflurane-induced cognitive impairment in aged rats. Brain Res. 2013;1496:84–93. doi: 10.1016/j.brainres.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: A systematic review and meta-analysis. Clinics (Sao Paulo) 2014;69:777–786. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason KP, Lerman J. Review article: Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129–1142. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 4.Dexmedetomidine for Sedation in the ICU or PICU: A Review of Cost-Effectiveness and Guidelines. Ottawa ON: 2014 Canadian Agency for Drugs and Technologies in Health. 2014 [PubMed] [Google Scholar]
- 5.Zhang Q, Li SZ, Feng CS, Qu XD, Wang H, Zhang XN, Liu Y, Wang Y, Wu AS, Yue Y. Serum proteomics of early postoperative cognitive dysfunction in elderly patients. Chin Med J (Engl) 2012;125:2455–2461. [PubMed] [Google Scholar]
- 6.Liu J, Wang P, Zhang X, Zhang W, Gu G. Effects of different concentration and duration time of isoflurane on acute and long-term neurocognitive function of young adult C57BL/6 mouse. Int J Clin Exp Pathol. 2014;7:5828–5836. [PMC free article] [PubMed] [Google Scholar]
- 7.Maekawa K, Baba T, Otomo S, Morishita S, Tamura N. Low pre-existing gray matter volume in the medial temporal lobe and white matter lesions are associated with postoperative cognitive dysfunction after cardiac surgery. PLoS One. 2014;9:e87375. doi: 10.1371/journal.pone.0087375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Y, Hu H, Liu P, Feng G, Dong W, Yu B, Zhu Y, Song J, Zhao M. Association between the apolipoprotein E4 and postoperative cognitive dysfunction in elderly patients undergoing intravenous anesthesia and inhalation anesthesia. Anesthesiology. 2012;116:84–93. doi: 10.1097/ALN.0b013e31823da7a2. [DOI] [PubMed] [Google Scholar]
- 9.Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH, Liu KX. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta-analysis of randomized clinical trials. J Surg Res. 2013;185:833–843. doi: 10.1016/j.jss.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 10.Kunnimalaiyaan S, Sokolowski KM, Balamurugan M, Gamblin TC, Kunnimalaiyaan M. Xanthohumol inhibits notch signaling and induces apoptosis in hepatocellular carcinoma. PLoS One. 2015;10:e0127464. doi: 10.1371/journal.pone.0127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cakir M, Polat A, Tekin S, Vardi N, Taslidere E, Rumeysa Duran Z, Tanbek K. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren Fail. 2015;37:704–708. doi: 10.3109/0886022X.2015.1011550. [DOI] [PubMed] [Google Scholar]
- 12.Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 13.Tian F, Zhang YJ, Li Y, Xie Y. Celecoxib ameliorates non-alcoholic steatohepatitis in type 2 diabetic rats via suppression of the non-canonical Wnt signaling pathway expression. PLoS One. 2014;9:e83819. doi: 10.1371/journal.pone.0083819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Penta A, Chiba A, Alloza I, Wyssenbach A, Yamamura T, Villoslada P, Miyake S, Vandenbroeck K. A trifluoromethyl analogue of celecoxib exerts beneficial effects in neuroinflammation. PLoS One. 2013;8:e83119. doi: 10.1371/journal.pone.0083119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su SC, Tanimoto K, Tanne Y, Kunimatsu R, Hirose N, Mitsuyoshi T, Okamoto Y, Tanne K. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:845–851. doi: 10.1016/j.joca.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Xiong M, Nadavaluru PR, Zuo W, Ye JH, Eloy JD, Bekker A. Dexmedetomidine Attenuates Neurotoxicity Induced by Prenatal Propofol Exposure. J Neurosurg Anesthesiol. 2015;28:51–64. doi: 10.1097/ANA.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zeng M, Chen W, Liu C, Wang F, Han X, Zuo Z, Peng S. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS One. 2014;9:e93639. doi: 10.1371/journal.pone.0093639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jomha NM, Pinczewski LA, Clingeleffer A, Otto DD. Arthroscopic reconstruction of the anterior cruciate ligament with patellar-tendon autograft and interference screw fixation. The results at seven years. J Bone Joint Surg Br. 1999;81:775–779. doi: 10.1302/0301-620x.81b5.8644. [DOI] [PubMed] [Google Scholar]
- 19.Williams RJ 3rd, Hyman J, Petrigliano F, Rozental T, Wickiewicz TL. Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. Surgical technique. J Bone Joint Surg Am. 2005;87(Suppl 1):51–66. doi: 10.2106/JBJS.D.02805. [DOI] [PubMed] [Google Scholar]
- 20.Zhou TT, Wu JR, Chen ZY, Liu ZX, Miao B. Effects of dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in spinal microglia in rats with spared nerve injury. Brain Res. 2014;1568:21–30. doi: 10.1016/j.brainres.2014.04.025. [DOI] [PubMed] [Google Scholar]


